Endothelial functional activity in women of reproductive age and menopausal women with metabolic syndrome
Shevlyukova T.P., Bulatova I.A.
Objective: This study aimed to investigate the functional state of the endothelium in patients with metabolic syndrome (MS) during the late reproductive age and early postmenopausal period.
Materials and methods: The study included 85 women with MS, of whom 35 patients (group 1) were of late reproductive age (mean age 44.3 (5.8) years) and 46 women (group 2) were of early postmenopausal age (mean age 51.5 (3.4) years). The control group consisted of 29 healthy women with a mean age of 41.4 (11.3) years. Biometric and metabolic parameters, lipid spectrum, follicle-stimulating hormone (FSH), and estradiol levels were measured. Laboratory markers of endothelial dysfunction (ED) including exfoliated endothelial cells (EEC), endothelin-1, vascular endothelial growth factor (VEGF), and von Willebrand factor activity were also assessed. The functional state of the endothelium was evaluated using local skin thermometry using a Microtest device (Russia).
Results: Laboratory signs of MS were more pronounced in postmenopausal patients in both groups. Hormonal status assessment revealed a significant increase in FSH and a decrease in estradiol levels in menopausal women with MS compared to menstruating patients with MS. The number of laboratory markers of ED in both subgroups of women with MS was significantly higher than that of the control group. Additionally, the activity of von Willebrand factor, which indicates thrombogenic potential and degree of endothelial damage, and the blood level of the vasoconstrictor endothelin-1 in patients with menopausal MS exceeded those in fertile women (p = 0.041 and p = 0.031, respectively).
Conclusion: Late reproductive age and early postmenopausal period in women with MS are accompanied by the development of dyslipidemia and significant changes in the vascular system, resulting in ED with increased levels of endothelin-1, EEC, VEGF, and von Willebrand factor activity, as well as impaired physiological vasodilation in response to local heating. As estrogen levels decrease, the severity of MS components and dyslipidemia increases, and ED progresses in the form of persistent vasoconstriction.
Authors' contributions: Shevlyukova T.P., Bulatova I.A. – conception and design of the study; Bulatova I.A., Shevlyukova T.P. – material collecting and processing, drafting of the manuscript; Shevlyukova T.P. – statistical analysis, editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the Tyumen SMU, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Shevlyukova T.P., Bulatova I.A. Endothelial functional activity in women of reproductive age and menopausal women with metabolic syndrome.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (6): 91-97 (in Russian)
https://dx.doi.org/10.18565/aig.2024.41
Keywords
Women spend at least one-third of their lives in the hypoestrogenic state. Currently, approximately 10% of the female population is postmenopausal. The mean age of menopause worldwide is 48.8 years, while in Russia, it ranges between 49 and 51 years [1]. An ESSE-RF-2 study conducted in 2017 examined a large sample from four regions in Russia and identified metabolic syndrome (MS) in 33% of Russians aged 25–64 years based on the 2006 IDF criteria. The prevalence of MS was significantly higher among women in older age groups [2, 3]. Postmenopausal women have a higher prevalence of MS compared to premenopausal women, with rates of 37–69% and 19–59%, respectively [4–6].
The most common components of MS in postmenopausal women are central obesity, hypertension, and dyslipidemia. Multivariate analysis showed that age and a high body mass index (BMI) increased the risk of developing MS [4]. A 2018 study of the nutritional status of adults in the Russian Federation found that 63.0% of women were overweight (BMI≥25.0 kg/m2) and 27.4% were classified as obese (BMI≥30.0 kg/m2). The incidence of obesity in women increases slowly from 19 to 40 years of age and then rapidly from 40 to 65 years of age. The highest incidence of overweight is found in women over 50 years of age (75–83%), and obesity rates in women over 50 years of age are 12–16% higher than those in men of the same age [7]. According to a large meta-analysis, changes in fat mass between premenopausal and postmenopausal women were mainly associated with increasing age. However, menopause leads to redistribution of fat mass and the development of abdominal obesity, probably due to hormonal changes [8].
Menopause is characterized by changes in ovarian hormone levels, increased cardiovascular risk factors, and vasomotor symptoms that can negatively impact vascular health. Estrogen plays a critical role in maintaining endothelial health by increasing nitric oxide bioavailability, reducing oxidative stress and inflammation, and influencing estrogen receptor signaling in women. Studies have shown that estrogen receptor expression is reduced during both acute and long-term estrogen deficiency [9].
