Diagnostic significance of determining the expression of energy metabolism genes in fetal growth retardation
Objective: To determine the level of expression of energy metabolism genes, namely visfatin (NAMPT), ghrelin (GHRL) and leptin (LEP) in maternal and umbilical cord blood and in the placenta in case of fetal growth retardation.Kan N.E., Soldatova E.E., Tyutyunnik V.L., Volochaeva M.V., Sadekova A.A., Krasnyi A.M.
Materials and methods: The study included 52 pregnant women: the main group consisted of 27 patients diagnosed with fetal growth retardation postnatally; the control group included 25 women with normal course of pregnancy. Real-time PCR was used to determine the expression level of energy metabolism genes.
Results: The level of expression of the NAMPT and GHRL genes in maternal blood was found to be statistically significantly reduced in fetal growth retardation (p=0.012 and p=0.019, respectively). The level of expression of the NAMPT and GHRL genes in umbilical cord blood was also reduced in comparison with the control group, but it was not statistically significant (p=0.30 and p=0.23, respectively). LEP gene expression in maternal and umbilical cord blood was not found. The level of leptin expression in the placenta was found to be statistically significantly increased in the main group (p=0.045), though these differences were not associated with gestational age at the time of delivery.
Conclusion: The decreased levels of expression of the NAMPT and GHRL genes in maternal blood can become an objective marker for diagnosing fetal growth retardation during pregnancy. The increased expression of LEP in the placenta in fetal growth retardation may give a better understanding of its pathogenesis and new opportunities for its diagnosis.
Authors’ contributions: Kan N.E., Soldatova E.E., Tyutyunnik V.L., Volochaeva M.V., Sadekova A.A., Krasnyi A.M. – developing the concept and design of the study, obtaining data for analysis, review of publications, processing and analysis of material on the issue, writing the text of the manuscript, editing the article.
Conflicts of interest: The authors declare no possible conflicts of interest.
Funding: The study was conducted without sponsorship.
Ethical Approval: The study was approved by the Ethical Review Board of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia (Protocol No.11 – 11/11/2021).
Patient Consent for Publication: All patients signed an informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Kan N.E., Soldatova E.E., Tyutyunnik V.L., Volochaeva M.V.,
Sadekova A.A., Krasnyi A.M. Diagnostic significance of determining the expression of energy metabolism genes in fetal growth retardation.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (8): 48-55 (in Russian)
https://dx.doi.org/10.18565/aig.2023.93
Keywords
Despite ongoing research, fetal growth retardation (FGR) remains one of the leading problems of modern obstetrics and perinatology. According to the fetal origin of adult diseases hypothesis, non-optimal intrauterine environment for fetal development may result in metabolic, cardiovascular, and other conditions, namely obesity, type 2 diabetes mellitus (DM), atherosclerosis, and hypertension in adults [1]. This concept confirms the idea that fetal nutrition and hormonal status during pregnancy can irreversibly affect the metabolic processes and lead to pancreatic β-cell dysfunction; they are also likely to change the insulin sensitivity of tissues causing disorders of carbohydrate metabolism in postnatal life. A great number of studies have shown the important role of growth hormone and insulin-like growth factors 1 and 2 in the growth and development of the fetus and child [2–5]. Moreover, considerable attention has recently been paid to adipose tissue and particularly to adipocytokines [4, 6], which include structurally and functionally diverse and highly active peptides, proteins and hormones that regulate glucose homeostasis. Some of them are synthesized by the placenta; therefore, they may play a role in the intrauterine development of the fetus, regulation of its growth and energy metabolism.
The scientists have recently been interested in studying the role of ghrelin during pregnancy. Ghrelin is a peptide hormone and it is mainly produced by endocrine cells of the mucous membrane of the gastrointestinal tract and pancreas [4]. The expression and synthesis of ghrelin are also found in the placenta (cytotrophoblast) and are suggestive of its role during pregnancy; however, its functions are not fully understood [7].
