Cardiometabolic risk factors in women undergoing the menopausal transition and the potential of drospirenone-containing contraceptive in the correction of the identified abnormalities
Objective: To investigate changes in cardiometabolic risk factors in women during menopausal transition and the feasibility of correcting the identified abnormalities using cyclic hormone therapy with drospirenone-containing COC.Tolstov S.N., Salov I.A., Rebrov A.P.
Materials and methods: The study enrolled 178 women who underwent a menopausal transition with manifestations of the menopausal syndrome. The study group included 87 women who received COC consisting of drospirenone 3 mg/ethinyl estradiol 20 μg. The control group included 91 women who had not received COC. Kupperman–Uvarova MMI was used to assess the severity of menopausal symptoms. Clinical evaluation included measurements of lipid metabolism, fasting blood glucose, IRI, HOMA-IR, WC, and WC/HC ratio.
Results: More than half of the women had excessive body weight and signs of abdominal obesity, one-third had lipid metabolism disorders, and 41 (23%) were diagnosed with metabolic syndrome. At the end of the study, women in the study group showed a significant reduction in body weight and abdominal obesity. By the end of the study, women in the control group had higher HOMA-IR values, concurrent with higher postprandial blood glucose levels. Women in the study group did not show significant changes in these parameters, and the levels of total and LDL cholesterol were reduced.
Conclusion: The study findings showed a high incidence of metabolic risk factors in women during the menopausal transition. Administration of COC to menopausal women prevents an increase in body weight and visceral obesity, contributes to a decrease in IR and impaired carbohydrate metabolism, and favorably affects lipid metabolism.
Authors' contributions: Tolstov S.N., Salov I.A., Rebrov A.P. – conception and design of the study, analysis and interpretation of results, manuscript editing; Tolstov S.N. – data acquisition (conducting experiments and research), statistical analysis, manuscript drafting.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was approved by the Research Ethics Committee of the V.I. Razumovsky Saratov State Medical University (Ref. No: 2/04.10.2016).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Tolstov S.N., Salov I.A., Rebrov A.P. Cardiometabolic risk factors in women undergoing the menopausal transition and the potential of drospirenone-containing contraceptive in the correction of the identified abnormalities.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (2): 98-107 (in Russian)
https://dx.doi.org/10.18565/aig.2022.257
Keywords
Although classic cardiovascular risk factors are increasingly well controlled, there has been a steady increase in what is known as cardiometabolic risk [1].
The "hormonal chaos" that occurs in women during the menopausal transition not only precipitates bothersome vasomotor symptoms, but also leads to a wide range of metabolic disorders, the accumulation of major cardiometabolic risk factors and contributes significantly to the development of cardiovascular disease in the postmenopausal period [2, 3]. Metabolic syndrome, which develops during menopausal transition long before menstruation stops, appears to be one of the causes of increased cardiovascular risk.
Despite conflicting data on the prevalence of metabolic syndrome in menopausal women, previous studies have shown a clear association with an increased cardiovascular mortality risk [4, 5].
Therefore, it is of interest to assess changes in cardiovascular risk factors in women at different stages of reproductive aging and investigate the possibilities of early correction of emerging metabolic disorders.
One of the promising treatment options for vasomotor manifestations of menopausal syndrome and metabolic disorders is the early prescription of hormonal therapy to women of menopausal age, from contraception to menopausal hormone therapy [6–8]. However, underestimating age-related changes in the functioning of the reproductive system and the specific metabolic disorders that occur in menopausal women may lead to inappropriate administration of such therapies. This suggests that early and wider use of low-dose combination therapy (estrogens with progestogens) in women in menopausal transition should be used in a cyclic regimen [9].
The choice of a particular combined oral contraceptive (COC) is not limited by current clinical guidelines; however, the safety in women with cardiovascular risk factors varies [7–9]. The nature of the changes in cardiometabolic risk factors and the cardiovascular safety of COCs containing drospirenone in transitional women still requires investigation.
This study aimed to investigate changes in cardiometabolic risk factors in women during menopausal transition and the feasibility of correcting the identified abnormalities using cyclic hormone therapy with COCs containing drospirenone.
Materials and methods
This two-stage, open, prospective, stratified study included women undergoing late menopausal transition with manifestations of menopausal syndrome of varying severity and duration.
The inclusion criteria were manifestations of menopausal syndrome of varying severity and duration in women diagnosed with late menopausal transition according to the STRAW+10 criteria for characterizing reproductive aging through menopause [10].
