Carbohydrate metabolism and hemostatic system in women with gestational diabetes mellitus, preeclampsia, and fetal growth restriction
Aim. To investigate changes in carbohydrate metabolism and hemostatic system during pregnancy in women with gestational diabetes mellitus, preeclampsia, and fetal growth restriction.Palieva N.V., Botasheva T.L., Petrov Yu.A., Pogorelova T.N., Drukker N.A., Levkovich M.A., Gun'ko V.O.
Materials and methods. The study included pregnant women with gestational diabetes mellitus who had preeclampsia (n=65), fetal growth restriction (n=71), and no obstetric pathology (n=75) in the third trimester of pregnancy. Clinical evaluation included testing for the levels of glucose, insulin, C-peptide, insulin resistance index, D-dimer, activated partial thromboplastin time, soluble fibrin-monomeric complexes, and fibrinogen.
Results. Parameters of carbohydrate metabolism and hemostasis in women with gestational diabetes mellitus and preeclampsia differed from those who had gestational diabetes mellitus and fetal growth retardation. Preeclampsia was associated with higher hyperglycemia and D-dimer levels, while fetal growth restriction was characterized by the predominance of fibrinogen and soluble fibrin-monomeric complexes.
Conclusion. Changes in the examined peripheral blood parameters of women with gestational diabetes mellitus indicate higher demands for the maternal vascular bed's endothelial system. The severity of endothelial function decompensation determines the formation of a local "catastrophe" in the fetal-placental unit in the form of fetal growth restriction or systemic (maternal) in the form of preeclampsia.
Keywords
Preserving maternal and fetal health is the primary goal of obstetrics and a major objective of states' socio-economic policy because reproductive losses determine mortality and fertility rates, reflecting the negative trends in population growth [1–4]. Pregnancy complications with high perinatal losses include preeclampsia (PE), a possible cause of annual 50–60 thousand maternal deaths globally [5, 6], and fetal growth restriction syndrome (FGR) associated with more than 1 million annual fetal and infant mortality across the globe [7, 8]. These obstetric pathologists are the result of two counter directional "catastrophe" pathways in a pregnant woman. One causes adaptation breakdown in maternal and the other in the fetal functional systems. Pathogenetically, PE, and FGR are similar [1, 9–11]. In both cases, there is a reduced vascular network and steal syndrome formed in the maternal-fetal–placental unit due to functional failure of the endothelium [12].
Functional decompensation of the vascular system during pregnancy can be associated with maternal chronic somatic comorbidities, such as obesity and diabetes mellitus, which are currently common among women of reproductive age, reaching 50% in some countries [13–16]. This may explain the persistently high proportions of PE and FGRP in the structure of obstetric morbidity (2.4–31.1%) [1, 17] despite the available knowledge about their causal mechanisms [18]. Therefore, contemporary pregnant women's changed biological profile dictates the need to continue the research in this area of pathological obstetrics.
The present study aimed to investigate changes in carbohydrate metabolism and hemostatic system during pregnancy in women with gestational diabetes mellitus (GDM), preeclampsia (PE), and fetal growth restriction (FGR).
Materials and methods
A retrospective five-year cohort study (2013–2018) was conducted at the Research Institute of Obstetrics and Pediatrics of the Rostov State Medical University of Minzdrav of Russia. A total of 1483 primigravida women aged 18 to 40 with a singleton pregnancy complicated by GDM (inclusion criteria) were divided into three clinical groups. Group I (study group) included 65 women with PE. Group II included 71 women with FGR. Group III (control group) comprised 75 women without obstetric pathology (nAP). GDM, PE and FGR were defined according to the criteria set out in the National Clinical Guidelines: "Gestational diabetes mellitus: diagnosis, treatment, postpartum follow-up" (2013), "Hypertensive disorders during pregnancy, childbirth and the postpartum period. Preeclampsia. Eclampsia" (2013) and based on ultrasound data (fetometry). Exclusion criteria were all endocrine diseases except GDM, non-obstetric pathology in the stage of exacerbation or decompensation, pregnancy after assisted reproductive technology programs, multiple pregnancy, oncological and mental illnesses, alcoholism and drug addiction, maternal and fetal malformations.
The study was approved by Research Ethics Committee, and all participants signed an informed consent form as per the Helsinki Declaration (The World Medical Association's Declaration of Helsinki, Finland, 2013, 9th revision).
