В последние годы синдром поликистозных яичников (СПКЯ) рассматривается не только как заболевание, связанное с репродукцией, но и все чаще в контексте метаболических нарушений, в основе которых лежит инсулинорезистентность (ИР). Так, ИР имеют от 50 до 80% женщин с СПКЯ [1, 2], избыточную массу тела – 61% [3], сахарный диабет 2 типа и метаболический синдром до 40 лет – более половины [4]. Более того, широко обсуждается тот факт, что характерная для СПКЯ яичниковая гиперандрогения и ассоциированные с ней репродуктивные проблемы могут являться следствием метаболических нарушений у женщин, и потому ряд экспертов называют ее «метаболической гиперандрогенией», а СПКЯ – «метаболическим репродуктивным синдромом» [5]. В то же время следует отметить и взаимное влияние повышенного уровня андрогенов и ИР. Гиперандрогения способствует накоплению и перераспределению жировой ткани у женщин, вызывая повреждение сигнальных путей инсулина на клеточном уровне, и является независимым фактором риска нарушения метаболизма глюкозы и развития ИР [6].
Как известно, яичники, в отличие от других органов и тканей, не бывают инсулинорезистентными, поэтому стероидогенез в них осуществляется в условиях гиперинсулинемии, компенсаторно развивающейся при ИР [7]. Высокий уровень инсулина снижает чувствительность гранулезных клеток и ооцитов к действию фолликулостимулирующего гормона (ФСГ), одновременно повышая пульсацию гонадотропин-рилизинг-гормона (ГнРГ) и лютеинизирующего гормона (ЛГ) через свои центральные рецепторы, тем самым ингибируя фолликулогенез и созревание ооцитов [8]. Чрезмерная активация ЛГ и непосредственная стимуляция инсулином пролиферации тека-клеток повышают синтез андрогенов в яичниках, а блокирование активности ароматазы нарушает их конвертацию в эстрогены. Также гиперинсулинемия ингибирует образование глобулина, связывающего половые гормоны (ГСПГ), в печени, в результате чего увеличиваются как общий уровень андрогенов, так и их свободная фракция, что приводит к клиническим проявлениям гиперандрогении и нарушениям менструального цикла [9]. Таким образом, ИР и компенсаторная гиперинсулинемия выступают триггерами основных патогенетических изменений при СПКЯ.
Помимо прямого участия в стероидогенезе, инсулин влияет на него путем изменения соотношения стереоизомеров инозитола в ткани яичника – миоинозитола (МИ) и Д-хироинозитола (ДХИ). МИ и ДХИ являются вторичными мессенджерами инсулина в клетках, где МИ отвечает за внутриклеточный транспорт глюкозы и параллельно конвертируется в ДХИ, необходимый для ее включения в цикл Кребса или депонирования. Конвертацию МИ в ДХИ осуществляет фермент эпимераза под влиянием инсулина, который регулирует внутриклеточное соотношение МИ и ДХИ [10, 11]. Физиологическое соотношение МИ и ДХИ в сыворотке крови составляет 40:1, тогда как в яичниках значительно выше, достигая 100:1, что обусловлено активным участием МИ в реализации сигналов ФСГ [10]. При гиперинсулинемии эпимеризация МИ в ДХИ усиливается, что вызывает дефицит МИ. Это сопровождается нарушением фолликулогенеза, ановуляцией и снижением качества яйцеклеток [12]. Увеличение уровня ДХИ стимулирует инсулинопосредованный синтез андрогенов тека-клетками яичников, нарушает конвертацию андрогенов в эстрогены путем подавления активности ароматазы, вызывая гиперандрогенное состояние у женщин с ИР [13]. Экспериментальные и клинические исследования показывают, что МИ, один или вместе с ДХИ в соотношении 40:1, соответствующем физиологическому содержанию в плазме крови, является перспективным в лечении метаболических, гормональных и репродуктивных нарушений у женщин с ИР и СПКЯ [14, 15].