Exclusion criteria were any acute cardiovascular events, including acute coronary syndrome, stroke, and thromboembolic events currently and in the past; the presence of chronic atherosclerotic cardiovascular diseases (coronary heart disease, peripheral artery disease, and cerebrovascular disease); a history of smoking and at the time of inclusion; diabetes mellitus type 1 or 2; thyroid disease with dysfunction; arterial hypertension in the reproductive period; polycystic ovary syndrome; hysterectomy with adnexectomy; renal and liver disease with dysfunction; cancer at any stage; and current and past treatment with estrogens and progestogens.
A total of 178 women with a median age of 48.7 (48.0;50.0) years were included in the study.
At study inclusion, baseline evaluation included collection of anamnestic data, physical examination, cervical speculum examination, and oncocytological screening by a gynecologist according to current clinical practice [7–9]. All the patients underwent transvaginal ultrasound examination.
The first phase of the study aimed to characterize the cardiometabolic status of study participants.
The Kupperman–Uvarova modified menopausal index (MMI) was used to evaluate menopausal symptoms. Neurovegetative (vasomotor), psychoemotional, and neuroendocrine (metabolic) symptoms were also assessed. The questionnaire data were analyzed separately by group and cumulatively. Neurovegetative manifestations of menopausal syndrome were diagnosed with a total score between 10 and 20, moderate severity with a total score between 21 and 30, and severe severity with a total score greater than 30.
Psycho-emotional and neuroendocrine manifestations of menopausal syndrome were assessed similarly: mild impairment with a total score of 1 to 7, moderate impairment with a total score of 8 to 14, and severe impairment when the total score exceeded 14 points.
A score of less than 10 was considered absent for neurovegetative disorders, 10-20 mild, 21–30 moderate, and >30, severe. Metabolic and psycho-emotional disturbances were assessed similarly: 1–7 points for mild disturbances, 8–14 points for moderate, and >14 points for severe symptoms.
The final MMI score was determined by taking the sum of all data obtained: mild severity if the total score was 12–34, moderate symptoms if the total score exceeded 58, and severe menopausal syndrome was diagnosed.
Anthropometric parameters, including Body Mass Index (BMI), calculated as body weight divided by the square of height (kg/m²), waist circumference (WC), and hip circumference, and the waist circumference/hip circumference (WC/HC) ratio were determined.
Lipid profile included total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), non-high-density lipoprotein cholesterol (non-HDL-C), and the TG/HDL-C ratio.
Blood glucose concentrations were measured in the fasting state and 2 h after ingestion of 75 g of anhydrous glucose dissolved in 250 ml of water (postprandial glycemia). Indicators characterizing insulin resistance (IR) included plasma immunoreactive insulin (IRI) levels determined by enzyme immunoassay and Homeostasis Model Assessment of Insulin Resistance (HOMA-IR), which was calculated using the formula: HOMA-IR = fasting IRI (µU/ml) × fasting glucose (mmol/l)/22.5. IRI values ≥12 µEU/ml and HOMA-IR >2.77 c.u. were indicative of IR.
The second phase of the study investigated the feasibility of correcting detected cardiometabolic abnormalities with cyclic hormonal therapy using drospirenone.
The study was conducted in routine clinical practice, and cyclic hormone therapy containing drospirenone was administered by a gynecologist, taking into account the gynecological status, the presence of contraindications, and the woman's desire. The COC was administered to all patients who consented and had no contraindications.
All patients in the treatment group, who consented and had no contraindications (n=87), received the COC, Yaz (Bayer Pharma AG), containing 24 0.02 mg tablets of ethinyl estradiol and 3 mg drospirenone in one blister and four inactive tablets. Yaz was administered according to the instructions for use, that is, one tablet daily for 28 consecutive days, in the order shown on the pack. The COC was administered before the onset of menopause. The groups were stratified according to COC use. The control group included 91 women who had not received COCs.
The study duration was limited to the onset of menopause and/or COC withdrawal. The median duration of the COC use was 1.8 (1.6;2.0) years.
The possibility of correcting existing cardiometabolic disturbances was assessed by changes in BMI, WC, WC/HC, lipid profile, and carbohydrate metabolism parameters. Relevant parameters were assessed at baseline, after six months, and at the end of the study. The primary endpoints were 1) reduction or no increase in BMI by study completion, 2) reduction or no increase in WC by study completion, 3) achievement of target LDL-C (≤2.6 mmol/L, based on current clinical guidelines), and 4) reduction or no increase in IRI≥12 µED/ml [11].