Ultrasound examination was carried out on a Siemens Sonoline G 50 ultrasound system (Germany) (3.5 MHz). The body mass index was estimated according to the formula of A. Quetelet (body weight, kg/height, squared, m); the initial weight of the pregnant woman in the first trimester was used for the calculation. Activated partial thromboplastin time (APTT), soluble fibrin-monomer complexes (SFMK), and fibrinogen were determined by enzyme immunoassay (ELISA Siemens (Germany) and D-dimer kits, Technozym D-dimer ELISA (Austria). In the blood plasma, basal levels of glucose were determined (photometric method, set "Glucose," Biosystems (Spain)), immunoreactive insulin (IRI) (ELISA, "DRG Insulin ELISA EIA-2935" (Germany)) and C-peptide (radioimmunological method, Beringwerke-AG (Germany)). The insulin resistance index (IR) HOMA-IR (D. Matthews (1985) was calculated by the formula: glucose × IRI/22.5. The HOMA-IR value> 2.77 was regarded as IR. Fasting venous blood was sampled from pregnant women with obstetric pathology (FGR and PE) before starting therapy.
Statistical analysis
Many biological variables do not meet the normality assumption [19]. Quantitative variables were expressed as the median (Me) and interquartile range (Q1; Q3). Statistical analysis was performed using the EXCEL 2010 and IBM SPSS 25.0.0.2 software. Kruskal–Wallis test was used for comparing numerical data between unpaired groups followed by pairwise comparison using the Mann–Whitney U-test with Bonferroni correction. Differences between the groups were considered statistically significant at p<0.05.
Results and discussion
The age of pregnant women provides important information about their reproductive health. Median age of the study participants was 28.51 [20.41; 38.02], 26.05 [21.30; 29.12] and 28.16 [20.04; 33.18] years among women with PE, FGR, and nAP. In the whole study cohort, 44.6% of women were in the range of 24–29 years. Women in this age range constituted 42.3%, 43.2%, and 45.5% in the I, II, and III groups, respectively. Since maternal obesity is an important risk factor for GDM and pregnancy complications, the body mass index was assessed. The highest BMI was in the group with PE (24.19 kg/m2 [23.71; 26.05]) and the lowest among pregnant women with FGR (23.16 kg/m2 [19.04; 24.18]); in the nAP group, it was 24.12 kg/m2 [18.28; 27.43], but no statistically significant differences between groups were found.
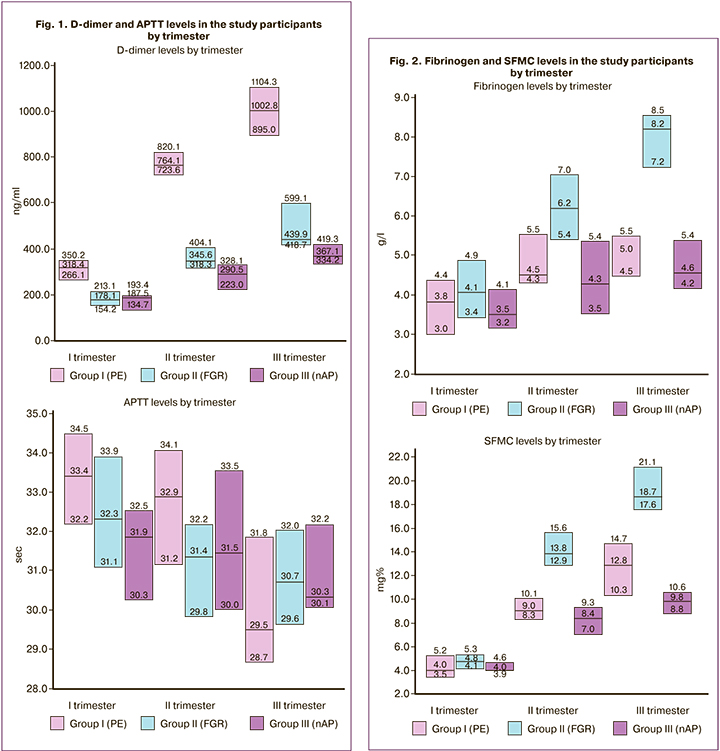
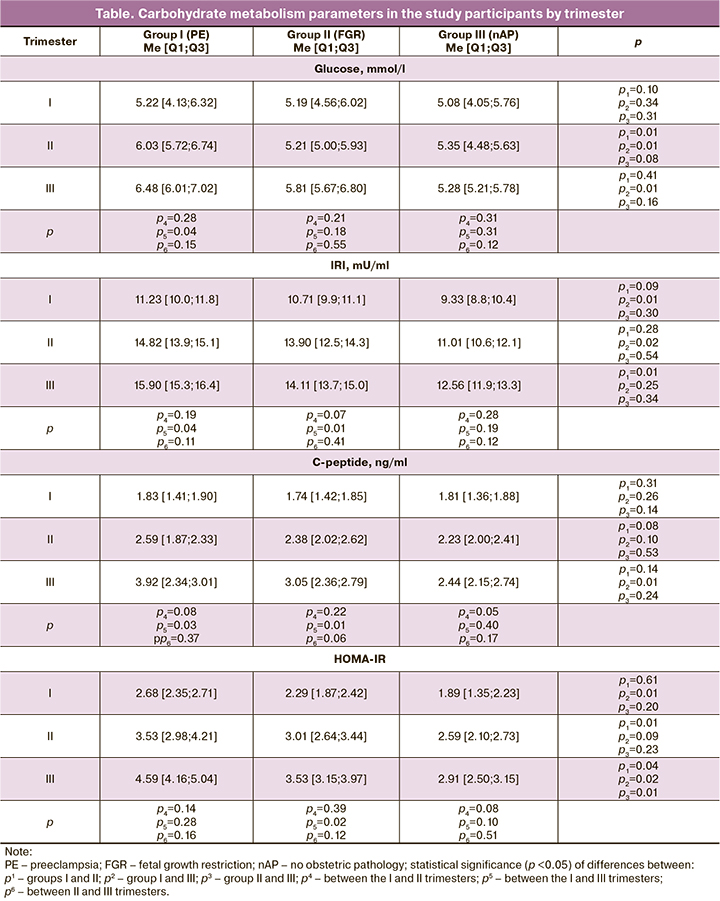