According to some data, a decline in endothelial function begins during perimenopause, independent of vasomotor symptoms, and worsens with the loss of ovarian function and long-term estrogen deficiency [10]. Vascular dysfunction at different stages of menopause has been associated with more frequent and severe menopausal symptoms and a lower quality of life [11–13].
Endothelial dysfunction (ED) plays an important role in atherosclerosis development. Therefore, after menopause and the associated loss of estrogen, women are at increased risk for adverse cardiovascular events [14, 15]. However, data on the effects of MS and its components on endothelial function in women with different reproductive and hormonal statuses are limited.
This study aimed to investigate the functional state of the endothelium in patients with MS during late reproductive age and early postmenopause.
Materials and methods
We examined 81 women with MS, established based on the criteria of the International Diabetes Federation (IDF, 2005), of which 35 patients (group 1) were of late reproductive age (44.3 (5.8) years) and 46 (group 2) were in the early postmenopause (51.5 (3.4) years). The control group included 29 practically healthy women of reproductive age (17 women) and early postmenopausal women (12 women) with a mean age of 41.4 (11.3) years. All the women signed an informed consent form to participate in the study. Baseline evaluation included anthropometric and body composition measurements. BMI was calculated using Quetelet's index formula: body weight (kg)/height (m)2. The women underwent laboratory examinations, including determination of blood glucose, total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and very low-density lipoprotein cholesterol (VLDL), using an automatic biochemical analyzer Architect-4000 (USA). An enzyme immunoassay was used to determine insulin levels using the ELISA Monobind Inc. kit (Germany), endothelin-1 using a kit from Biomedica Medizinprodukte GmbH & Co RG (Austria), and vascular endothelial growth factor (VEGF) using a kit from JSC Vector-Best (Russia). The levels of estradiol and follicle-stimulating hormone (FSH) were determined by chemiluminescent immunoassay using a Siemens analyzer (Germany) (postmenopausal levels were 5–46 pg/mL).
To determine blood plasma exfoliated endothelial cells (EEC), a modified method of Hladovec (1978) was used, which requires the separation of endothelial cells and platelets by adenosine diphosphate precipitation [16]. The von Willebrand factor activity was assessed on a BIOLA aggregometer (Russia) using a Renam kit (Russia). The insulin resistance index was calculated using the formula IR-HOMA=basal insulin level (μIn/ml) × basal glucose level (mmol/l)/22.5. The presence of liver steatosis was determined using ultrasound examination (US).
The functional state of the endothelium was assessed by local skin thermometry using a Microtest device (Russia; RU Roszdravnadzor No. FSR 2012/14175), with a temperature resolution of up to 0.001°C. Fluctuations in skin temperature were recorded during the local heating of the distal phalanx of the middle finger to 42°C for 10 min, with further observation of the process of equalization of the temperature of the finger to room temperature. Low-amplitude temperature fluctuations on the skin surface are caused by changes in the tone and velocity of the blood flow in the small arteries and arterioles in the subcutaneous tissue. Time-frequency analysis of temperature variations was performed using wavelet analysis, which allowed the isolation of variations within a certain frequency range. Spectral analysis of fluctuations in the tone of the skin vascular system provides additional information about local, hormonal, and neurogenic factors of microcirculation regulation [17]. Fluctuations in skin temperature were measured in specific frequency ranges, including 0.05–0.14 Hz (myogenic interval, scales 7–20 s), 0.02–0.05 Hz (neurogenic 20–50 s), and 0.0095–0.02 Hz (endothelial 50–105 s). To assess the contribution of different mechanisms of regulation of vascular tone, we selected the root mean square value of the amplitude of fluctuations in skin temperature in the corresponding frequency range, the thermal vasodilation index (TVI), which was calculated using the formula TVI=(ST1-ST2)/ST2, where ST1 is the root mean square amplitude of heating, and ST2 is the amplitude after local heating.