This study was conducted in accordance with the Declaration of Helsinki of the World Medical Association. The study protocol was approved by the Research Ethics Committee of V.I. Razumovsky Saratov State Medical University (Ref. No: 2/04.10.2016). All the patients provided informed consent to participate in the study.
Statistical analysis
Statistical analysis was performed using SPSS 21.0 for Windows software. At the planning stage, the sample size was calculated based on a planned comparison of proportions and statistical hypothesis testing for continuous variables. The expected proportions and standard deviations (SD) were obtained from the literature and tested with a sample size of approximately 2/3. The test power (1-β) was taken as 80%, with a significance level of α of 5% [12]. The normality of the distribution was tested using the Shapiro-Wilk test. Continuous variables are described using the median (Me) and interquartile range (Q1; Q3). Categorical variables were reported as frequencies and percentages. Mann-Whitney, Wilcoxon, and Kruskal-Wallis tests were used to test the statistical hypotheses. The effect size was described as the median difference with 95% confidence intervals (95% CI) (Hodges-Lehman estimate). Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated by using two-by-two contingency tables. The Pearson's χ2 test was used to compare categorical variables. Differences were considered statistically significant at p<0.05.
Results
The clinical characteristics of the study participants are summarized in Table 1.
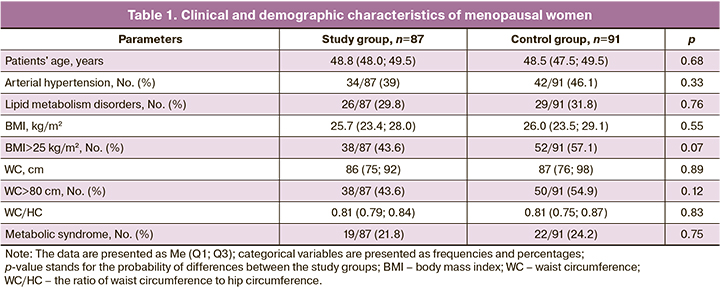
Women in the study groups did not differ significantly in their main clinical and demographic characteristics, except for the prevalence of overweight women in the control group.
The study was completed by 83 women in the study group and 87 women in the control group. Cholelithiasis was diagnosed in one woman in the study group, two women discontinued the drug owing to dyspeptic symptoms, and a blood TG increase was noted in one woman. No cases of deep venous thrombosis, pulmonary embolism, ischemic stroke, or acute coronary events and no cases of cancer were observed during COC administration.
Women in the study group were more likely to have vasomotor symptoms of menopausal syndrome, which is an additional argument for administering low-dose hormone therapy.
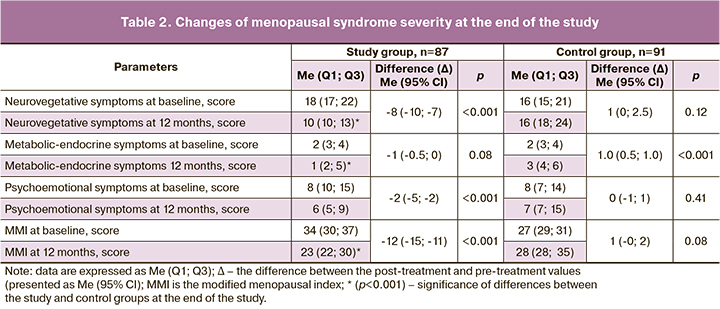
At the end of the study, vasomotor and metabolic endocrine manifestations of menopausal syndrome persisted and increased in the women in the control group, while there was a significant regression of the entire symptom complex of menopausal syndrome in the women in the study group at the end of the COC treatment (Table 2).
An increase in body weight was observed in 38 (43.6%) and 52 (57.1%) women in the study and control groups, respectively (χ2=3.2, p=0.07). There were no significant differences in the severity and frequency of abdominal obesity or metabolic syndrome among the women examined.
Changes in body weight and severity of abdominal obesity during treatment with drospirenone-containing contraceptives are shown in the figure.