The gestational glycemic thresholds used herein are based on international consensus on the risk of adverse pregnancy outcomes. Maternal hyperglycemia, which first appeared during pregnancy as a moderate increase in the maternal glucose, results in diabetic fetopathy (hypo- or hypertrophic variants), fetal/newborn death, newborn respiratory/cerebral disorders, etc. [20, 21]. But GDM can also cause irreparable harm to maternal health and become a trigger of complications such as PE/eclampsia, placental abruption, HELLP syndrome, type 2 diabetes mellitus, etc. [20, 22, 23]. However, it is not clear under what conditions, against the background of GDM, adaptation breakdown occurs in the fetal organism and under what conditions in the maternal organism. Considering this, we analysed the levels of glucose, IRI, C-peptide, and HOMA IR index, reflecting the severity of maternal hyperglycemia and IR (table), and hemostasis parameters (D-dimer, APTT, fibrinogen, SFMC), indicating the tension of the blood coagulation system (Fig. 1, 2).
The glycemic level was significantly increased only in group I: by 19.4% at delivery, 11.3% for the second trimester, and 18.5% for the third trimester compared with the nAP group, as well as by 13.6% in the second trimester compared with the group with FGR (table). IRI values in the whole cohort ranged from 8.8 to 16.4 μU/ml. The highest absolute values (significant maximum peak at 29.6% by the III trimester) were determined in the PE group, which were significant in the first (by 17.0%) and second trimesters (by 25.7%) compared with nAP and in the III trimester (by 11.3%) compared with FGR. For the FGR group, a significant increase of 24.1% was established at delivery. Thus, the level of glucose and IRI increases as the date of delivery approaches. Moreover, all indicators were normal, up to 20–26 μU/ml (according to age reference range). However, IRI values> 12.5 μU/ml are already associated with the presence of IR.
C-peptide is a fragment of proinsulin, which is not destroyed by the liver, has a longer half-life (up to 30 minutes) than insulin, is secreted in equimolar amounts to insulin, and does not cross with it. The level of C-peptide in the groups corresponded normal range during pregnancy. There was a significant increase by 53.3% and 43.0% at delivery with PE and FGR by 53.3% and 43.0%, respectively, and in the third trimester by 37.7% with PE compared with nAP. This trend in the C-peptide levels is possibly associated with higher IRI levels in PE compared with the FGR and nAP group.