Клиническая картина СПКЯ разнообразна и представлена 4 различными фенотипами [16], среди которых выделяют классический фенотип А, объединяющий все 3 Роттердамских критерия (гиперандрогения клиническая и/или биохимическая, овуляторная дисфункция и поликистозные яичники по данным ультразвукового исследования (УЗИ)), фенотип В – гиперандрогенный ановуляторный, фенотип С – гиперандрогенный овуляторный и фенотип D – нормоандрогенный. Фенотипы В, С и D имеют только 2 из 3 критериев, поэтому часто остаются недиагностированными. Например, у пациенток с фенотипом D овуляторная дисфункция не сопровождается гиперандрогенией, а при фенотипе С, наоборот, гиперандрогения не сопряжена с репродуктивными нарушениями. Также разделение на фенотипы не привело к дифференциации терапевтических подходов. Рекомендации по питанию, изменению образа жизни, приему половых стероидов, лечению кожных проявлений гиперандрогении, нормализации менструального цикла и восстановлению репродуктивной функции назначаются у большинства женщин без учета патогенетических различий [17]. Более того, редко акцентируют внимание у пациенток с СПКЯ на необходимости коррекции метаболических нарушений и лечении ИР, причем не только в целях профилактики сахарного диабета 2 типа и сердечно-сосудистых заболеваний, но и лечения гинекологических нарушений. При этом научный и клинический опыт применения инсулинсенситайзеров, таких как метформин и инозитол, в дополнение к общим рекомендациям по коррекции пищевого поведения и физической активности показывает хороший терапевтический эффект у ряда женщин с СПКЯ, хотя результаты остаются противоречивыми [18–21]. С учетом вышеизложенного мы решили оценить частоту метаболических нарушений у пациенток с классическим фенотипом СПКЯ и влияние комбинации МИ и ДХИ в соотношении 40:1 на гормональные и метаболические параметры с целью определить наиболее комплаентные категории к терапии инсулинсенситайзерами.
Материалы и методы
Проспективное клиническое исследование проведено в соответствии с Хельсинкской декларацией Всемирной медицинской ассоциации (2008 г., Сеул), правилами Надлежащей клинической практики и другими применимыми в Российской Федерации нормативными документами. Все пациентки подписали информированное добровольное согласие на участие в исследовании.
Обследованы 40 женщин репродуктивного возраста, обратившихся за амбулаторной гинекологической помощью в медицинские центры г. Москвы с января 2022 г. по февраль 2023 г., с верифицированным диагнозом СПКЯ (код по МКБ-10 Е28.2).
Критериями включения в исследование явились: возраст 20–40 лет, верифицированный диагноз СПКЯ (фенотип А). Диагноз СПКЯ фенотипа А устанавливался согласно клиническим протоколам ESHRE/ASRM 2018 г. и клиническим рекомендациям «Синдром поликистозных яичников» Министерства здравоохранения РФ 2021 г. при наличии следующих признаков: 1) хроническая олиго/ановуляция; 2) гиперандрогения (клинические и/или биохимические признаки); 3) поликистозные яичники по данным УЗИ.
Хроническая олиго/ановуляция устанавливалась на основании олигоменореи (менее 9 менструаций в год) и/или наличии бесплодия вследствие овуляторной дисфункции (тип IV – СПКЯ, согласно обновленной классификации овуляторной дисфункции FIGO [22]). К клиническим признакам гиперандрогении относились акне и/или гирсутизм; биохимическим подтверждением гиперандрогении яичникового генеза считался сывороточный уровень общего тестостерона на 2–3-й день менструального цикла выше 1,72 нмоль/л. Поликистозная морфология яичников определялась с помощью трансвагинального УЗИ при наличии ≥20 фолликулов в каждом яичнике.
Критерии исключения: использование гормональных методов лечения (средств, содержащих производные эстрогенов и прогестерона), прием метформина, диагноз СПКЯ с фенотипами В, С и D, другие эндокринные причины олиго/ановуляции (гиперпролактинемия, заболевания щитовидной железы, надпочечников, сахарный диабет 2 типа), трубно-перитонеальный и маточный фактор бесплодия, тяжелые соматические заболевания, нарушение протокола или отказ от участия в исследовании.
Всем пациенткам было назначено 2255 мг инозитола в сутки в течение 3 месяцев путем приема двух растворимых таблеток «Актиферт-Гино», содержащих 1100 мг МИ и 27,5 мг ДХИ (в соотношении 40:1) с 400 мкг фолиевой кислоты. При включении в исследование и по окончании терапии на 2–3-й день менструального цикла производился забор венозной крови из локтевой вены. Оценивались сывороточные показатели ФСГ, ЛГ, эстрадиола, общего и свободного тестостерона, ГСПГ, антимюллерова гормона (АМГ), уровня глюкозы, инсулина и индекс ИР (НОМА). Индекс ИР рассчитывался по формуле: НОМА=(гликемия натощак (ммоль/л)×инсулин натощак (мкЕД/л))/22,5. Оценивалась динамика индекса массы тела (ИМТ), рассчитанного по формуле: вес (кг), разделенный на квадрат роста (м).