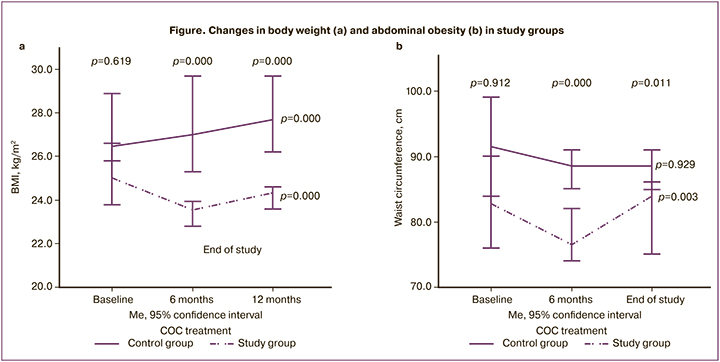
At baseline, there were no significant initial differences in BMI and WC among women in the study groups. However, by the end of the study, there were significant differences in the studied parameters, including a significant decrease in BMI and WC in the study group and a significant increase in BMI and no change in WC in the control group. BMI in the control group was 5.1 times as likely to increase compared to the study group treated with the COC containing drospirenone [OR 5.1 (95% CI 2.7;9.6), χ2=46.1 (p<0.001)].
There were no significant changes in the WC/HC ratios of the women examined during the follow-up period. At comparable baseline WC/HC ratio values in the women of the study group, the WC/HC ratio tended to decrease from 0.81 (0.79;0.84) to 0.80 (0.78;0.83) units at the end of the study (p=0.07); no significant changes in WC/HC ratio were observed in the control group [0.81 (0.75;0.87) and 0.82 (0.75;0.85) units (p=0.18)].
There were no statistically significant differences in the risk of increased abdominal obesity: OR 0.42 (95% CI 0.11;1.48), χ2=1.8 (p=0.16).
Given the close relationship between visceral obesity and IR, it was of interest to examine the dynamics of IR and carbohydrate metabolism in women on the COC (Table 3).
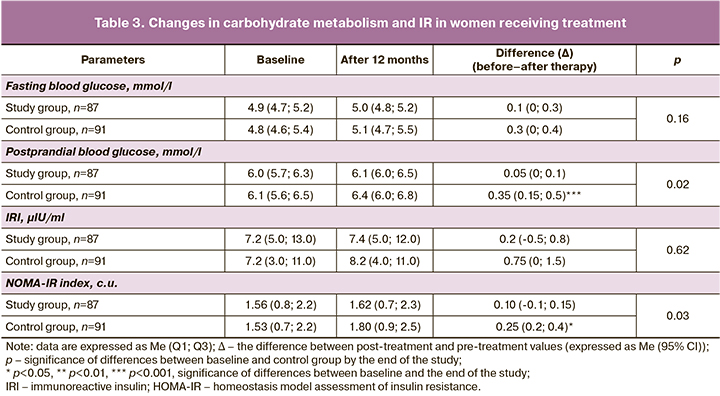
At baseline, no significant differences were observed between the groups.
There were no significant changes in the fasting blood glucose levels in the women in the two groups at the end of the study. Postprandial blood glucose levels showed no significant changes in the study group and a significant increase in the control group (p=0.02).
The women in the control group had an increased IRI by the end of the study, with a clear tendency toward higher IRI levels and a significant increase in the IR NOMA-IR index; no significant changes in the values of the same indices were found in the women in the study group.
There were no statistically significant differences in the risk of increased IR: OR 1.6 (95% CI 0.7;3.7), χ2=1.9 (p=0.17).
There were no significant differences in lipid metabolism between the two groups at baseline:26 (29.8%) women in the study group and 29 (31.8%) in the control group were diagnosed with lipid metabolism disorders (χ2=0.9, p>0.05).
Changes in the parameters of blood lipid spectra are presented in Table 4.
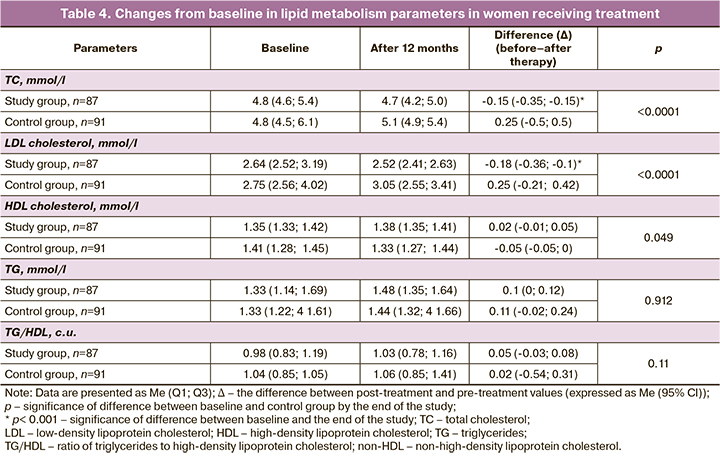
By the end of the study, no significant changes in blood lipid parameters were detected in the control group. In patients undergoing COC treatment, changes in lipid metabolism were different and more pronounced: there was a significant reduction in atherogenic lipid fractions, including total and LDL cholesterol. At the same time, a decrease in the risk of adverse changes in the lipid profile was observed in the study group: the probability of no change – OR 3.1 (95% CI 1.8;5.1), χ2=26.9 (p<0.001).
