Polycystic ovary syndrome in adolescents suffering from excessive body weight and insulin resistance
Objective. Evaluate the features of phenotypic variants of PCOS during adolescence with regard to body mass index and insulin resistance (IR).Khashchenko E.P., Vysokikh M.Yu., Batyrova Z.K., Kumykova Z.Kh., Uvarova E.V., Ivanetc T.Yu., Tsvirkun D.V., Vtorushina V.V.
Materials and methods. A study in parallel groups was conducted. The total of 95 girls with PCOS from 15 to 17 years old were divided into four groups according to the presence or absence of IR and excessive body weight. The control group consisted only of lean healthy girls without IR with regular menstrual cycle. All participants underwent full clinical and instrumental examination, assessment of the level of C-reactive protein, leptin.
Results. The obese insulin resistant PCOS group in comparison with lean nonIR PCOS group was characterized with the most unfavorable atherogenic lipid profile and biochemical hyperandrogenism due to the decreased level of sex hormone binding protein (SHBP), more pronounced pro-inflammatory activation according to higher levels of CRP (p = 0.0011), leptin (p = 0.0004) and white blood cell count (p = 0.0235), platelets (p = 0.0012), neutrophils (p = 0.0440) and ESR (p = 0.0036). The obese insulin resistant PCOS group compared with the control group characterized not only by significantly higher values of HOMA-IR (p = 0.0026), fasting glucose (p = 0.0021), higher TG levels (p = 0.0237), atherogenic coefficient (p = 0.0010) and cardiovascular risk by the VAI index (p = 0.0105), lower HDL content (p = 0.0459), and higher value of CRP ( p = 0.0239) and leptin (p = 0.0034) levels. Lean PCOS insulin resistant group significantly differed from lean nonIR PCOS group with a higher iron content (p = 0.0423), higher TG (p = 0.0056) and atherogenic coefficient values (p = 0.0262), lower levels of HDL (p = 0.0391), which proved dyslipidemia, despite the normative BMI in this group. Lean PCOS insulin resistant group differed significantly from the control group with higher levels of total and direct bilirubin (p = 0.0076 and p = 0.0155, respectively) and iron (23.8 ± 8.0 versus 16.6 ± 6 , 8, p = 0.0343), and higher atherogenic coefficient values (p = 0.0206). Obese PCOS nonIR group differed from lean PCOS nonIR group with higher levels of CRP (p = 0.0226) and leptin (p = 0.0267), which indicates that proinflammatory activation occurs in PCOS patients with excessive weight already in adolescence even in the absence of metabolic disorders. Lean PCOS nonIR group differed from the control group with a higher content of total protein (p = 0.0431) and direct bilirubin (p = 0.0251).
Conclusions: the combination of excessive body weight and metabolic disorders since adolescence is associated with increasing dyslipidemia and atherosclerosis, proinflammatory activation as well as an unfavorable cardiovascular risk.
Keywords
Polycystic ovary syndrome (PCOS) is one of the common causes of gynecological and endocrine disorders in adolescence [1-3]. The clinical manifestations of PCOS include not only gynecological, but also multiple metabolic disorders: insulin resistance (IR) and dyslipidemia, obesity, especially visceral obesity, fatty hepatosis, diabetes [4, 5]. The frequency of metabolic disorders in adolescents with PCOS is 3-5 times higher than that in the group of healthy girls of the same age and weight, up to 33% [6]. At the same time, excessive weight is recorded in 38-88% of patients with PCOS [7, 8].
IR and obesity are independent factors of the pathogenesis of PCOS [3, 4, 9]. Overweight girls are at increased risk for the development of IR, hyperinsulinemia, and carbohydrate intolerance [9]. At the same time, patients with PCOS have increased risk of obesity.