Since the glucose levels, IRI and C-peptide separately cannot correctly reflect the state of IR, and the IR index was calculated using the small homeostasis model assessment of insulin resistance HOMA-IR, as optimal for GDM, where fasting hyperglycemia is mainly up to 7.0 mmol/l. Considering an increase in the fasting glycemia and IRI before delivery in all groups, an increase in HOMA-IR was also natural. It was significantly higher in pregnant women with PE (by 14.7% and 23.1%, respectively) compared with FGR in the II and III trimesters and compared with nAP in I (by 29.5%) and III (by 36.6%) trimesters. In patients with FGR, there was a significant increase in this parameter compared with nAP in the third trimester by 17.6% and at delivery by 35.1%. In PE, the maximum number of pregnant women from early gestational age had IR (26.1%/17 women), with FGR (30.9%/22) and nAP (8.0% / 6) only from the second trimester. In the PE and FGR group, RI by delivery was 100%, and only 42.7% in nAP (n=32). Therefore, patients with PE had significantly more significant shifts in carbohydrate metabolism than women with FGR and nAP.
Obstetric complications in patients with GDM are often associated with thrombophilia due to impaired vascular endothelial function in the maternal-fetal– placental unit [24]. Still, it is not clear which changes in hemostasis and their severity accompany PE and which FGR. In pregnant women with GDM, we analyzed plasma hemostasis considering the obstetric situation. It was found that in PE, the level of D-dimer is significantly higher in the first trimester – by 44.1% to FGR, in the second trimester – by 61.9% to nAP, and in the third trimester – to both groups (by 56.1% – to FGR and 63.4% to nAP). An increase in these fibrin degradation products' levels was observed in all groups before delivery, which indicates an increase in the blood coagulation potential. However, only pregnant women with PE had a significant increase in the D-dimer level (by 58.3% by the second trimester and by 68.2% by the third trimester). The APTT level in all groups was stable and did not have significant differences (Fig. 1). A possible explanation for this stability can be that APTT reflects the time of blood clot formation (activation of the internal blood coagulation pathway). In the absence of a bleeding source, there is no need for hemostasis activation. This assumption is supported by Erhabor O. et al. (2013) [25], who suggest that the APTT should be used to monitor obstetric bleeding.
Fibrinogen and SFMC levels were significantly higher in patients with FGR (Fig. 2). Fibrinogen was 30.8% more elevated in the II trimester compared with nAP, by 38.6% and 44.4% in the III trimester compared with PE and nAP, respectively, and increased by delivery (I–III trimester – by 50, 5% and II–III trimester – by 24.5%). In patients with FGR, SFMC was higher than in those with PE and nAP in II (by 34.8% and 39.6%, respectively) and III (by 31.1% and 47.3%, respectively) trimester, with a significant 74.4%increase. In patients with PE, the levels of fibrinogen and SFMC significantly increased by delivery (by 24.1% and 68.6%).
Therefore, the hemostatic system in PE is characterized by an increase in D-dimer level, while in FGR by higher levels of fibrinogen and SFMC. That is, a higher coagulation tension is present in PE. Hale S. A. et al. (2012) also reported that in PE, there is a more pronounced procoagulant activity manifested by high levels of D-dimer. Only they did not take into account the presence of GDM [26].
At excessive blood glucose concentrations, vascular endothelium's functioning is disrupted due to glucose flux into its insulin-independent cells [12, 14, 27]. We can assume that in decompensated GDM, endotheliopathy in the maternal-fetal–placental unit activates the procoagulant hemostasis, thereby disrupting maternal-fetal sensitive interaction mechanisms, leading to PE or FGR. This hypothesis is confirmed by the results of our study and provides a rationale for prophylactic treatment of patients with drugs reducing the blood coagulation potential (anticoagulants, low molecular weight heparins, etc.), which are approved for use during pregnancy.
For the objectivity of the assessment of carbohydrate metabolism and the blood coagulation system in GDM, we analyzed the study participants' somatic morbidity. In the general sample, blood disorder constituted the largest proportion (57.1%), with anemia as the leading diagnosis. This is followed by eye diseases (31.9%), mainly myopias, and gastrointestinal diseases in third place (24.2%), primarily gastritis and cholecystitis. There were cardiovascular diseases (10.5%), including lower limb varicose veins, mitral valve prolapse without circulatory disorders, heart rhythm disturbances. Urinary (cystitis and pyelonephritis) and respiratory diseases (tonsillitis, bronchitis, pneumonia, etc.) constituted 9.1% and 8.2%, respectively. The groups were comparable in terms of somatic morbidity rates, and no significant differences were found.