Despite the described risk of increased TG levels during treatment with COC, no significant changes in TG levels or HDL/HDL ratio were observed at the end of the study.
Thus, at the end of the study, the women in the control group, compared to women taking the contraceptive containing drospirenone, had significantly higher levels of TC and LDL cholesterol, and lower HDL cholesterol.
Discussion
Metabolic syndrome is one of the most important factors contributing to an increased risk of death in women [13].
Some studies have shown that the onset of metabolic syndrome occurs earlier in the female reproductive system, several years before the onset of menopause, which calls for earlier interventions aimed at preventing the main components of metabolic syndrome [14–16]. However, it remains unclear whether prescribing COCs during menopausal transition can slow the rate of progression of metabolic disorders and how this affects the risk of future cardiovascular disease [16].
It is clear that the different components of metabolic syndrome contribute differently to increased cardiovascular risk, and the presence of menopausal vasomotor symptoms is an independent factor for cardiovascular distress.
Recent studies have shown an association between the severity of vasomotor symptoms and increased cardiovascular risk [17–19]. Women with severe menopausal symptoms were more likely to develop metabolic syndrome [19, 20].
As menopausal transition is characterized by "hormonal chaos,” vasomotor symptoms, and an accumulation of cardiovascular risk factors, we were interested in studying the severity of metabolic risk factors, IR, and dyslipidemia in women during menopausal transition.
More than half of the women in the study had excess body weight and abdominal obesity, which are the basis for the development of metabolic syndrome [7, 8, 14, 15].
A third of the women [55/178 (30.9%)] had lipid metabolism disorders and 41/178 (23%) were diagnosed with metabolic syndrome. In our study, the incidence of metabolic syndrome in women during menopausal transition was consistent with results reported in other studies [15].
Due to the high incidence of metabolic abnormalities in women undergoing menopausal transition, it is of interest to further develop the concept of early hormone therapy, initiated while still in the transition period from contraception to menopausal hormone therapy, which not only effectively addresses the early manifestations of menopausal syndrome but may also further reduce the risk of late cardiovascular complications of menopause [3, 6].
The nature and characteristics of the changes in cardiovascular metabolic risk factors and cardiovascular safety issues associated with the use of COCs in menopausal women have not been sufficiently explored.
The main concern is the potential for COC to increase the risk of thrombotic complications (myocardial infarction, ischemic stroke, and venous thromboembolism) [21–23].
Several studies have reported an increased risk of venous thromboembolism and arterial thrombosis associated with combined oral contraceptives containing drospirenone, largely due to the use of high-dose drospirenone and the type and dose of estrogen in COC [24, 25].
In our study, we used a low-dose combination of 20 µg of ethinyl estradiol and 3 mg of drospirenone. During the entire follow-up, none of the patients reported the development of acute thrombotic events on drospirenone-containing oral contraceptives.
Some researchers have attributed a small increase in these risks to undiagnosed baseline thrombophilia in women with an initially unfavorable background (obesity, diabetes, and hypertension) [23, 24].
However, given the age of women, the absolute risk of complications is generally low and is considered medically acceptable given the benefits that women can gain from using COCs [21, 23].
The current literature reports mixed findings regarding the effects of oral contraceptives on carbohydrate and lipid metabolism, and body weight.
It is clear that the active ingredients in oral contraceptives have a multidirectional effect, depending on their ratio and biological properties. Thus, according to some studies, the use of high-dose COCs or COC containing progestins with an androgenic effect in most cases was associated with impaired glucose tolerance and increased IR, increased body weight, and increased blood pressure [9, 21], offsetting the positive effects of estrogen in relation to blood lipids and increasing thrombogenic risks [26].
The choice of COCs in our study was due to the fact that ethinyl estradiol is a synthetic estradiol with more pronounced estrogenic properties, and is more resistant to metabolism in the liver than natural estradiol, and can be used in overweight or obese women [26].
Drospirenone has a pharmacological profile most similar to that of endogenous progesterone and has a high affinity for mineralocorticoid receptors, moderate anti-androgenic effect, and no residual glucocorticoid activity, which is particularly important when used in women with metabolic risk factors and obesity [27, 28].