Statistical analysis
Statistical analysis was performed using the built-in analysis package of the Excel 2016 MSO spreadsheet processor (Microsoft, 2016), and individual calculations were carried out using MedCalc 15.8 Portable (MedCalc Software, 1993–2014). The distribution of continuous variables was tested for normality using the Shapiro–Wilk test. Quantitative variables showing normal distribution were expressed as means (M) and standard deviation (SD) and presented as M (SD); otherwise, the median (Me) with its confidence interval (xJ–xK) were reported according to GOST R ISO 16269-7–2004. Normally distributed continuous variables were compared between the two groups using the t-test; in the absence of a normal distribution, the Mann–Whitney (U) test was used. For multiple comparisons (three groups), the Kruskal–Wallis test (H) was used. Differences were considered statistically significant at p<0.05. The Bonferroni correction (critical significance level 0.017) was applied for pairwise comparisons using t-tests. The relationship between the quantitative variables was determined using the correlation coefficient (rXY) at a significance level of p<0.05).
Results and discussion
In both groups of patients with MS, the anthropometric parameters exceeded those of healthy women. Eight of the 35 (23%) women in group 1 with MS in late reproductive age and 18/46 (39%) patients in group 2 with menopausal metabolic syndrome (MMS) were overweight. Obesity of varying severity was observed in 27/35 (77%) and 28/46 (61%) women, respectively. Type 2 diabetes mellitus was diagnosed in 9/35 (26%) and 8/46 (17%) patients in groups 1 and 2, respectively, glucose intolerance in 15/35 (43%) and 15/46 (33%), arterial hypertension in 21/35 (60%) and 39/46 (85%), and fatty liver disease detected by US in 10/35 (29%) and 24/46 (52%), respectively.
The evaluation of metabolic parameters in both groups of patients revealed hyperinsulinemia, insulin resistance, and dyslipidemia of varying severity. Analysis of lipid profile parameters in patients in groups 1 and 2 showed a significant increase in triglycerides by 1.3 and 1.75 times, respectively, in LDL-C by 1.1 and 1.3 times, and in VLDL-C, as well as a decrease in HDL-C with aging. Moreover, in the majority of fertile women, the TC level remained within the reference values or at the upper limit of normal; in the group of patients with MMS it was significantly higher than normal (Table 1).
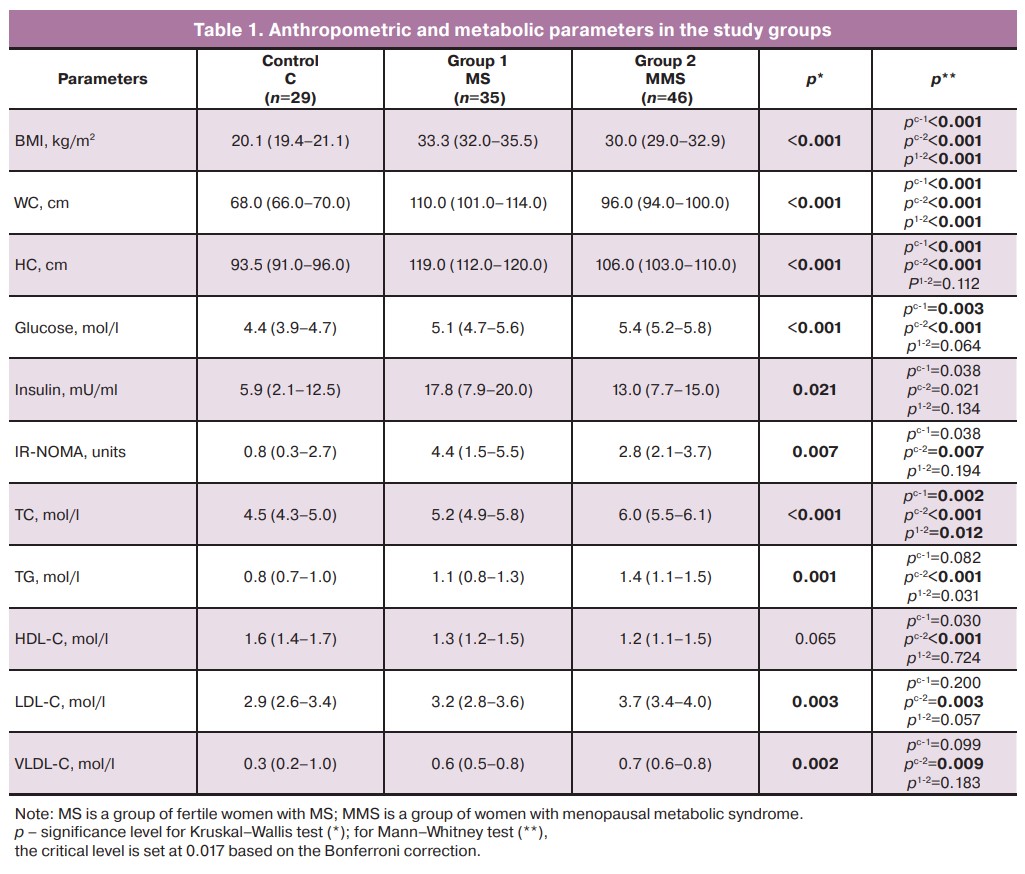
Thus, we observed laboratory signs of MS in both groups, which were more pronounced in postmenopausal patients. Assessment of hormonal status showed a significant increase in FSH and decrease in estradiol in the group of women with MMS compared with menstruating patients with MS: FSH levels were 67.8 (57.8–75.1) and 6.7 (4.7–12.0) mIU/mL (p<0.001), and estradiol levels were 52.0 (38.0–92.7) and 17.2 (13.3–23.5) pg/mL (p<0.001), respectively. Obese women have a greater amplitude of fluctuation in sex hormone levels within the menstrual cycle than women of normal weight, which may contribute to the development of ED [18].