Статистический анализ
Статистическую обработку результатов исследования проводили с использованием методов параметрического и непараметрического анализа. Для накопления, корректировки, систематизации исходной информации и фиксации полученных результатов применяли электронные таблицы Microsoft Office Excel 2016. Статистический анализ проводили с использованием программы Statistica 13.3 (StatSoft. Inc, США). Первоначально оценивали характер распределения данных при помощи критерия Шапиро–Уилка и нормальных вероятностных графиков. Для определения однородности дисперсий по группам был использован критерий Левена. Сравнение количественных показателей для 2 независимых групп осуществлялось с помощью t-критерия Стьюдента при нормальном распределении значений, в противном случае использовали критерий Манна–Уитни. Сравнение количественных показателей для 2 зависимых групп осуществлялось с помощью t-критерия Стьюдента для зависимых групп при нормальном распределении значений, в противном случае использовали критерий Вилкоксона. Критерием статистической значимости различий принято значение p<0,05.
Результаты
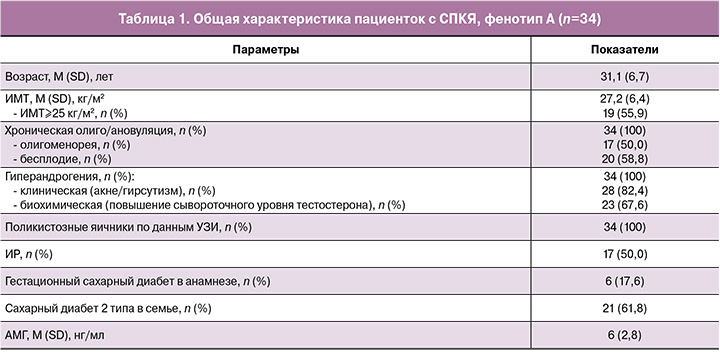
В исследовании приняли участие 40 пациенток с верифицированным диагнозом СПКЯ с фенотипом А, 6 из которых впоследствии были исключены как не прошедшие повторное лабораторное обследование. Общая характеристика 34 пациенток, завершивших исследование, представлена в таблице 1. Соответственно критериям фенотипа А все пациентки имели хроническую олиго/ановуляцию, гиперандрогению и поликистозные яичники по данным УЗИ. У половины были редкие нерегулярные менструации (менее 9 в год) и у 58,8% – бесплодие, ассоциированное с овуляторной дисфункцией (тип IV – СПКЯ). Гиперандрогения клинически проявлялась у 82,4% женщин акне/гирсутизмом, у 67,6% был отмечен повышенный уровень общего тестостерона в крови. Первичное обследование подтвердило высокую частоту метаболических нарушений у женщин с СПКЯ: 55,9% имели избыточную массу тела или ожирение (ИМТ³25 кг/м2), у половины обнаружена ИР, у 17,6% – гестационный сахарный диабет в анамнезе и у 61,8% – семейный анамнез сахарного диабета 2 типа. Характерным признаком для обследуемых пациенток был высокий уровень АМГ, который в среднем составил 6±2,8 нг/мл, при этом у 41,2% превышал верхнюю границу нормы.
Сравнительные данные гормональных и метаболических параметров до и после 3 месяцев приема комбинации МИ и ДХИ 40:1 представлены в таблице 2 и на рисунке. Было отмечено статистически значимое увеличение сывороточного уровня эстрадиола на фоне снижения общего и свободного тестостерона и увеличения ГСПГ. Показатель ЛГ в начале исследования превышал уровень ФСГ на 2–3-й день менструального цикла у 70,6% пациенток с СПКЯ, и, хотя у большинства (64,7%) такая тенденция сохранилась через 3 месяца приема инозитола, средний уровень ЛГ достоверно снизился (р=0,0058). При этом уровень ФСГ оставался стабильным. Прием комбинации МИ и ДХИ в соотношении 40:1 оказал значимое положительное влияние на маркеры метаболизма: достоверно снизились уровень инсулина, индекс ИР (НОМА) и ИМТ. Каких-либо нежелательных явлений в период терапии у пациенток отмечено не было.