In this study, a significant reduction in body weight, severity of abdominal obesity, no changes in carbohydrate metabolism and IR, and a decrease in blood plasma atherogenicity were observed in the women in the study group. Treatment with COCs containing drospirenone was associated with a reduction in the risk of increases in BMI and WC and adverse changes in lipid profile. It is possible that the cardiovascular effects of hormone therapy depend on the timing of its initiation (early administration of combined hormone therapy), which will allow the development of optimal regimens for its use.
Conclusion
This study identified a high incidence of metabolic risk factors in women undergoing menopausal transition. More than half of the study participants had excessive body weight and abdominal obesity, one-third of the women had lipid metabolism disorders, and almost a quarter of the women had metabolic syndrome.
Early administration (at the onset of the first symptoms of menopausal transition) of combined contraceptive containing drospirenone to menopausal women prevents an increase in body weight and visceral obesity, helps reduce IR and impaired carbohydrate metabolism, and has a beneficial effect on lipid metabolism, which is important for the prevention of menopausal metabolic syndrome.
Limitations of the study
A limitation of this study is the relatively short follow-up duration of women taking COCs containing drospirenone. The small sample size did not allow separate groups of obese women with metabolic syndrome to be identified to assess the efficacy and safety of COC in these groups.
There is clear interest in studying the long-term cardioprotective effects of COCs in women undergoing menopausal transition. Further research is needed to determine whether interventions during menopausal transition, including the use of COCs, can slow the rate of progression of metabolic syndrome severity and reduce the risk of future disease.
References
- Шляхто Е.В., Звартау Н.Э., Виллевальде С.В., Яковлев А.Н., Соловьева А.Е., Алиева А.С. и др. Система управления сердечно-сосудистыми рисками: предпосылки к созданию, принципы организации, таргетные группы. Российский кардиологический журнал. 2019; 24(11): 69-82. [Shlyakhto E.V., Zvartau N.E., Villevalde S.V., Yakovlev A.N., Soloveva A.E., Alieva A.S. et al. Cardiovascular risk management system: prerequisites for developing, organization principles, target groups. Russian Journal of Cardiology. 2019; 24(11): 69-82. (in Russian)]. https://dx.doi.org/10.15829/1560-4071-2019-11-69-82.
- Юренева С.В., Ильина Л.М., Сметник В.П. Старение репродуктивной системы женщин: от теории к клинической практике. Часть I. Эндокринные и клинические характеристики стадий репродуктивного старения женщин. Акушерство и гинекология. 2014; 3: 21-7. [Yureneva S.V., Ilyina L.M., Smetnik V.P. Female reproductive system aging: From theory to clinical practice. Part 1. The endocrine and clinical characteristics of female reproductive system aging stages. Obstetrics and Gynecology. 2014; (3): 21-7. (in Russian)].
- Подзолков В.И., Брагина А.Е., Подзолкова Н.М. Менопаузальная гормональная терапия и сердечно-сосудистая профилактика: желаемое или действительное? Кардиоваскулярная терапия и профилактика. 2019; 18(3): 94-106. [Podzolkov V.I., Bragina A.E., Podzolkovа N.M. Menopausal hormone therapy and heart disease prevention: desired or valid? Cardiovascular Therapy and Prevention. 2019; 18(3): 94-106. (In Russian)].https://dx.doi.org/10.15829/1728-8800-2019-3-94-106.
- Mumusoglu S., Yildiz B.O. Metabolic syndrome during menopause. Curr. Vasc. Pharmacol. 2019; 17(6): 595-603. https://dx.doi.org/10.2174/1570161116666180904094149.
- Эседова А.Э., Уруджева Н.Г., Ильина И.Ю. Менопаузальный метаболический синдром и риски назначения менопаузальной гормональной терапии. Пути решения. РМЖ. Мать и дитя. 2020; 3(4): 260-6. [Esedova A.E., Urudzheva N.G., Il’ina I.Yu. Menopausal metabolic syndrome and the risks of menopausal hormone therapy: the solutions. Russian Journal of Woman and Child Health. 2020; 3(4): 260-6. (in Russian)].https://dx.doi.org/10.32364/2618-8430-2020-3-4-260-266.