ED can be defined as an imbalance in the synthesis by the endothelium of various biologically active substances that affect the tone, elasticity and hemostatic properties of the vascular wall. Our study showed a significant increase in the content of one of the important factors of vasoconstriction, endothelin-1, in the blood serum of patients with MMS compared to that in the control group (p=0.001) (Table 2).
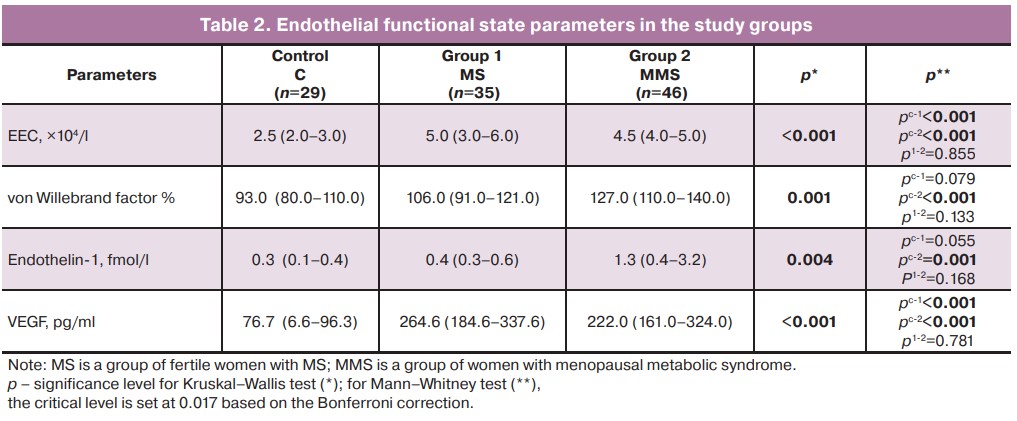
According to some researchers, MS and systemic inflammation are synergistically associated with ED in postmenopausal women who have a significantly higher risk of adverse cardiovascular events with these components [15]. The loss of estradiol effects, including the synthesis of the vasodilator nitric oxide and reduction of endothelin-1 synthesis, underlies the development of vascular dystonia and subsequent arterial hypertension [13]. We found a negative correlation between the level of endothelin-1 in postmenopausal patients and the level of estradiol (rXY =-0.529; p=0.035), confirming the dependence of vascular endothelial vasoconstriction on sex hormone levels.
The amount of EEC and the level of von Willebrand factor were higher in both subgroups of women with MS than in the control group, which may be a consequence of damage to the inner layer of the vascular intima and the activation of the procoagulant properties of the endothelium. Endothelial damage with the development of hypercoagulation against a background of vasoconstriction may lead to thrombosis, especially in postmenopausal women.
An increase in vascular endothelial growth factor by 3.5 and 2.9 times in fertile women and patients with MMS, respectively, compared to normal subjects indicates a disruption of the normal function of endothelial cells and their responsibility for the angiogenic function of the endothelial lining. VEGF levels may reflect arterial reactivity in postmenopausal women [19].
Obesity appears to be a significant contributor to ED. Metabolic products and inflammation of adipose tissue, nitric oxide bioavailability, insulin resistance and oxidized LDL-C are, according to some researchers, the main stimulators of endothelial imbalance [20, 21].