There is a growing evidence of the significance of systemic inflammation in the pathogenesis of PCOS due to direct stimulation of androgen production in the ovaries. Thus, TNF-α stimulates in vitro the proliferation of theca cells and thus production of androgens, and also potentiates follicle atresia [10]. Concentration of C-reactive protein and proinflammatory cytokines in PCOS closely correlates with the level of circulating androgens [11-14]. In patients with the risk of PCOS, chronic systemic inflammation accompanied by physiological hyperinsulinemia, insulin resistance and hyperandrogenism at puberty, may trigger the dysregulation of cellular interactions within the ovary and cause hyperandrogenism, anovulation and metabolic complications in future [15].
The aim of the study was to evaluate the features of phenotypic variants of PCOS in adolescence with regard to body mass index and insulin resistance (IR).
Materials and Methods
The study included 95 girls aged from 15 to 17 years, with at least two out of three Rotterdam PCOS criteria: the oligo/amenorrhea, clinical and/or biochemical hyperandrogenism, polycystic ovaries revealed by ultrasound. In addition, the including criteria were menarche at least 2 years ago; exclusion of other endocrine disorders, somatic and infective diseases; no drug administration over 3 months preceding the study; written informed consents. Exclusion criteria were tumors of the pelvic organs, chronic or acute somatic or infectious diseases, mental illness, genetic syndromes and malformations. The control group consisted of 30 lean healthy girls with regular menstrual cycle without metabolic, gynecological and endocrine pathology.
All participants of the study underwent complete clinical examination, including a medical history, anthropometric indicators, evaluation of severity of hirsutism. All girls were determined the concentration of total protein, uric acid, creatinine, direct and total bilirubin, glucose, Ca2+, Fe2+/3+, highly sensitive C-reactive protein (CRP), leptin. They were also measured the level of lipids, including total cholesterol, triglycerides (TG), low density lipoproteins (LDL) and high density lipoproteins (HDL), atherogenic coefficient (CA). Evaluation of the biochemical parameters was performed by photometric and turbidimetric methods on automatic analyzers BA-400 and A-25 using Biosystems reagents (Spain).
Oral glucose tolerance test (OGTT) was performed 12-16 hours after the last meal. The level of glucose and immunoreactive insulin (IRI) was determined in venous blood on an empty stomach twice in 0 and 120 minutes after 75 g glucose administration. The homeostatic index HOMA-IR was calculated. For an indirect measurement of abdominal adipose tissue, the Visceral Adiposity Index (VAI) [16] was used according to the formula:
VAI = (WC ÷ (36.58+ (1.89 × BMI)) × (TG ÷ 0.81) × (1.52 ÷ HDL), where WC is Waist Circumference measured in cm, BMI is measured in kg/m2, TG and HDL are measured in mmol/L.
All girls were performed an ultrasound examination of the pelvic organs on days 3-5 of a spontaneous or progestogen-induced menstrual cycle; besides, there was an analysis of their hormonal profile (LH, FSH, E2, T, SHBP, PRL, DGA-S, 17-OHP, androstendione, AMG, cortisol, TSH, T4, AT-TPO). The determination of hormonal profile was carried out by electrochemiluminescent and immunochemiluminescent methods on automatic analyzers Cobas e 411 (F. Hoffmann-La Roche, Switzerland), Immulite 2000, Immulite 1000 (Siemens, USA) using reagents of the same companies. Determination of AMG, 17-OH-progesterone was carried out by the enzyme immunoassay (ELISA) method on automatic analyzers DYNEX DSX System. The concentration of SHBG was determined by ELISA using the DPC system (USA) on an Immulite analyzer.
Statistical analysis of data was performed with MS Excel and Statistica 8 programs using ANOVA methods. A variety of groups were compared in pairs using post hoc method of Least Significant Difference test, LSD. The variables with non-normal distribution were compared using the Kruskal-Wallis rank tests and post hoc test according to Dunn’s criterion. Correlations were evaluated using the Spearman’s rank correlation coefficient.