- Юренева С.В., Ильина Л.М. Старение репродуктивной системы женщин: от теории к клинической практике. Часть II. Роль гормональной терапии в решении проблем переходного периода и ранней постменопаузы. Акушерство и гинекология. 2014; 4: 17-24. [Yureneva S.V., Ilyina L.M. Female reproductive system aging: From theory to clinical practice. Part 2. Role of hormone therapy in solving the problems of transition stage and early postmenopause. Obstetrics and Gynegology. 2014; (4): 17-24.(in Russian)].
- Baber R.J., Panay N., Fenton A.; IMS Writing Group. 2016 IMS Recommendations on women’s midlife health and menopause hormone therapy. Climacteric. 2016; 19(2): 109-50. https://dx.doi.org/10.3109/13697137.2015.1129166.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of the North American Menopause Society. Menopause. 2017; 24(7): 728-53. https://dx.doi.org/10.1097/GME.0000000000000921.
- Юренева С.В., Ермакова Е.И. Ведение женщин с менопаузальными расстройствами (обзор клинических рекомендаций). Проблемы репродукции. 2017; 23(5): 115-22. [Yureneva S.V., Ermakova E.I. The management of women with menopausal disorders (review of clinical guidelines). Russian Journal of Human Reproduction. 2017; 23(5):115-22. (in Russian)].https://dx.doi.org/10.17116/repro2017235115-122.
- Harlow S.D., Gass M., Hall J.E., Lobo R., Maki P., Rebar R.W. et al.; STRAW 10 Collaborative Group. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause. 2012; 19(4): 387-95. https://dx.doi.org/10.1097/gme.0b013e31824d8f40.
- Mach F., Baigent C., Catapano A.L., Koskinas K.C., Casula M., Badimon L. et al.; ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020; 41(1): 111-88. https://dx.doi.org/10.1093/eurheartj/ehz455.
- Peacock J.L., Peacock P.J. Oxford handbook of medical statistics. Oxford university press; 2011. 517p.
- Lin J.W., Caffrey J.L., Chang M.H., Lin Y.S. Sex, menopause, metabolic syndrome, and all-cause and cause-specific mortality--cohort analysis from the Third National Health and Nutrition Examination Survey. J. Clin. Endocrinol. Metab. 2010; 95(9): 4258-67. https://dx.doi.org/10.1210/jc.2010-0332.
- Heidari R., Sadeghi M., Talaei M., Rabiei K., Mohammadifard N., Sarrafzadegan N. Metabolic syndrome in menopausal transition: Isfahan Healthy Heart Program, a population based study. Diabetol. Metab. Syndr. 2010; 2: 59. https://dx.doi.org/10.1186/1758-5996-2-59.
- Hallajzadeh J., Khoramdad M., Izadi N., Karamzad N., Almasi-Hashiani A., Ayubi E. еt al. Metabolic syndrome and its components in premenopausal and postmenopausal women: a comprehensive systematic review and meta-analysis on observational studies. Menopause. 2018; 25(10): 1155-64.https://dx.doi.org/10.1097/GME.0000000000001136.
- Gurka M.J., Vishnu A., Santen R.J., DeBoer M.D. Progression of metabolic syndrome severity during the menopausal transition. J. Am. Heart Assoc. 2016; 5(8): e003609. https://dx.doi.org/10.1161/JAHA.116.003609.
- Zhu D., Chung H.F., Dobson A.J., Pandeya N., Anderson D.J., Kuh D. et al. Vasomotor menopausal symptoms and risk of cardiovascular disease: a pooled analysis of six prospective studies. Am. J. Obstet. Gynecol. 2020; 223(6): 898. e1-898. e16. https://dx.doi.org/10.1016/j.ajog.2020.06.039.
- Dam V., Dobson A.J., Onland-Moret N.C., van der Schouw Y.T., Mishra G.D. Vasomotor menopausal symptoms and cardiovascular disease risk in midlife: A longitudinal study. Maturitas. 2020; 133: 32-41. https://dx.doi.org/10.1016/j.ajog.2020.06.039.
- Толстов С.Н., Салов И.А., Ребров А.П. Взаимосвязь выраженности эндотелиальной дисфункции и метаболических нарушений с тяжестью климактерического синдрома. Акушерство и гинекология. 2021; 8: 119-26. [Tolstov S.N., Salov I.A., Rebrov A.P. Relationship between the severity of endothelial dysfunction and metabolic disorders and the severity of climacteric syndrome. Obstetrics and Gynecology. 2021; (8): 119-26. (in Russian)].https://dx.doi.org/10.18565/aig.2021.8.119-126.
- Tuomikoski P., Savolainen-Peltonen H. Vasomotor symptoms and metabolic syndrome. Maturitas. 2017; 97: 61-5. https://dx.doi.org/10.1016/j.maturitas.2016.12.010.