Another hormone synthesized by adipocytes and placenta during pregnancy is leptin and it is involved in the growth and development of the fetus as well. According to some studies, synthesis of such adipocytokine increases in pregnancy complications, namely placental insufficiency, preeclampsia and gestational diabetes mellitus [4, 8].
Visfatin plays an important role in the course of normal pregnancy and in the development of complications. This adipocytokine is mainly produced by adipose tissue, namely visceral fat, and it can be expressed in the placenta, fetal membranes, myometrium, macrophages and neutrophils [9]. It should be noted that visfatin has an immunoregulatory characteristic as it can regulate the pro- and anti-inflammatory activity of cytokines with human monocytes and its metabolic properties. After binding to insulin receptors, visfatin causes an insulin-mimicking effect, stimulates glucose uptake by peripheral tissues and inhibits gluconeogenesis in the liver [10].
Taking into account the differences in the literature data on various concentrations of adipocytokines in pregnancy complications [2, 4, 11] including FGR, we paid particular attention to the study of the expression of visfatin, ghrelin and leptin genes in the blood of the mother and fetus, as well as in the placenta. Thus, we can obtain new data on the pathogenetic features of FGR and identify potential markers of this pregnancy complication.
The aim of the study is to determine the level of expression of energy metabolism genes, namely visfatin (NAMPT), ghrelin (GHRL) and leptin (LEP) in maternal and umbilical cord blood and in the placenta in case of FGR.
Materials and methods
The study was carried out at the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow, Russia. The study included 52 pregnant women: group I (main) consisted of 27 patients diagnosed with FGR postnatally; group II (control) included 25 women with a normal pregnancy without FGR according to the data of clinical examination and instrumental diagnostic tests.
The study was approved by the Ethical Review Board of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia (Protocol No.11 – 11/11/2021).
FGR diagnosis at the antenatal stage was made according to the criteria of Delphi consensus methodology for early and late forms. The postnatal diagnosis of low birth weight or small for gestational age was made on the basis of birth weight less than the 10th percentile according to the percentile scales of INTERGROWTH-21 for full-term and premature infants.
There were the following criteria for the inclusion of pregnant women in the study: gestation period of 22–40 weeks, age of pregnant women from 18 to 49 years, single pregnancy, pregnancy complicated by FGR (main group), normal pregnancy (control group), signed informed consent to participate in the study.
The exclusion criteria for both groups were as follows: multiple pregnancy, the use of a donor egg, severe extragenital pathology (type I diabetes and others), antiphospholipid syndrome, acute infectious and genetic (balanced chromosomal rearrangements) diseases of a pregnant woman, fetal malformations, hemolytic disease of the fetus.
In order to determine the level of gene expression in the blood, samples of venous blood of pregnant women and venous umbilical cord blood which were obtained during delivery were collected in vacuum tubes containing ethylenediaminetetraacetic acid (EDTA) in the volume of 5 ml. The samples were processed within 30 minutes after the biological material was collected. Then, 200 µl of ExtractRNA lysis buffer (Evrogen JSC, Russia) was added to 200 µl of whole blood; the samples were subsequently stored at a temperature of -80°C.
The fragments of placental tissue from the paracentral zone including the villous chorion, sized 1.0×0.5 cm, were placed in a cryoprobe containing RNAlater solution for 10 minutes after delivery; the fragments were subsequently stored at a temperature of -80°C.
After obtaining a complete collection, the blood and placenta samples were thawed. Total RNA was isolated according to the manufacturer’s protocol by phenolic extraction using ExtractRNA reagent (Evrogen JSC, Russia). The level of purity and concentration of RNA was determined spectrophotometrically on the DeNovix device (Thermo Fisher, USA). cDNA synthesis was carried out using the OT-1 Reverse Transcription Kit (Syntol, Russia) according to the manufacturer’s protocol.