Local heating leads to vascular hypotension, vasodilation, a decrease in the amplitudes of skin temperature fluctuations, and an increase in TVI, which was recorded in the control group. In patients with MS in the late reproductive and early postmenopausal periods, there was an insufficient increase in TVI in the muscle and endothelial frequency ranges, which reflects a violation of the mechanisms of regulation of vascular tone (Table 3).
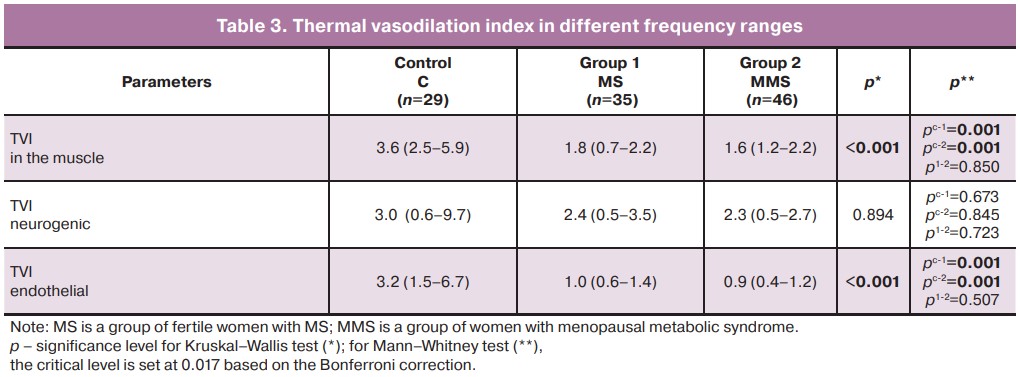
A significant decrease in the vascular response of the endothelium to local heating in obese menopausal women has also been reported by other investigators [22]. In postmenopausal women, hypoestrogenism precedes the development of ED and the weakening of endothelial resistance to ischemia-reperfusion syndrome. Research has also shown that endothelial resistance to damage is weakened in healthy women during early post-menopause [23]. Thus, MS components, in combination with estrogen deficiency, contribute to the development of ED, which is manifested by vascular wall damage, vasoconstriction, and impaired vasodilation.
Conclusion
The course of late reproductive age and early postmenopause in women with MS is accompanied by the development of dyslipidemia and significant changes in the vasculature leading to ED with increased levels of laboratory markers, including endothelin-1, EEC, VEGF, and Willebrand factor activity, as well as impaired physiological vasodilation to local heating. As estrogen levels decrease, the severity of MS components, dyslipidemia, and ED progression increases in the form of persistent vasoconstriction by endothelin-1 levels and growth of the thrombogenic potential of the vessel wall with increased functional activity of Willebrand factor. The informative markers of ED are endothelin-1, von Willebrand factor, and the assessment of the thermal vasodilation index during local heating.
References
- Мазитова М.И., Мардиева Р.Р., Талипова И.Р., Антропова Е.Ю. Климактерический синдром. Клинико-эпидемиологический анализ. Российский вестник акушера-гинеколога. 2021; 21(5): 66-72. [Mazitova M.I., Mardieva R.R., Talipova I.R., Antropova E.Y. Menopausal syndrome. Clinical and epidemiological analysis. Russian Bulletin of the Obstetrician-Gynecologist. 2021; 21(5): 66-72. (in Russian)]. https://dx.doi.org/10.17116/rosakush20212105166.
- Баланова Ю. А., Имаева А/.Э., Куценко В.А., Капустина А.В., Муромцева Г.А., Евстифеева С.Е., Максимов С.А., Карамнова Н.С., Яровая Е.Б., Шальнова С.А., Драпкина О.М. Метаболический синдром и его ассоциации с социально-демографическими и поведенческими факторами риска в российской популяции 25-64 лет. Кардиоваскулярная терапия и профилактика. 2020; 19(4): 2600. [Balanova Yu.A., Imaeva A.E., Kutsenko V.A., Kapustina A.V., Muromtseva G.A., Evstifeeva S.E., Maksimov S.A., Karamnova N.S., Yarovaya E.B., Shalnova S.A., Drapkina O.M. Metabolic syndrome and its associations with socio-demographic and behavioral risk factors in the Russian population 25-64 years old. Cardiovascular Therapy and Prevention. 2020; 19(4): 2600. (in Russian)]. https://dx.doi.org/10.15829/1728-8800-2020-2600.