Results
Patients with PCOS were divided into four subgroups according to the results of oral glucose tolerance test, HOMA-IR index and BMI; the presence or absence of metabolic disorders (MD) and excessive body weight was also taken into consideration:
- patients with PCOS without metabolic disorders (MD─) and normal weight (BMI<25kg/m2, n = 48; PCOS_MD─/NW);
- overweight PCOS group without MD (BMI≥25kg/m2, n = 10; PCOS_MD─/OW);
- normal weight patients with PCOS and metaboliс disorders (n = 13; PCOS_MD+/NW);
- overweight patients with PCOS and metaboliс disorders (n = 24; PCOS_MD+/OW);
- normal weight healthy girls without MD (control group, n = 30).
Analysis of the anthropometric parameters of PCOS groups in comparison with the control group is presented in Table 1. It was predictable that two overweight PCOS groups significantly differed from normal weight PCOS groups and the control group in such indicators as BMI, WC, hip circumference (HC), waist-to-hip ratio.
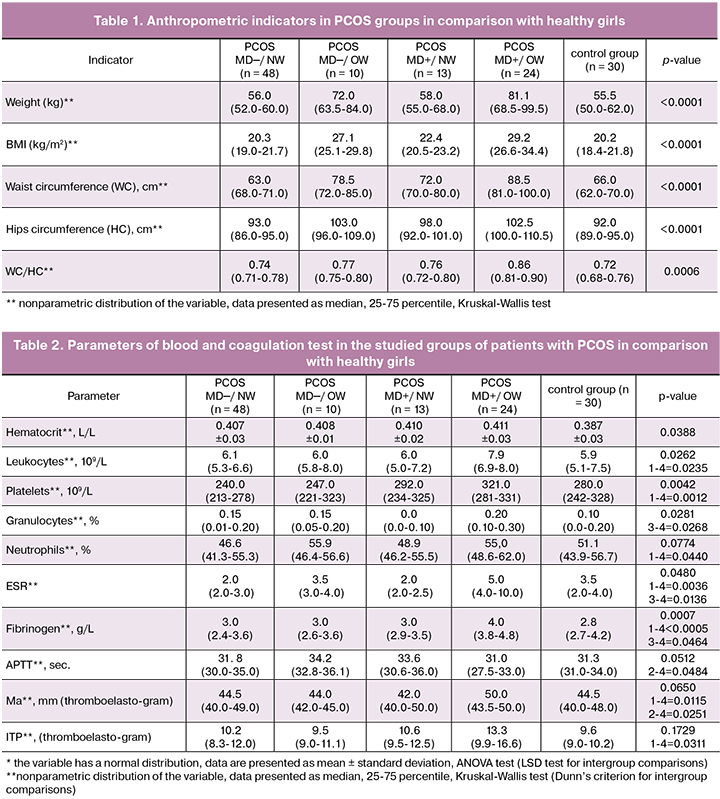
In addition, PCOS_MD+/OW group compared with PCOS_MD─/OW was characterized by significantly higher body weight, BMI, WC and waist-to-hip ratio (p = 0.0135, p = 0.0031, p = 0.0015 and p = 0.0207, hereinafter, the Dunn’s criterion of multiple comparisons). Patients from PCOS_MD+/NW group in comparison with patients of similar weight without MD were characterized by higher WC and HC (p = 0.0435, p = 0.0230), and differed from healthy girls in higher WC (p = 0.0233), that fact proves the risk of atherosclerosis in the group of PCOS patients with MD, even in the absence of excessive weight.
The most essential parameters of the blood and coagulation test of the selected groups are summarized in Table 2. Patients of PCOS_MD+/OW group differed from ones of PCOS_MD─/NW group in a significantly higher content of blood leukocytes (p = 0.0235, hereinafter, the Dunn’s criterion), platelets (p = 0.0012), neutrophils (p = 0.00440), and ESR (p = 0.0036). Despite the fact that these indicators in all groups were within the reference intervals, their increase in the PCOS_MD+/OW group may indicate proinflammatory activation.
Patients of PCOS_MD+/OW group compared with patients of PCOS_MD─/NW group were characterized by simultaneous activation of the blood coagulation system according to thromboelastography data and an increase in fibrinogen concentration (Table 2). Despite the fact that the studied parameters were within the normal intervals, procoagulant activation was detected in the PCOS_MD+/OW group without treatment and may be a risk factor for cardiovascular complications along with dyslipidemia and atherosclerosis.