Conclusion
1. The study findings suggest that starting from the second trimester, pregnant women with GDM and PE have significantly higher levels of glycemia ≥6.03 [5.72; 6.74] mmol/L and IR ≥3.53 [2.98; 4.21], along with an increase in the level of D-dimer ≥764.08 [723.57; 820.12] ng/ml. Patients with GDM and FGR had high levels of fibrinogen ≥6.19 [5.40; 7.04] g/l and SFMC ≥ 13.84 [12.92; 15.62] mg/%. The tension of the plasma coagulation pathway of hemostasis indirectly indicates endothelial dysfunction of the maternal-fetal– placental unit vascular network in pregnant women with GDM (against the background of increasing hyperglycemia). When decompensated, it contributes to local disadaptation of the fetal system in the form of FGR or systemic (in the maternal body), with the development of PE.
2. Pregnant women with uncompensated GDM are most at risk for developing clinically significant gestational coagulopathies, contributing to the development of severe obstetric maternal (PE) and fetal (FGR) complications. Along with glycemic control, they warrant dynamic control of the IRI level (to assess the severity of IR and carbohydrate disorders) and testing coagulation parameters including fibrinogen, SFMC, and D-dimer at least one time per trimester.
References
- Иванов Д.О., ред. Руководство по перинатологии. 2-е изд. т. 1. СПб.: Информ-Навигатор; 2019. 936 с. [Ivanov D.O., ed. Guidance on Perinatology. 2-nd ed. Vol. 1. SPb.: Inform-Navigator; 2019. 936 p.(in Russian)].
- Янкина С.В., Шатрова Н.В., Берстнева С.В., Павлов Д.Н. Особенности течения и исходы беременности у женщин с гестационным сахарным диабетом. Российский медико-биологический вестник имени академика И.П. Павлова. 2018; 26(1): 96-105. [Yankina S.V., Shatrova N.V., Berstneva S.V., Pavlov D.N. Features of the course and outcomes of pregnancy in women with gestational diabetes. Rossijskij mediko-biologicheskij vestnik imeni I.P. Pavlova / I.P. Pavlov Russian medical biological herald. 2018; 26 (1): 96-105. (in Russian)]. https://dx.doi.org/10.23888/PAVLOVJ201826196-105.
- Харитонова Л.А., Папышева О.В., Катай Г.А., Юдина Т.М., Богомаз Д.С. Состояние здоровья детей, рожденных от матерей с сахарным диабетом. Российский вестник перинатологии и педиатрии. 2018; 63(3): 26-31. [Kharitonova L.A., Papysheva O.V., Katai G.A., Yudina T.M., Bogomaz D.S. The health status of children born by mothers with diabetes. Rossijskij vestnik perinatologii i pediatrii / Russian Bulletin of perinatology and pediatrics. 2018; 63 (3): 26-31. (in Russian)]. https://dx.doi.org/10.21508/1027-4065-2018-63-3-26-31.
- Филиппов О.С., Гусева Е.В., Малышкина А.И., Михайлов А.В., Зубенко Н.В., Фаткуллин И.Ф., Башмакова Н.В., Артымук Н.В., Пестрикова Т.Ю., Палиева Н.В. Материнская смертность в Российской Федерации в 2016 году. Методическое письмо Министерства здравоохранения Российской Федерации №15-4/10/2-7339 от 23.10.17. [Filippov O.S., Guseva E.V., Malyshkina A.I., Mikhailov A.V., Zubenko N.V., Fatkullin I.F., et al. Maternal mortality in the Russian Federation in 2016. Methodological letter of the Ministry of Health of the Russian Federation №15-4/10/2-7339 on 23.10.2017. 90. (in Russian)].
- Say L., Chou D., Gemmill A., Tunçalp Ö., Moller A.-B., Daniels J. et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob. Health. 2014; 2(6): 323-33. https://dx.doi.org/10.1016/S2214-109X(14)70227-X.
- World Health Organization. Trends in maternal mortality 2000 to 2017: estimates by WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division: executive summary. WHO; 2019. Available at: https://apps.who.int/iris/handle/10665/327596.
- Sharma D., Shastri S., Sharma P. Intrauterine growth restriction: antenatal and postnatal aspects. Clin. Med. Insights Pediatr. 2016; 10: 67-83. https://dx.doi.org/10.4137/CMPed.S40070.