- Вильсон Н.И., Беленькая Л.В., Шолохов Л.Ф., Игумнов И.А., Наделяева Я.Г., Сутурина Л.В. Метаболический синдром: эпидемиология, критерии диагностики, расовые особенности. Acta biomedica scientifica. 2021; 6(4): 180-91. [Wilson N.I., Belenkaya L.V., Volokhov L.F., Igumnov I.A., Nadelyaeva Ya.G., Suturina L.V. Metabolic syndrome: epidemiology, diagnostic criteria, racial characteristics. Acta biomedica scientifica. 2021; 6(4): 180-91. (in Russian)]. https://dx.doi.iorg/10.29413/ABS.2021-6.4.
- Jeenduang N., Trongsakul R., Inhongsa P., Chaidach P. The prevalence of metabolic syndrome in premenopausal and postmenopausal women in Southern Thailand. Gynecol. Endocrinol. 2014; 30(8): 573-6. https://dx.doi.org/10.3109/09513590.2014.907261.
- Maharlouei N., Bellissimo N., Ahmadi S.M., Lankarani K.B. Prevalence of metabolic syndrome in pre- and postmenopausal Iranian women. Climacteric. 2013; 16(5): 561-7. https://dx.doi.org/10.3109/13697137.2012.727504.
- Мухамедова Н.Х. К механизму дисфункции эндотелия при метаболическом синдроме у женщин репродуктивного и постменопаузального периода. Молодой ученый. 2015; 11(91): 683-7. [Mukhamedova N.Kh. The mechanism of endothelial dysfunction in MS in women of reproductive and postmenopausal. Young Scientist. 2015; 11(91): 683-7. (in Russian)].
- Мартинчик А.Н., Лайкам К.Э., Козырева Н.А., Кешабянц Э.Э., Михайлов Н.А., Батурин А.К., Смирнова Е.А. Распространение ожирения в различных социально-демографических группах населения России. Вопросы питания. 2021; 90(3): 67-76. [Martinchik A.N., Laikam K.E., Kozyreva N.A., Keshabyants E.E., Mikhailov N.A., Baturin A.K., Smirnova E.A. The prevalence of obesity in various socio-demographic groups of the population of Russia. Problems of Nutrition. 2021; 90(3): 67-76. (in Russian)]. https://dx.doi.org/10.33029/0042-8833-2021-90-3-67-76.
- Ambikairajah A., Walsh E., Tabatabaei-Jafari H., Cherbuin N. Fat mass changes during menopause: a metaanalysis. Am. Obstet. Gynecol. 2019; 221(5): 393-409. https://dx.doi.org/10.1016/j.ajog.2019.04.023.
- Somani Y.B., Pawelczyk J.A., De Souza M.J., Kris-Etherton P.M., Proctor D.N. Aging women and their endothelium: probing the relative role of estrogen on vasodilator function. Am. J. Physiol. Heart Circ. Physiol. 2019; 317(2): 395-404. https://dx.doi.org/10.1152/ajpheart.00430.2018.
- Moreau K.L., Hildreth K.L., Meditz A.L., Deane K.D., Kohrt W.M. Endothelial function is impaired across the stages of the menopause transition in healthy women. J. Clin. Endocrinol. Metab. 2012; 97(12): 4692-700. https://dx.doi.org/10.1210/jc.2012-2244.
- Hildreth K.L., Ozemek C., Kohrt W.M., Blatchford P.J., Moreau K.L. Vascular dysfunction across the stages of the menopausal transition is associated with menopausal symptoms and quality of life. Menopause. 2018; 25(9): 1011-19. https://dx.doi.org/10.1097/GME.0000000000001112.
- Keller A.C., Klawitter J., Hildreth K.L., Christians U., Putnam K., Kohrt W.M. et al. Elevated plasma homocysteine and cysteine are associated with endothelial dysfunction across menopausal stages in healthy women. J. Appl. Physiol. 2019; 126(6): 1533-40. https://dx.doi.org/10.1152/japplphysiol.00819.2018.
- Кузнецова И.В. Эндотелиальная дисфункция как связующее звено климактерического синдрома и сердечно-сосудистых заболеваний. Эффективная фармакотерапия. 2019; 15(32): 32-40. [Kuznetsova I.V. Endothelial dysfunction as a link between menopausal syndrome and cardiovascular diseases. Effective Pharmacotherapy. 2019; 15(32): 32-40. (in Russian)]. https://dx.doi.org/10.33978/2307-3586-2019-15-32-32-40.