Patients of PCOS_MD─/NW group differed from patients of the control group in the increased total protein content (73.2 ± 5.3 vs. 70.6 ± 4.6, respectively, p = 0.0431) and direct bilirubin (4.5 ± 2.0 vs. 3.1 ± 1.0, respectively, p = 0.0251). The PCOS_MD+/NW group significantly differed from the PCOS_MD─/NW only in the increased iron content (23.8 ± 8.0 vs. 16.6 ± 6.8, respectively, p = 0.0423), and from the control group in the increased total and direct bilirubin (19.0 ± 5.5 vs. 11.1 ± 4.2, p = 0.0076 and 5.1 ± 3.6 vs. 3.1 ± 1.0, p = 0.0155, respectively) and iron concentration (23.8 ± 8.0 vs. 16.6 ± 6.8, p = 0.0343) (Table 3). It is well known that bilirubin is a strong lipid soluble antioxidant, its elevated concentration in normal weight PCOS patients can be considered as a protective adaptive mechanism against the oxidative stress and the proinflammatory activation observed in PCOS.
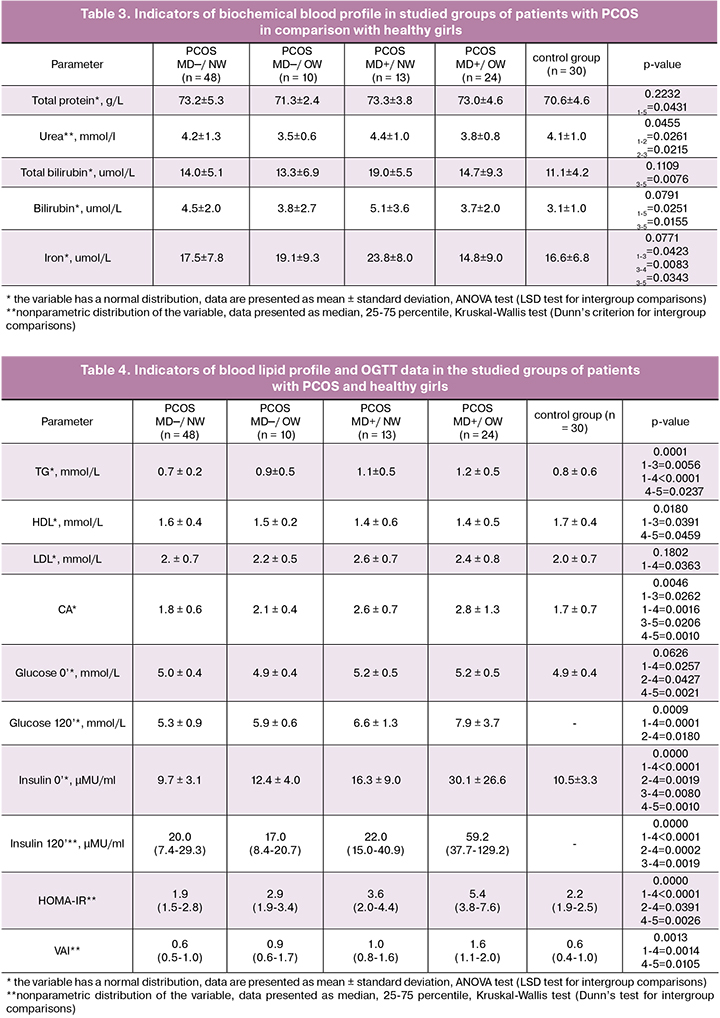
Overweight patients with PCOS showed significantly lower blood urea level than patients with PCOS with normal weight, both with MD (p = 0.0215) and without MD (p = 0.0261) (Table 3). Reduced formation of urea, which is the final product of degradation of nitrogenous bases for excretion, can be a predisposing factor for increasing the number of products of nitrogen metabolism and their damaging effects.