- Kozuki N., Katz J., Lee A.C., Vogel J.P., Silveira M.F., Sania A. et al. Short maternal stature increases risk of small-for-gestational-age and preterm births in low- and middle-income countries: individual participant data meta-analysis and population attributable fraction. J. Nutr. 2015; 145 (11): 2542-50. https://dx.doi.org/10.3945/jn.115.216374.
- Юсупова З.С., Новикова В.А., Оленев А.С. Современные представления о преэклампсии – патогенез, диагностика, прогнозирование. Практическая медицина. 2018; 16(6): 45-1. [Yusupova Z.S., Novikova V.A., Olenev A.S. Modern ideas about preeclampsia – pathogenesis, diagnosis, prognosis. Prakticheskaya medicina/Journal «Practical medicine». 2018; 16 (6): 45-51. (in Russian)]. https://dx.doi.org/10.32000/2072-1757-2018-16-6-45-51.
- Горюнова А.Г., Симонова М.С., Мурашко А.В. Синдром задержки роста плода и адаптация плаценты. Архив акушерства и гинекологии им. В.Ф. Снегирева. 2016; 3(2): 76-80. [Goryunova A.G., Simonova M.S., Murashko A.V. Fetal growth retardation syndrome and adaptation of placenta. Arkhiv Akusherstva i Ginekologii im. V.F. Snegiryova/V.F. Snegirev Archives of Obstetrics and Gynecology, Russian journal. 2016; 3(2): 76-80. (in Russian)]. https://dx.doi.org/10.18821/2313-8726-2016-3-2-76-80..
- Lockwood C.J., Huang S.J., Krikun G., Caze R., Rahman M., Buchwalder L.F. et al. Decidual hemostasis, inflammation, and angiogenesis in pre-eclampsia. Semin. Thromb. Hemost. 2011; 37(2): 158-64. https://dx.doi.org/10.1055/s-0030-1270344.
- Погорелова Т.Н., Гунько В.О., Никашина А.А., Палиева Н.В., Аллилуев И.А., Ларичкин А.В. Нарушение регуляции редокс-процессов в плаценте при ее дисфункции. Проблемы репродукции. 2019; 25(6): 112-8. [Pogorelova T.N., Gunko V.O., Nikashina A.A., Palieva N.V., Alliluev I.A., Larichkin A.V. Violation of the regulation of redox processes in the placenta during its dysfunction. Problemy reproduktsii/Human Reproduction. 2019; 25 (6): 112-8. (in Russian)]. https://dx.doi.org/10.17116/repro201925061112.
- Комилова М.С., Пахомова Ж.Е. Значение эндотелия в развитии осложнений гестационного периода. Российский вестник акушера-гинеколога. 2015; 15(1): 18-23. [Komilova M.S., Pakhomova Gh.E. Endothelium meaning in the development of complications of the gestational period. Rossiyskiy vestnik akushera-ginekologa/Russian Bulletin of Obstetrician-Gynecologist. 2015; 15(1): 18-23. (in Russian)]. https://dx.doi.org/10.17116/rosakush201515118-23.
- Палиева Н.В., Боташева Т.Л., Линде В.А., Авруцкая В.В., Железнякова Е.В. Особенности некоторых вазоактивных гормонов и сосудистых факторов у женщин с метаболическим синдромом и их влияние на формирование акушерских осложнений. Акушерство и гинекология. 2017; 6: 48-54. [Palieva N.V., Botasheva T.L., Linde V.A., Avrutskaya V.V., Zheleznyakova E.V. Peculiarities of some vasoactive hormones and vascular factors in women with metabolic syndrome and their influence on the formation of obstetric complications development. Akusherstvo i ginekologija/Obstetrics and gynecology. 2017; 6: 48-54. (in Russian)]. https://dx.doi.org/10.18565/aig.2017.6.48-54.
- Радзинский В.Е., Боташева Т.Л., Котайш Г.А., ред. Ожирение. Диабет. Беременность. Версии и контраверсии. Клинические практики. Перспективы. М.: ГЭОТАР-Медиа; 2020. 528 с. [Radzinskij V.E., Botasheva T.L., Kotajsh G.A., ed. Obesity. Diabetes. Pregnancy. Versions and contraverses. Clinical practices. Prospects. Moscow: GEOTAR-Media; 2020. 528 р. (in Russian)].