Amplification of the mRNA level of the NAMPT, GHRL, LEP genes and the HPTR1 housekeeping gene was performed by polymerase chain reaction (PCR) with the detection of accumulation of reaction products in real time (RealTime PCR) using the CFX-96 amplifier (Bio-Rad, USA) and specific primers and probes to the studied sections of genes. The threshold cycle was defined as the arithmetic mean of three repetitions. The primer and probe sequences are presented in Table 1.
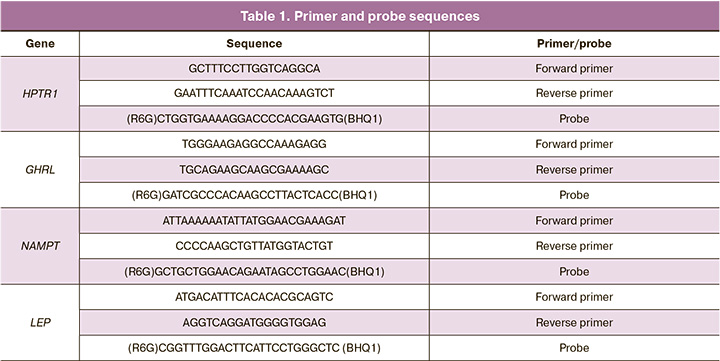
PCR was performed at 95°C for 5 min followed by 40 cycles of 95°C for 10 s and 60°C for 25 s.
Three placenta samples were used for positive control of primers and the validity of all four primers was confirmed. The HPTR1 housekeeping gene was used to normalize the results.
Statistical analysis
Statistical processing of the results was carried out using Microsoft Excel, OriginPro 8 and SPSS Statistics 17. The hypothesis of a normal distribution was tested using the Shapiro–Wilk test (with the number of subjects less than 50). When two groups were compared by a quantitative indicator with a distribution other than normal, the Mann–Whitney U test was used. The data are described using median (Me), lower and upper quartiles (Q1;Q3); the diagrams in the figures are presented as percentiles (5; 25; 50; 75; 95). Qualitative parameters were compared using Fisher’s exact test. Categorical data were described in absolute values and percentage (%). The diagnostic accuracy of the obtained data was evaluated using ROC analysis. The area under the ROC curve, sensitivity and specificity were determined. The indicators of diagnostic accuracy were calculated by constructing conjugacy tables, 95% confidence interval (CI) was calculated for each indicator. To identify the correlation between the variables, the Spearman’s correlation coefficient was calculated. The differences between the compared values were considered statistically significant when p-value was less than 0.05.
Results and discussion
All the patients included in the study were compared in initial clinical and anamnestic characteristics (Table 2).
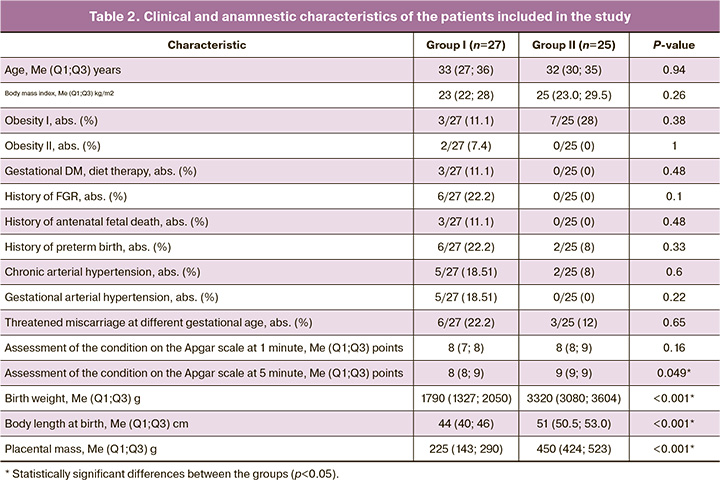
The age of pregnant women ranged from 24 to 46 years and it was 33 (27; 36) years in the main group and 32 (30; 35) years in the control group (p=0.94). The weight and height parameters did not deviate from the population norms. Body weight was 63 (57.0; 70.5) and 73.8 (62.5; 79.0) kg; height was 162 (160; 164) cm and 165 (160; 168) cm in groups, respectively, (p>0.05).