- Keshikova D.D., Olshevskaya O.K., Khidirova L.D. Endothelial dysfunction as the main component of menopausal syndrome and cardiovascular diseases. Lechaschi Vrach. 2023; 26(6): 78-82. https://dx.doi.org/10.51793/OS.2023.26.6.011.
- Zhang H., Sun T., Cheng Y., Zhang J., Zhang H., Krittanawong C. et al. Impact of metabolic syndrome and systemic inflammation on endothelial function in postmenopausal women. Turk. Kardiyol. Dern. Ars. 2022; 50(1): 57-65. https://dx.doi.org/10.5543/tkda.2022.47443.
- Васина Л.В., Петрищев Н.Н., Власов Т.Д. Эндотелиальная дисфункция и ее основные маркеры. Регионарное кровообращение и микроциркуляция. 2017; 16(1): 4-15. [Vasina L.V., Petrishchev N.N., Vlasov T.D. Markers of endothelial dysfunction. Regional Blood Circulation and Microcirculation. 2017; 16(1): 4-5. (in Russian)]. https://dx.doi.org/10.24884/1682-6655-2017-16-1-4-15.
- Podtaev S., Stepanov R., Smirnova E., Loran E. Wavelet-analysis of skin temperature oscillations during local heating for revealing endothelial dysfunction. Microvasc. Res. 2015; 97: 109-14. https://dx.doi.org/10.1016/j.mvr.2014.10.003.
- Yeung E.H., Zhang C., Albert P.S., Mumford S.L. Adiposity and sex hormones across the menstrual cycle: the BioCycle Study. Int. J. Obes. (Lond.). 2013; 37(2): 237-43. https://dx.doi.org/10.1038/ijo.2012.9.
- Lam P.M., Yim S.F., Chung T.K., Haines C. Serum vascular endothelial growth factor as a possible indicator of arterial reactivity in postmenopausal women. Gynecol. Endocrinol. 2006; 22(8): 460-4. https://dx.doi.org/10.1080/09513590600902986.
- Engin A. Endothelial dysfunction in obesity. Adv. Exp. Med. Biol. 2017; 960: 345-79. https://dx.doi.org/10.1007/978-3-319-48382-5_15.
- Чусова Н.А. Роль эндотелиальной дисфункции при ожирении. Международный студенческий научный вестник. 2019; 5(часть 2). [Chusova N.A. The role of endothelial dysfunction in obesity. International Student Scientific Bulletin. 2019; 5(Part 2). (in Russian)]. Available at: https://eduherald.ru/ru/article/viewid=19809.
- Смирнова Е.Н., Лоран Е.А., Шулькина С.Г., Подтаев С.Ю. Взаимосвязь эндотелиальной дисфункции и метаболических проявлений ожирения. Регионарное кровообращение и микроциркуляция. 2020; 19(1): 53-9. [Smirnova E.N., Lоran E.A., Shulkina S.G., Podtaev S.Yu. The relationship of endothelial dysfunction and metabolic manifestations of obesity. Regional Blood Circulation and Microcirculation. 2020; 19(1): 53-9. (in Russian)]. https://dx.doi.org/10.24884/1682-6655-2020-19-1-53-59.
- Delgado Spicuzza J.M., Proctor D.N., Thijssen D.H.J., Somani Y.B. Menopausal stage differences in endothelial resistance to ischemia-reperfusion injury. Physiol. Rep. 2023; 11(18): e15768. https://dx.doi.org/10.14814/phy2.15768.
Received 27.02.2024
Accepted 05.06.2024
About the Authors
Tatyana P. Shevlyukova, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology, Tyumen State Medical University, Ministry of Health of Russia,625023, Russia, Tyumen, Odesskaya str., 54, +7(922)394-28-08, tata21.01@mail.ru, https://orcid.org/0000-0002-7019-6630
Irina A. Bulatova, Dr. Med. Sci., Head of the Department of Normal Physiology, Professor at the Department of Faculty Therapy No. 2, Occupational Pathology and Clinical Laboratory Diagnostics, Academician E.A. Wagner Perm State Medical University, Ministry of Health of Russia, 614990, Russia, Perm, Petropavlovskaya str., 26,
+7(922)315-92-88, bula.1977@mail.ru, https://orcid.org/0000-0002-7802-4796
Corresponding author: Irina A. Bulatova, bula.1977@mail.ru