Analysis of the blood lipid profile of PCOS_MD−/NW patients did not reveal any differences from the profile in patients of control group (Table 4). The PCOS_MD+/NW group was different from the PCOS_MD−/NW group in higher TG and CA level (p = 0.0056 and p = 0.0262, respectively) and lower HDL level (p = 0.0391). At the same time, a significant difference from the control group was observed only in CA parameter which was higher in the PCOS_MD+/NW (p = 0.0206) group.
The PCOS_MD+/OW group compared with the group of healthy girls showed, as it was expected, dyslipidemia according to an elevated TG level (p = 0.0237) and CA (p = 0.0010), reduced HDL content (p = 0.0459), higher values of HOMA-IR (p = 0.0026) and fasting glucose (p = 0.0021), as well as increased cardiovascular risk according to the VAI index (p = 0.0105). In addition, PCOS_MD+/OW patients differed from PCOS_MD−/NW patients in the same parameters as from the control group: higher TG concentration (p <0.0001), LDL (p = 0.0363) and CA (p = 0.0016), elevated glucose and insulin levels, high HOMA-IR (p <0.0001 for each indicator), as well as higher VAI index (p = 0.0105) (Table 4).
According to our results, excessive weight related to PCOS in the absence of metabolic disorders did not cause significant differences in blood lipid spectrum compared to the control group in adolescence. At the same time, PCOS_MD+/OW differed from the control group and other PCOS subgroups in number of parameters of proinflammatory and procoagulant blood activation (Tables 3 and 4). Some studies show that PCOS in adulthood is associated with impairments of the lipid profile and higher values of HOMA-IR, which are observed not only in overweight patients, but also in patients with normal weight and without metabolic disorders [3, 4]. In adolescent patients in the studied groups, combination of excessive weight and metabolic disorders in PCOS group led to atherogenic lipid profile and increased cardiovascular risk.
Analysis of CRP as the marker of inflammation revealed its higher level in both groups of overweight patients in comparison with normal weight patients regardless of the MD presence (p = 0.0127 and p = 0.0226, respectively) (Table 5). In the PCOS_MD+/OW group the level of CRP was significantly higher compared with the PCOS_MN─/NW group (p = 0.0011) and the group with healthy girls (p = 0.0239). A positive relationship was found between the body weight and CRP level (r = 0.29; p <0.05). The positive association of excessive weight and CRP level was confirmed by two-factor analysis (p = 0.0028). However, according to the literature data, in adult patients the results of meta-analyses revealed an increased level of CRP in PCOS which was associated not only with obesity, but with the disease itself [13, 14]. In our study in adolescent patients CRP level was not influenced by pronounced factors of PCOS and MD, it was affected by excessive weight.
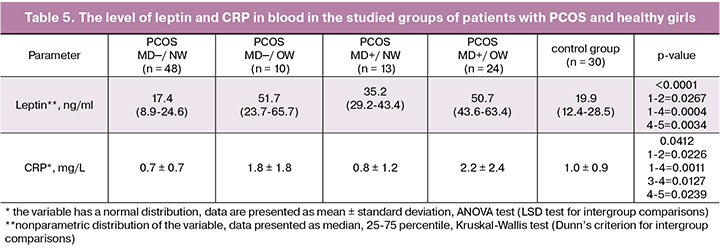
As it was predicted, in patients with PCOS with excessive weight and metabolic disorders hyperleptinemia was detected because this adipokine is secreted mainly by subcutaneous adipose tissue (Table 5). At the same time, the level of leptin was significantly higher in overweight patients regardless of the presence of MD compared with normal weight groups (p = 0.0267 and p = 0.0004, respectively). In the PCOS_MD+/OW group leptin level was also higher than in control group (p = 0.0034). Factorial analysis revealed the independent influence of factor of excessive body weight (p = 0.0007) and the presence of metabolic disorders (p = 0.0083) on the level of leptin in the blood. Both factors independently led to an increase of leptin level, which can also be considered as a factor of the systemic inflammatory response activation in PCOS patients with excessive weight and metabolic disorders already in adolescence. Thus, according to the results of our study, we suggest that in adolescent girls, PCOS itself is not associated with proinflammatory activation. Elevated levels of inflammatory markers (leukocyte count, CRP, leptin) were observed only in subgroups with obesity and/or in the presence of metabolic disorders. Along with the activation of the coagulation system, proinflammatory status is a factor of endothelial dysfunction in PCOS related to MD in patients already in adolescence.