Some studies showed the importance of the body mass index and disorders of fat metabolism in pregnant women and their relationship with the above hormones; therefore, we studied these characteristics. Body mass index in the main group was 23 (22; 28) kg/m2, and it was 25 (23.0; 29.5) kg/m2 in the control group (p=0.26). The incidence of grade I and II obesity, as well as gestational diabetes did not differ in the study groups.
The analysis of obstetric history showed that FGR (6/27, 22.2%), antenatal fetal death (3/27, 11.1%), as well as premature birth (6/27, 22.2%) occurred more often in group I, but it was not statistically significant.
The course of pregnancy in the patients of the main group was complicated by threatened miscarriage at different stages of pregnancy (6/27, 22.2%, p=0.65), by the development of chronic (5/27, 18.51%, p=0.6) and gestational hypertension (5/27, 18.51%, p=0.22).
The patients with FGR gave birth at 36.5 (34.4; 37.3) weeks gestation in comparison with the control group where the delivery was at 38.3 (37.7; 39.4) weeks gestation (p=0.001); it can be explained that early delivery of the patients was necessary due to the deterioration of the fetus according to the instrumental diagnostic tests. The rate of preterm birth in this group was 14/27 (51.85%) versus 2/25 (8%) in the control group, p=0.007.
The children were born with an Apgar score from 5 to 9 points. The analysis of anthropometric data showed that the birth weight of children in the main group was 1790 (1327; 2050) g, and birth weight of children from the control group was 3320 (3080; 3604) g (p<0.001). According to the INTERGROWTH-21 scale, the percentile of body weight after birth was 1 (1;2) and 70 (55;85) in groups, respectively (p<0.001).
The analysis of the expression level of the LEP, NAMPT, GHRL genes by real-time PCR did not reveal LEP gene expression in maternal blood in any of the two groups.
However, the expression level of the NAMPT and GHRL genes in maternal blood was statistically significantly lower in the group with FGR. Thus, the expression level of GHRL in the main group was 1 (0.8; 2.4) relative expression unit (REU) and it was 2.34 (2.06; 2.82) REU in the control group, p=0.019. The NAMPT expression was also statistically significantly lower in women with FGR and amounted to 1 (0.84;1.47) REU and it was 1.89 (1.23; 2.07) REU in the control group, p=0.012, which is indicative of the role of visfatin and ghrelin in FGR (Fig. 1).
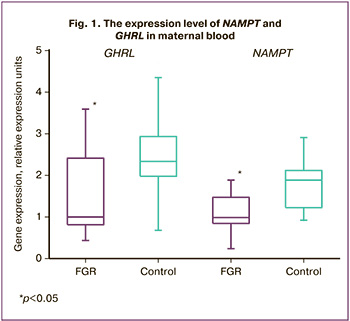
Visfatin is known to be involved not only in glucose and insulin metabolism, but also in the production of pro- and anti-inflammatory cytokines, thereby it can influence the immune system regulation [12]. The visfatin expression increases with the activation of the immune system, thus it becomes an important mechanism for providing cells with sufficient energy due to the insulin mimetic action and glucose uptake by cells [13]. The decrease in the expression of visfatin in leukocytes can be explained by reduced cell activity which may be associated with the suppression of the immune response in FGR.
The studies of ghrelin and its effect on the regulation of growth hormone / insulin-like growth factor-1 axis are of particular interest. The prolonged action of ghrelin increases their level and contributes to the normal growth and differentiation of fetal cells and tissues, as well as the placenta [14]. In our study, there was a statistically significant decrease in the expression of the ghrelin gene in maternal blood, as well as a statistically significant decrease in the length and weight of the fetus at birth in the group with FGR, as well as a lower weight of the placenta in comparison with the control group. The obtained results are consistent with the literature data on the relationship between ghrelin levels and growth hormone / insulin-like growth factor-1 axis; however, this requires further study.