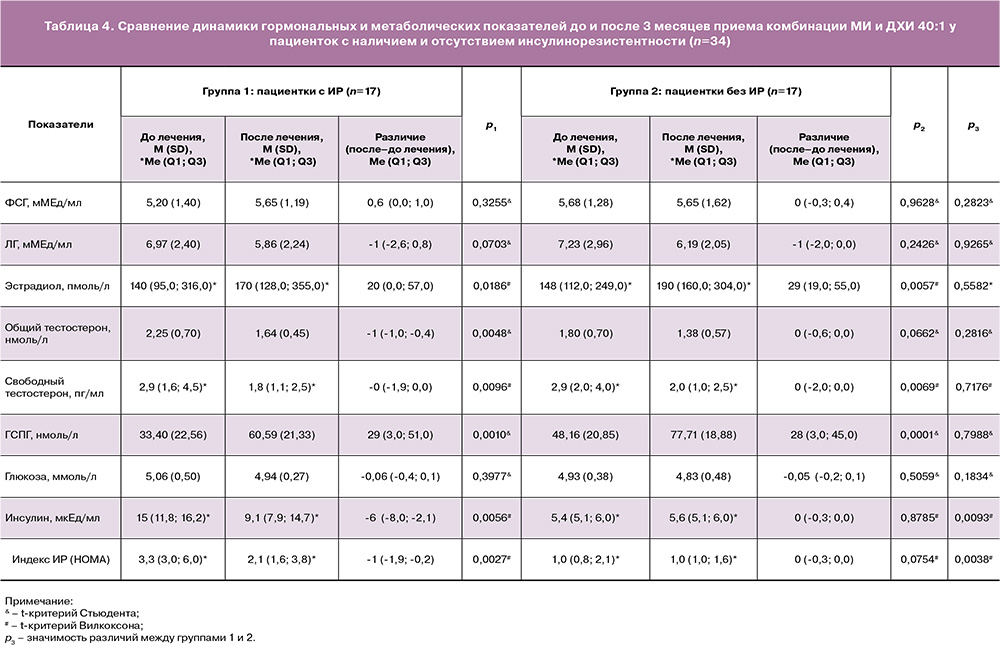
Также мы провели сравнительную оценку динамики гормональных и метаболических показателей после приема инозитола у пациенток с СПКЯ при наличии ИР (группа 1, n=17) и отсутствии ИР (группа 2, n=17) (табл. 3). Большинство пациенток с ИР (82,4%) имели избыточную массу тела или страдали ожирением, тогда как без ИР – только 29,4% (р=0,0019). Большинство из них (88,2% в сравнении с 47,1% в группе пациенток без ИР) имели повышенный уровень тестостерона в крови. Следует отметить, что семейный анамнез нарушений углеводного обмена имели почти две трети пациенток, независимо от текущего инсулинового статуса.
В таблице 4 представлен сравнительный анализ динамики гормональных и метаболических показателей у пациенток с наличием и отсутствием ИР. В обеих группах после 3 месяцев приема комбинации МИ и ДХИ 40:1 статистически значимо увеличился сывороточный уровень эстрадиола и ГСПГ, уменьшились показатели ЛГ, общего и свободного тестостерона. В группе женщин с ИР значительно снизились уровни инсулина и индекс ИР (НОМА), которые в группе без ИР находились в пределах нормативных показателей.
Обсуждение
Результаты проведенного нами исследования выявили высокую частоту ИР, гестационного сахарного диабета в анамнезе, избыточной массы тела и ожирения у обследованных нами женщин, что свидетельствует о значительной ассоциации метаболических нарушений и клинико-лабораторных проявлений классического фенотипа СПКЯ. Более 60% пациенток имели семейный анамнез сахарного диабета 2 типа, причем частота последнего не отличалась между группами с нормальным уровнем инсулина и с ИР. Семейный анамнез сахарного диабета 2 типа является доказанным фактором риска развития СПКЯ и метаболических нарушений [23], поэтому с большой долей вероятности мы можем предполагать наличие нарушений углеводного обмена и у многих пациенток с нормальным уровнем инсулина в крови. Также это может свидетельствовать о недостаточной информативности определения сывороточного уровня инсулина и индекса ИР (НОМА) для выявления начальных признаков ИР. С этой целью может быть полезен пероральный тест с 75 г глюкозы для определения толерантности не только к глюкозе, но и инсулину.