A comparative analysis of the hormonal profile of blood revealed significantly higher concentrations of LH, LH/FSH ratio, T, FAI, 17-OHP, cortisol and androstendione levels in patients with PCOS compared to healthy girls. Both subgroups of overweight PCOS patients differed from normal weight patients in the hormonal profile with the concentration of sex hormone binding protein (SHBP) and with free androgen index. In the group of PCOS_MD+/OW compared with the group of PCOS_MD─/NW a significant decrease in SHBG was found (25.1 (20.3-32.0) vs. 47.5 (36.4-68.4); p = 0.0003, Dunn’s criterion), and, accordingly, a significant increase in FAI (5.9 (4.8-9.2) vs. 3.6 (1.6-6.0), p = 0.0143).
Thus, all patients with PCOS compared with healthy girls were characterized not only by higher rates of ovarian hyperandrogenism, but activation of the hypothalamic-pituitary-ovarian and adrenal axis, which was confirmed by higher levels of LH, LH/FSH, cortisol, 17-OHP, DGA-S and androstendione. Moreover, patients with PCOS were characterized by higher concentrations of AMH compared with the group of healthy adolescents. At the same time, patients with PCOS related to MD and excessive weight were characterized by the highest levels of the free androgen index and free testosterone in the blood in these subgroups.
Analyzing patients with PCOS and taking into account the presence of metabolic disorders and excessive BMI, we revealed that the PCOS_MD+/OW group in comparison with the PCOS_MD─/NW group was characterized not only by the most unfavorable atherogenic lipid profile and biochemical hyperandrogenism due to the decreased level of SHBP, but also more pronounced proinflammatory activation according to higher levels of CRP (p = 0.0011), leptin (p = 0.0004) and white blood cell count (p = 0.0235), platelets (p = 0.0012), neutrophils (p = 0.0440) and ESR (p = 0.0036).
The PCOS_MD+/OW group compared with the control group characterized not only by significantly higher values of HOMA-IR (p = 0.0026), fasting glucose (p = 0.0021), higher TG levels (p = 0.0237), atherogenic coefficient (p = 0.0010) and cardiovascular risk by the VAI index (p = 0.0105), lower HDL content (p = 0.0459), but also higher CRP ( p = 0.0239) and leptin (p = 0.0034) levels that are the markers of systemic inflammation activation.
The PCOS_MD+/NW group significantly differed from PCOS_MD─/NW group with a higher iron content (p = 0.0423), higher TG (p = 0.0056) and atherogenic coefficient values (p = 0.0262), lower levels of HDL (p = 0.0391) which proved dyslipidemia, despite the normative BMI parameters in this group.
The PCOS_MD+/NW group differed significantly from the control group in higher levels of total and direct bilirubin (p = 0.0076 and p = 0.0155, respectively) and iron (23.8 ± 8.0 vs. 16.6 ± 6.8; p = 0.0343), and higher atherogenic coefficient values (p = 0.0206) that evidenced the activation of heme metabolism antioxidant mechanism against oxidative stress related to dyslipidemia in this group.
The PCOS_MD─/OW group differed from PCOS_MD─/NW group with higher levels of CRP (p = 0.0226) and leptin (p = 0.0267), which indicates that proinflammatory activation in PCOS patients with excessive weight already in adolescence even in the absence of metabolic disorders.
The PCOS_MD─/NW group differed from the control group with a higher content of total protein (p = 0.0431) and direct bilirubin (p = 0.0251).