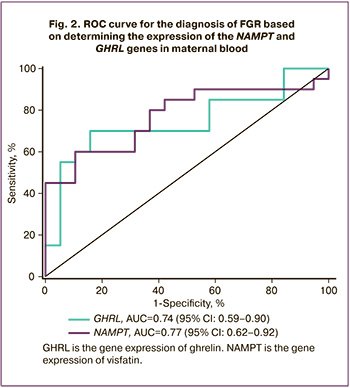
To determine the diagnostic significance of the gene expression of NAMPT and GHRL in maternal blood, the ROC analysis was performed (Fig. 2). The area under the ROC curve for ghrelin gene expression was found to be 0.74 (95% CI: 0.59-0.90) with a threshold value of 1.27 REU, sensitivity was 70% and specificity was 84.20% (p=0.02). The prognostic value of a positive result was 82.35% (95% CI: 56.57–96.20), and negative result was 72.73% (95% CI: 49.78–89.27). The area under the ROC curve for the gene expression of visfatin was 0.77 (95% CI: 0.62-0.92) with a threshold value of 1.01 REU, sensitivity was 60%, and specificity was 89.40% (p=0.01). The prognostic value of a positive result was 85.71% (95% CI: 57.19 – 98.22), and negative result was 68.00% (95% CI: 46.50–85.05). Thus, according to the expert scale, both parameters have a good diagnostic value and they can be considered as potential markers of FGR.
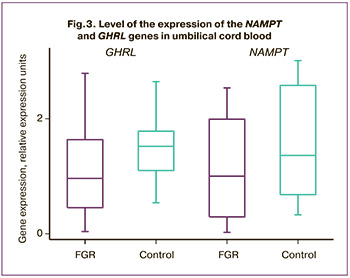
Taking into account the correlation of various pathological processes which occur in the mother-placenta-fetus system, we studied their expression in umbilical cord blood and in the placenta. The analysis of LEP gene expression in umbilical cord blood did not reveal expression in any of the two groups. The expression level of the NAMPT and GHRL genes was reduced in comparison with the control group, but it was not statistically significant. Thus, the level of NAMPT expression in the main group was 1 (0.29; 1.99) REU and it was 1.36 (0.7; 2.4) REU in the control group, p=0.30; GHRL expression level was 0.96 (0.45; 1.64) and 1.52 (1.10; 1.77) REU, p=0.23, respectively (Fig. 3).
The study of gene expression in the placenta is of particular interest as during pregnancy the placenta is not only the source of genes, but it also expresses them depending on the complications of pregnancy. Thus, the expression level of the NAMPT and GHRL genes in the placenta turned out to be the same in the study groups, but the results were not statistically significant. The level of the NAMPT gene in the main group was 0.87 (0.65;1.13) and it was 1 (0.75; 1.44) REU in the control group, p=0.24; the GHRL level was 0.97 (0.67; 1.56) and 1 (0.68; 1.36) REU, respectively, p=0.57 (Fig. 4).
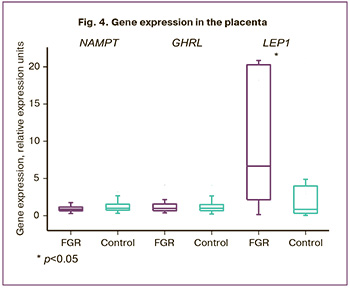
The analysis of the LEP gene expression in the placenta revealed that its expression level was statistically significantly increased in the main group and amounted to 5.6 (1.64; 18.8) REU and it was 1 (0.36; 4.28) REU in the control group, p=0.045 (Fig. 4).