- Сидорова И.С., Никитина Н.А. Преэклампсия как гестационный иммунокомплексный комплементопосредованный эндотелиоз. Российский вестник акушера-гинеколога. 2019; 19(1): 5-11. [Sidorova I.S., Nikitina N.A. Preeclampsia as a gestational immunocomplex complement-mediated endotheliosis. Rossiyskiy vestnik akushera-ginekologa/Russian Bulletin of Obstetrician-Gynecologist. 2019; 19 (1): 5-11. (in Russian)]. https://dx.doi.org/10.17116/rosakush2019190115.
- Regal J.F., Burwick R.M., Fleming S.D. The сomplement system and preeclampsia. Curr. Hypertens. Rep. 2017; 19(11): 87. https://dx.doi.org/10.1007/s11906-017-0784-4.
- Погорелова Т.Н., Гунько В.О., Палиева Н.В., Аллилуев И.А., Каушанская Л.В., Ларичкин А.В. Дисбаланс свободных аминокислот в околоплодных водах при преэклампсии. Акушерство и гинекология. 2019; 2: 60-7. [Pogorelova T.N., Gunko V.O., Palieva N.V., Alliluev I.A., Kaushanskaya L.V., Larichkin A.V. Imbalance of free amino acids in the amniotic fluid during preeclampsia. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; 2: 60-7. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.2.60-67.
- Ланг Т.А., Сесик М. Как описывать статистику в медицине. Аннотированное руководство для авторов, редакторов и рецензентов. Пер. с англ. Леонов В.П., ред. М.: Практическая медицина; 2016. 480 с. [Lang T.A., Secic M. How to report statistics in medicine. Annotated Guidelines for Authors, Editors, and Reviewers; transl. from eng., V.P. Leonov eds. M.: Practical medicine; 2016. 480 p.(in Russian)].
- Karakash S.D., Einstein F.H. Diabetes in pregnancy: glycemia control guidelines and rationale. Curr. Opin. Endocrinol. Diabetes Obes. 2011; 18(2): 99-103. https://dx.doi.org/10.1097/MED.0b013e3283446ed2.
- Ахметова Е.С., Ларева Н.В., Мудров В.А., Гергесова Е.Е. Особенности течения беременности при гестационном сахарном диабете и прогнозирование диабетической фетопатии. Журнал акушерства и женских болезней. 2017; 66(4): 14-24. [Ahmetova E.S., Lareva N.V., Mudrov V.A., Gergesova E.E. Features of the course of pregnancy during the gestational diabetes mellitus and prediction of diabetic fetopathy. Jurnal akusherstva i genskih boleznej/Journal of obstetrics and women's diseases. 2017; 66(4): 14-24. (in Russian)]. https://dx.doi.org/10.17816/JOWD66414-24.
- Радзинский В.Е., Палиева Н.В., Боташева Т.Л., Железнякова Е.В. Влияние эндотелий-опосредованных факторов на формирование акушерской патологии при метаболических нарушениях. Современные проблемы науки и образования. 2016; 5: 30. [Radzinskij V.E., Palieva N.V., Botasheva T.L., Zheleznyakova E.V. The effect of endothelium-mediated factors on the formation of obstetric pathology during metabolic disorders. Sovremennie problemy nauki i obrazovaniya/Modern problems of science and education. 2016; 5: 30 (in Russian)]. Available at: https://www.science-education.ru/ru/article/view?id=25145
- Линде В.А., Палиева Н.В., Боташева Т.Л., Авруцкая В.В., Дударева М.В. Роль про- и контринсулярных факторов в формировании акушерской патологии. Акушерство и гинекология. 2017; 2: 32-8. [Linde V.A., Palieva N.V., Botasheva T.L., Avrutskaya V.V., Dudareva M.V. Role of pro- and contra-insular factors in the formation of obstetric pathology. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2017; (2): 32-8. (in Russian)]. https://dx.doi.org/10.18565/aig.2017.2.32-8.
- Shamshirsaz A.A., Paidas M., Krikun G. Preeclampsia, hypoxia, thrombosis, and inflammation. J. Pregnancy. 2012; 2012: 374047. https://dx.doi.org/10.1155/2012/374047.
- Erhabor O., Isaac I.Z., Muhammad A.M., Abdulrahaman Y., Ezimah A.C., Adias T.C. Some hemostatic parameters in women with obstetric hemorrhage in Sokoto, Nigeria. Int. J. Womens Health. 2013; 5: 285-91. https://dx.doi.org/10.2147/IJWH.S43503.