Conclusion
The results of our study in adolescents with PCOS, in contrast to adult patients, indicate that excessive weight itself does not have a significant influence on the studied parameters of the blood lipid spectrum in girls with PCOS, unless the development of the disease is associated with abnormalities of carbohydrate metabolism and insulin resistance. On the contrary, the combination of obesity and metabolic disorders already in the adolescence leads to the atherogenic dyslipidemia as well as an increase of cardiovascular risk.
We revealed a key role of obesity factor in the CRP level (p = 0.0028), as well as an independent effect of obesity (p = 0.0007) and metabolic disorders (p = 0.0083) on leptin level in the blood in adolescents with PCOS. The findings suggest that in adolescence at the very onset of the disease, PCOS itself could not be associated with proinflammatory activation, in contrast to the results typical for adult women. At the same time, the presence of excessive weight and / or metabolic disorders, especially their combination, is to be considered as a factor of significant activation of the systemic inflammatory response and cardiovascular risk in PCOS girls already in adolescence. Due to the social significance of metabolic and cardiovascular complications in PCOS patients, the parameters of blood lipid profile, carbohydrate metabolism and BMI should be considered in management of PCOS phenotypes in adolescent patients.
References
1. Deligeoroglou E., Vrachnis N., Athanasopoulos N., Iliodromiti Z., Sifakis S., Iliodromiti S. et al. Mediators of chronic inflammation in polycystic ovarian syndrome. Gynecol. Endocrinol. 2012; 28(12): 974-8. doi: 10.3109/09513590.2012.683082.
2. Чернуха Г.Е., Блинова И.В., Купрашвили М.И. Эндокринно-метаболические характеристики больных с различными фенотипами синдрома поликистозных яичников. Акушерство и гинекология. 2011; 2: 70-6. [Chernukha G.Ye., Blinova I.V., Kuprashvili M.I. Endocrine and metabolic characteristics of patients with different phenotypes of polycystic ovary syndrome. Akusherstvo i ginekologiya/Obstetrics and Gynecology. 2011.-N 2. P.70-76. (in Russian)]
3. El Hayek S., Bitar L., Hamdar L.H., Mirza F.G., Daoud G. Poly cystic ovarian syndrome: an updated overview. Front. Physiol. 2016; 7: 124. doi: 10.3389/fphys.2016.00124.
4. Rojas J., Chávez M., Olivar L., Rojas M., Morillo J., Mejías J. Polycystic ovary syndrome, insulin resistance, and obesity: navigating the patophysiologic labyrinth. Int. J. Reprod. Med. 2014; 2014: 719050. doi: 10.1155/2014/719050.
5. De Sousa S.M., Norman R.J. Metabolic syndrome, diet and exercise. Best Pract. Res. Clin. Obstet. Gynaecol. 2016; 37: 140-51. doi: 10.1016/j.bpobgyn.2016.01.006.
6. Goodman N.F., Cobin R.H., Futterweit W., Glueck J.S., Legro R.S., Carmina E. American association of clinical endocrinologists, american college of endocrinology, and androgen excess and pcos society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome- part 1. Endocr. Pract. 2015; 21(11): 1291-300. doi: 10.4158/EP15748.DSC.
7. Oleszczak B., Szablewski L., Pliszka M., Głuszak O., Stopińska-Głuszak U. Transport of deoxy-d-glucose into lymphocytes of patients with polycystic ovary syndrome. Endocrine. 2014; 47(2): 618-24. doi: 10.1007/s12020-014-0174-5.
8. Dumesic D.A., Oberfield S.E., Stener-Victorin E., Marshall J.C., Laven J.S., Legro R.S. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr. Rev. 2015; 36(5): 487-525. doi: 10.1210/er.2015-1018.
9. Diamanti-Kandarakis E., Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr. Rev. 2012; 33(6): 981-1030.
10. González F. Inflammation in polycystic ovary syndrome: underpinning of insulin resistance and ovarian dysfunction. Steroids. 2012; 77(4): 300-5. doi: 10.1016/j.steroids.2011.12.003.
11. Yang Y., Qiao J., Li R., Li M.Z. Is interleukin-18 associated with polycystic ovary syndrome? Reprod. Biol. Endocrinol. 2011; 9: 7-18. doi: 10.1186/1477-7827-9-7.