According to the literature data, the level of leptin correlates with the body mass index [15]. In our study, we performed a correlation analysis to determine the relationship between the expression level of the leptin gene in the placenta and the weight of the newborn. After we assessed the expression level of the leptin gene in the placenta in the main group of patients, we found an inverse correlation between the level of LEP expression and fetal birth weight (Spearman’s correlation coefficient was -0.53, p=0.01). Though, the correlation analysis in the control group did not show a statistically significant correlation (Spearman’s correlation coefficient was 0.27, p=0.17).
In order to confirm the dependence of the expression level of leptin in FGR on the weight of the newborn, but not on the time of delivery, we conducted a correlation analysis between the level of LEP and the time of delivery. The analysis did not reveal a statistically significant correlation between these parameters (Spearman’s correlation coefficient was -0.24, p=0.17).
Thus, the expression of the leptin gene in the placenta was significantly higher in patients with the lowest newborn weight (p<0.05). These differences were not related to the gestational age at the time of delivery. It can be assumed that the highest expression of LEP in the placenta indicates greater intrauterine fetal suffering.
According to the literature data, leptin which is synthesized in trophoblast cells has mitogenic and antiapoptotic effects, regulates fetal growth and development by stimulating such signaling pathways as JAK/STAT, MAPK and PI3K [8, 16]. Previous studies have shown that leptin is a modulator of the endocrine function of the placenta which is an important source of maternal circulating leptin. Stefaniak M. and Dmoch-Gajzlerska E. [17] made a conclusion about a possible compensatory mechanism by which placentas small for gestational age produce more leptin. Moreover, Karakosta P. et al. found a connection between the weight of the newborn and the level of leptin in the umbilical cord blood in FGR [18]; the study showed a negative correlation between these parameters. Despite the fact that the role of leptin as a growth factor before birth remains uncertain, the increased expression of leptin in the placenta suggests the physiological significance of leptin in the life of the fetus. The change in intrauterine synthesis may indicate a change in the availability of nutrients, and therefore may play an important role in the control of gluconeogenesis and maturation of fetal tissues, as well as in the establishment of nerve pathways important for energy balance in the later life of the newborn.
The results of our study are consistent with the literature data [8, 16] that the placenta is the main source of leptin during pregnancy and the level of its expression is likely to increase in FGR.
Conclusion
Determining the level of expression of the visfatin, ghrelin and leptin genes in maternal blood and in the placenta in FGR gives a better understanding of its pathogenesis and new opportunities for its diagnosis.
The level of expression of the NAMPT and GHRL genes in maternal blood was statistically significantly decreased in FGR and thus it can become an objective marker for diagnosing such a condition during pregnancy.
Determining the level of LEP expression in the placenta confirms the data that the placenta is the main source of this adipocytokine during pregnancy. The inverse relationship between the increased level of leptin expression and the weight of the newborn indicates greater fetal suffering and confirms its role in the regulation of fetal growth.
It should be noted that there is a need for further in-depth research with a correlation analysis of the concentration of the adipocytokines in maternal and umbilical cord blood and the profile of DNA methylation in the placenta in order to clarify the pathogenetic mechanisms in the development of FGR.
References
- Gluckman P.D., Hanson M.A., Pinal C. The developmental origins of adult disease. Matern. Child Nutr. 2005; 1(3): 130-41. https://dx.doi.org/10.1111/j.1740-8709.2005.00020.x.
- Леонова И. А., Иванов Д. О. Фетальное программирование и ожирение у детей. Детская медицина Северо-Запада 2015; 6(3): 28-41. [Leonova I.A., Ivanov D.O. Fetal programming and obesity in children. Children's Medicine of the North-West. 2015; 6(3): 28-41. (in Russian)].
- Железова М.Е., Зефирова Т.П., Канюкова С.С. Задержка роста плода: современные подходы к диагностике и ведению беременности. Практическая медицина. 2019; 17(4): 8-14. [Zhelezova M.E., Zefirova T.A., Kanyukov S.S.Fetal growth restriction: modern approaches to the diagnosis and management of pregnancy. Practical Medicine. 2019; 17(4): 8-14. (in Russian)].https://dx.doi.org/10.32000/2072-1757-2019-4-8-14.