- Hale S.A., Sobel B., Benvenuto A., Schonberg A., Badger G.J., Bernstein I.M.Coagulation and fibrinolytic system protein profiles in women with normal pregnancies and pregnancies complicated by hypertension. Pregnancy Hypertens. 2012; 2(2): 152-7. https://dx.doi.org/10.1016/j.preghy.2012.01.004.
- Domokos G. Hyperglycemia-induced endothelial dysfunction. In: Endothelial dysfunction – old concepts and new challenge. 2018: 179-210. https://dx.doi.org/10.5772/intechopen.71433.
Received 03.09.2020
Accepted 01.02.2021
About the Authors
Natalia V. Palieva, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology №2, Principal Researcher at the Department of Medical andBiological Problems in Obstetrics, Gynecology and Pediatrics, Rostov State Medical University, Ministry of Health of the Russian Federation.
Tel.: +7(928)296-46-96. E-mail: nat-palieva@yandex.ru. ORCID: 0000-0003-2278-5198. 344022, Russia, Rostov-on-Don, Nakhichevanskiy str., 29.
Tatyana L. Botasheva, Dr. Med. Sci., Professor, Principal Researcher at the Department of Medical and Biological Problems in Obstetrics, Gynecology and Pediatrics,
Rostov State Medical University, Ministry of Health of the Russian Federation. Tel.: +7(906)424-81-03. E-mail: t_botasheva@mail.ru. ORCID: 0000-0001-5136-1752.
344022, Russia, Rostov-on-Don, Nakhichevanskiy str., 29.
Yuriy A. Petrov, Dr. Med. Sci., Professor, Head of the Department of Obstetrics and Gynecology №2, Rostov State Medical University, Ministry of Health of the Russian Federation. Tel.: +7(928)279-75-75. E-mail: mr.doktorpetrov@mail.ru. ORCID: 0000-0002-2348-8809. 344022, Russia, Rostov-on-Don, Nakhichevanskiy str., 29.
Tatyana N. Pogorelova, Dr. Bio. Sci., Professor, Principal Researcher at the Department of Medical and Biological Problems in Obstetrics, Gynecology and Pediatrics,
Rostov State Medical University, Ministry of Health of the Russian Federation. Tel.: +7(951)509-98-52. E-mail: tnp.rniiap@yandex.ru. ORCID: 0000-0002-0400-0652.
344022, Russia, Rostov-on-Don, Nakhichevanskiy str., 29.
Nina A. Drukker, Dr. Bio. Sci., Professor, Principal Researcher at the Department of Medical and Biological Problems in Obstetrics, Gynecology and Pediatrics,
Rostov State Medical University, Ministry of Health of the Russian Federation. Tel.: +7(951)509-98-52. E-mail: n.drukker@yandex.ru. ORCID: 0000-0002-1605-6354.
344022, Russia, Rostov-on-Don, Nakhichevanskiy str., 29.
Marina A. Levkovich, Dr. Med. Sci., Associate Professor, Leading Researcher at the Department of Medical and Biological Problems in Obstetrics, Gynecology and Pediatrics, Rostov State Medical University, Ministry of Health of the Russian Federation. Tel.: +7(918)570-64-36. E-mail: xlma@mail.ru. ORCID: 0000-0001-8047-7148.
344022, Russia, Rostov-on-Don, Nakhichevanskiy str., 29.
Viktoriya O. Gun’ko, Ph.D. (bio.sci.), Senior Researcher at the Department of Medical and Biological Problems in Obstetrics, Gynecology and Pediatrics, Rostov State Medical University, Ministry of Health of the Russian Federation. Tel.: +7(918)534-80-93. E-mail: rniiap@yandex.ru. ORCID: 0000-0001-8607-9052.
344022, Russia, Rostov-on-Don, Nakhichevanskiy str., 29.
For citation: Palieva N.V., Botasheva T.L., Petrov Yu.A., Pogorelova T.N., Drukker N.A., Levkovich M.A., Gun'ko V.O. Carbohydrate metabolism and hemostatic system in women with gestational diabetes mellitus, preeclampsia, and fetal growth restriction.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 2: 69-76 (in Russian)
https://dx.doi.org/10.18565/aig.2021.2.69-76