- Vinogradova Y., Coupland C., Hippisley-Cox J. Use of combined oral contraceptives and risk of venous thromboembolism: nested casecontrol studies using the QResearch and CPRD databases. BMJ 2015; 350: h2135.https://dx.doi.org/10.1136/bmj.h2135.
- FDA Office of Surveillance and Epidemiology. Combined Hormonal Contraceptives (CHCs) and the risk of cardiovascular disease endpoints. Available at: http://www.fda.gov/downloads/Drugs/DrugSafety/UCM277384.pdf
- Доброхотова Ю.Э., Боровкова Е.И. Персонализированный подход к выбору контрацептива: взвешиваем все за и против. Гинекология. 2017; 19(3): 40-4. [DobrokhotovaYu.E., Borovkova E.I. The personalized approach to choosing a contraceptive: weighing the pros and cons. Gynecology. 2017; 19(3): 40-4 (in Russian)]. https://dx.doi.org/10.26442/2079-5696_19.3.40-44.
- Wu C.Q., Grandi S.M., Filion K.B., Abenhaim H.A., Joseph L., Eisenberg M.J. Drospirenone-containing oral contraceptive pills and the risk of venous and arterial thrombosis: a systematic review. BJOG. 2013; 120(7): 801-10.https://dx.doi.org/10.1111/1471-0528.12210.
- Bird S.T., Delaney J.A., Etminan M., Brophy J.M., Hartzema A.G. Drospirenone and non-fatal venous thromboembolism: is there a risk difference by dosage of ethinyl-estradiol. J. Thromb. Haemost. 2013; 11(6): 1059-68.https://dx.doi.org/10.1111/jth.12224.
- Андреева Е.Н., Соколова Д.А., Григорян О.Р. Контрацепция у женщин с ожирением. Ожирение и метаболизм. 2016. 13(3): 65-9. [Andreeva E.N., Sokolova D.A., Grigoryan O.R. Contraception in obese women. Obesity and metabolism. 2016; 13(3): 65-9 (in Russian)]. https://dx.doi.org/10.14341/OMET2016365-69.
- Толстов С.Н., Салов И.А., Ребров А.П. Выраженность абдоминального ожирения и нарушений углеводного обмена у женщин в ранней постменопаузе и возможности коррекции выявленных нарушений. Фарматека. 2017; 3: 36-40. [Tolstov S.N., Salov I.A., Rebrov S.N. The severity of abdominal obesity and disorders of carbohydrate metabolism in women in early postmenopausal women and the possibility of correction of the revealed violations. Farmateka. 2017; (3): 36-40. (in Russian)].
- Прилепская В.Н., Новикова Е.П. Дроспиренон-содержащие контрацептивные средства – возможности расширяются. Фарматека. 2012; 12: 49-52. [Prilepskaya V.N., Novikova E.P. Drospirenone-containing contraceptives – options are expanding. Farmateka. 2012; (12): 49-52. (in Russian)].
Received 01.11.2022
Accepted 13.01.2023
About the Authors
Sergey N. Tolstov, Dr. Med. Sci., Professor at the Department of Therapy with Courses in Cardiology, Functional Diagnostics and Geriatrics, V.I. Razumovsky Saratov State Medical University, Ministry of Health of Russia, 112 B. Kazachya str., Saratov, 410012, Russian Federation; Head of the Vascular Center, Regional Clinical Cardiology Center, 16 Krymskiy proyezd str., Saratov, 410039, Russian Federation, +7(8452)39-28-09, tolstovsn@mail.ru, https://orcid.org/0000-0002-4546-9449Igor A. Salov, Dr. Med. Sci., Professor, Head of Obstetrics and Gynecology Department, V.I. Razumovsky Saratov State Medical University, Ministry of Health of Russia, +7(8452)51-51-21, salov.i.a@mail.ru, https://orcid.org/0000-0002-1926-5418, 112 B.Kazachya str., Saratov, 410012, Russian Federation.
Andrey P. Rebrov, Dr. Med. Sci., Professor, Head of Hospital Therapy Department, V.I. Razumovsky Saratov State Medical University, Ministry of Health of Russia,
+7(8452)49-14-37, andreyrebrov@yandex.ru, https://orcid.org/0000-0002-3463-7734, 112 B. Kazachya str., Saratov, 410012, Russian Federation.
Corresponding author: Sergey N. Tolstov, tolstovsn@mail.ru