- Dessì A., Pravettoni C., Cesare Marincola F., Schirru A., Fanos V. The biomarkers of fetal growth in intrauterine growth retardation and large for gestational age cases: from adipocytokines to a metabolomic all-in-one tool. Expert Rev. Proteomics. 2015; 12(3): 309-16. https://dx.doi.org/10.1586/14789450.2015.1034694.
- Кан Н.Е., Тютюнник В.Л., Хачатрян З.В., Садекова А.А., Красный А.М. Метилирование генов TLR2 и импринтинг-контролирующей области IGF2/H19 в плазме крови при задержке роста плода. Акушерство и гинекология. 2021; 5: 79-84. [Kan N.E., Tyutyunnik V.L., Khachatryan Z.V.,Sadekova A.A., Krasnyi A.M. Methylation of the TLR2 genes and the IGF2/H19 imprinting-control region in blood plasma in fetal growth retardation. Obstetrics and Gynecology. 2021; (5): 79-84. (in Russian)].https://dx.doi.org/10.18565/aig.2021.5.79-84.
- Cekmez F., Canpolat F.E., Pirgon O., Aydemir G., Tanju I.A., Genc F.A. et al. Adiponectin and visfatin levels in extremely low birth weight infants; they are also at risk for insulin resistance. Eur. Rev. Med. Pharmacol. Sci. 2013;17(4): 501-6.
- Lee M.H., Jeon Y.J., Lee S.M., Park M.H., Jung S.C., Kim Y.J. Placental gene expression is related to glucose metabolism and fetal cord blood levels of insulin and insulin-like growth factors in intrauterine growth restriction. Early Hum. Dev. 2010; 86(1): 45-50. https://dx.doi.org/10.1016/j.earlhumdev.2010.01.001.
- Maymó J.L., Pérez Pérez A., Gambino Y., Calvo J.C., Sánchez-Margalet V., Varone C.L. Review: Leptin gene expression in the placenta--regulationof a key hormone in trophoblast proliferation and survival. Placenta. 2011; 32(Suppl. 2): S146-53. https://dx.doi.org/10.1016/j.placenta.2011.01.004.
- Morgan S.A., Bringolf J.B., Seidel E.R. Visfatin expression is elevated in normal human pregnancy. Peptides. 2008; 29(8): 1382-9. https://dx.doi.org/10.1016/j.peptides.2008.04.010.
- Mazaki-Tovi S., Romero R., Kusanovic J.P., Vaisbuch E., Erez O., Than N.G. et al. Maternal visfatin concentration in normal pregnancy. J. Perinat. Med. 2009; 37(3): 206-17. https://dx.doi.org/10.1515/JPM.2009.054.
- Briana D.D., Malamitsi-Puchner A. The role of adipocytokines in fetal growth. Ann. N. Y. Acad. Sci. 2010; 1205: 82-7. https://dx.doi.org/10.1111/j.1749-6632.2010.05650.x.
- Moschen A.R., Kaser A., Enrich B., Mosheimer B., Theurl M., Niederegger H.,Tilg H. Visfatin, an adipocytokine with proinflammatory andimmunomodulating properties. J. Immunol. 2007; 178(3): 1748-58.https://dx.doi.org/10.4049/jimmunol.178.3.1748.
- Pavlová T., Novák J., Bienertová-Vašků J. The role of visfatin (PBEF/Nampt) in pregnancy complications. J. Reprod. Immunol. 2015; 112: 102-10.https://dx.doi.org/10.1016/j.jri.2015.09.004.
- Martín-Estal I., de la Garza R.G., Castilla-Cortázar I. Intrauterine growth retardation (IUGR) as a novel condition of insulin-like growth factor-1 (IGF-1) deficiency. Rev. Physiol. Biochem. Pharmacol. 2016; 170: 1-35.https://dx.doi.org/10.1007/112_2015_5001.