12. Tao T., Li S., Zhao A., Zhang Y., Liu W. Expression of the CD11c gene in subcutaneous adipose tissue is associated with cytokine level and insulin resistance in women with polycystic ovary syndrome. Eur. J. Endocrinol. 2012; 167(5): 705-13. doi: 10.1530/EJE-12-0340.
13. Escobar-Morreale H.F., Luque-Ramírez M., González F. Circulating inflammatory markers in polycystic ovary syndrome: a systematic review and meta-analysis. Fertil. Steril. 2011; 95(3): 1048-58. doi: 10.1016/j.fertnstert.2010.11.036.
14. Lee H., Oh J.Y., Sung Y.A. Adipokines, insulin-like growth factor binding protein-3 levels, and insulin sensitivity in women with polycystic ovary syndrome. Korean J. Intern. Med. 2013; 28(4): 456-63. doi: 10.3904/kjim.2013.28.4.456.
15. Conway G., Dewailly D., Diamanti-Kandarakis E., Escobar-Morreale H.F., Franks S., Gambineri A. et al. The polycystic ovary syndrome: a position statement from the European Society of Endocrinology. Eur. J. Endocrinol. 2014; 171: 1-29. doi: 10.1530/EJE-14-0253.
16. Amato M.C. Metabolically healthy polycystic ovary syndrome (MH-PCOS) and metabolically unhealthy polycystic ovary syndrome (MU-PCOS): a comparative analysis of four simple methods useful for metabolic assessment. Hum. Reprod. 2013; 28(7): 1919-28.
Received 15.06.2018
Accepted 23.06.2018
About the Authors
Khashchenko, Elena P., PhD, researcher at the Department of Pediatric Gynecology of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of Ministry of Healthcare of Russian Federation.117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954388542. Е-mail: khashchenko_elena@mail.ru
Vysokikh Mikhail Yu., PhD, Head of mitochondrial medicine research group, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of Ministry of Healthcare of Russian Federation, Head of aging molecular mechanism group, A.N. Belozersky Research Institute of Physicochemical Biology. 117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954387633 (1472). E-mail: m_vysokikh@oparina4.ru
Batyrova, Zalina K., PhD, researcher at the Department of Pediatric Gynecology of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of Ministry of Healthcare of Russian Federation. 117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954388542. Е-mail: linadoctor@mail.ru
Kumykova, Zaira K., PhD, senior researcher at the Department of Pediatric Gynecology of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of Ministry of Healthcare of Russian Federation.
117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954388542. E-mail: zai-kumykova@yandex.ru
Vtorushina, Valentina V., PhD, clinician of laboratory diagnostics at the department of clinical immunology at the Akademician V.I. Kulakov Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954381183. E-mail: v_vtorushina@oparina4.ru
Tsvirkun, Darya V., PhD, researcher at mitochondrial medicine research group, Akademician V.I. Kulakov Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954387633 (1472). E-mail: darunyat@gmail.com
Uvarova, Elena V., MD, PhD, Professor, Head of the Department of pediatric and adolescent gynecology, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of Ministry of Healthcare of Russian Federation.
117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954388509. E-mail: elena-uvarova@yandex.ru
Ivanets, Tatyana Yu., PhD, MD, Head of the Department of Research and Diagnostics Laboratory of the Federal State Budget Institution “Research Center for Obstetrics, Gynecology and Perinatology” Ministry of Healthcare of the Russian Federation. 117997, Russia, Moscow, Ac. Oparina str. 4. Е-mail: t_ivanets@oparina4.ru
For citations: Khashchenko E.P., Vysokikh M.Yu., Batyrova Z.K., Kumykova Z.Kh., Uvarova E.V., Ivanetc T.Yu., Tsvirkun D.V., Vtorushina V.V. Polycystic ovary syndrome in adolescents suffering from excessive body weight and insulin resistance. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2018; (12): 94-102. (in Russian)
http://dx.doi.org/10.18565/aig.2018.12.94-102