- Krasnyi A.M., Sadekova A.A., Smolnova T.Y., Chursin V.V.,Buralkina N.A., Chuprynin V.D., Yarotskaya E., Pavlovich S.V..,Sukhikh G.T. The levels of Ghrelin, Glucagon, Visfatin and Glp-1Are Decreased in the Peritoneal Fluid of women with endometriosisalong with the increased expression of the CD10 protease by themacrophages. Int. J. Mol. Sci. 2022; 23(18): 10361. https://dx.doi.org/10.3390/ijms2318103611.
- Barrientos G., Toro A., Moschansky P., Cohen M., Garcia M.G., Rose M. et al. Leptin promotes HLA-G expression on placental trophoblasts via the MEK/Erk and PI3K signaling pathways. Placenta. 2015; 36(4): 419-26.https://dx.doi.org/10.1016/j.placenta.2015.01.006.
- Stefaniak M., Dmoch-Gajzlerska E. Maternal serum and cord bloodleptin concentrations at delivery in normal pregnancies and in pregnancies complicated by intrauterine growth restriction. Obes. Facts. 2022; 15(1): 62-9. https://dx.doi.org/10.1159/000519609.
- Karakosta P., Roumeliotaki T., Chalkiadaki G., Sarri K., Vassilaki M., Venihaki M.et al. Cord blood leptin levels in relation to child growth trajectories. Metabolism. 2016; 65(6): 874-82. https://dx.doi.org/10.1016/j.metabol.2016.03.003.
Received 07.04.2022
Accepted 14.07.2023
About the Authors
Natalia E. Kan, Professor, Dr. Med. Sci., Professor, MD, PhD, Deputy Director of Science, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology Ministry of Health of Russia, +7(926)220-86-55, kan-med@mail.ru, Researcher ID: B-2370-2015, SPIN-код: 5378-8437, Authors ID: 624900, Scopus Author ID: 57008835600, https://orcid.org/0000-0001-5087-5946, 117997, Russia, Moscow, Ac. Oparina str., 4.Ekaterina E. Soldatova, postgraduate student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology Ministry
of Health of Russia, +7(906)110-51-13, katerina.soldatova95@bk.ru, https://orcid.org/0000-0001-6463-3403, 117997, Russia, Moscow, Ac. Oparina str., 4.
Victor L. Tyutyunnik, Professor, MD, PhD, Leading Researcher of Center of Scientific and Clinical Researches, Academician V.I. Kulakov National Medical Research Center
for Obstetrics, Gynecology and Perinatology Ministry of Health of Russia, +7(903)969-50-41, tioutiounnik@mail.ru, Researcher ID: B-2364-2015, SPIN-код: 1963-1359,
Authors ID: 213217, Scopus Author ID: 56190621500, https://orcid.org/0000-0002-5830-5099, 117997, Russia, Moscow, Ac. Oparina str., 4.
Maria V. Volochaeva, PhD, Senior Researcher, Department of Regional Cooperation and Integration, Physician at the 1st Maternity Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology Ministry of Health of Russia, +7(919)968-72-98, m_volochaeva@oparina4.ru,
https://orcid.org/0000-0001-8953-7952, 117997, Russia, Moscow, Ac. Oparina str., 4.
Alsu A. Sadekova, PhD, Researcher at the Cytology Laboratory, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-22-72, a_sadekova@oparina4.ru, https://orcid.org/0000-0003-4726-7477, 117997, Russia, Moscow, Ac. Oparina str., 4.
Aleksey M. Krasnyi, PhD (Bio), Head of the Cytology Laboratory, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology,
Ministry of Health of Russia, +7(495)438-22-72, alexred@list.ru, https://orcid.org/0000-0001-7883-2702, 117997, Russia, Moscow, Ac. Oparina str., 4.
Corresponding author: Ekaterina E. Soldatova, katerina.soldatova95@bk.ru