Мы продемонстрировали положительное влияние ежедневного приема 2255 мг инозитола (комбинация МИ и ДХИ в соотношении 40:1) на метаболические параметры у пациенток с классическим фенотипом СПКЯ при отсутствии каких-либо нежелательных явлений. Так, через 3 месяца терапии достоверно снизились показатели ИР – уменьшился ИМТ (р=0,0029), снизился индекс ИР (НОМА) (р=0,0007) и в 2,4 раза уменьшилось количество пациенток с повышенным уровнем инсулина в крови. Уровень глюкозы плазмы имел тенденцию к снижению, но находился в пределах нормативного диапазона, поэтому мы не можем оценивать динамику гликемии как положительную или отрицательную.
Характерным для СПКЯ считается нарушение соотношения уровня гонадотропных гормонов гипофиза, регулирующих стероидогенез в яичниках, когда секреция ЛГ в первую фазу менструального цикла превышает секрецию ФСГ [24], что мы наблюдали у 70,6% наших пациенток. Избыток или чрезмерная пульсация ЛГ вызывает пролиферацию тека-клеток яичников и продукцию ими андрогенов, подавляя при этом активность ароматазы, конвертирующей андрогены в эстрогены. Одновременно ЛГ активирует рецепторы в клетках гранулезы и вызывает преждевременную остановку роста антральных фолликулов и их накопление в яичнике [25]. Через 3 месяца приема комбинации МИ и ДХИ количество пациенток с нарушенным соотношением ЛГ/ФСГ уменьшилось незначительно, но средний уровень ЛГ достоверно снизился с 7,10±2,66 до 6,03±2,12 (р=0,0058), при этом показатель ФСГ оставался стабильным. Данная тенденция может свидетельствовать о необходимости более длительного приема инсулинсенситайзеров для коррекции гипоталамо-гипофизарно-яичниковых взаимодействий.
К дополнительным маркерам СПКЯ относится повышенный уровень АМГ, секретируемого преантральными фолликулами [26], которые в большом количестве были представлены в яичниках обследуемых нами женщин, у 41,2% уровень АМГ превышал верхнюю границу нормы. Какой-либо динамики показателя после приема инозитола мы не отметили, что также, возможно, обусловлено недостаточно длительным курсом терапии.
В отличие от АМГ и соотношения ЛГ/ФСГ, степень яичниковой гиперандрогении значительно снизилась уже через 3 месяца терапии. Если в начале исследования сывороточный уровень общего тестостерона превышал нормативный диапазон у 67,6% женщин, а свободного тестостерона – у 55,9%, то через 3 месяца – только у 23,5 и 14,7% соответственно (р=0,013; 0,0002). При этом средний показатель ГСПГ статистически достоверно вырос (р<0,0001).
Отмеченный факт особенно важен у пациенток с СПКЯ, планирующих беременность, так как повышенный синтез андрогенов в яичниках по механизму отрицательной обратной связи ведет к аномальной реакции ЛГ на триггерное воздействие ГнРГ и нарушению центральных механизмов регуляции гипоталамо-гипофизарно-яичниковой оси [27]. В большинстве случаев аномальная реакция формируется в период внутриутробного развития и ассоциирована с повышенной выработкой АМГ у беременных женщин с СПКЯ. Кроме непосредственного стимулирующего влияния на пульсационный выброс ЛГ, АМГ ингибирует активность ароматазы в плаценте, тем самым повышая биодоступность андрогенов для плода [28]. Таким образом, под влиянием гиперандрогенного статуса у матери происходит андроген-ассоциируемое перепрограммирование нейронального ответа на ГнРГ и рождение ребенка с нарушенным чрезмерным синтезом ЛГ и высоким риском развития СПКЯ [29].
Важным аспектом терапии МИ и ДХИ в соотношении 40:1 явилось статистически достоверное увеличение сывороточного уровня эстрадиола, которое в сочетании со снижением уровня общего тестостерона может свидетельствовать о ее восстановительном влиянии на стероидогенез в яичниках. Следует при этом учитывать, что увеличение дозы ДХИ в соотношении нежелательно, так как вызывает снижение экспрессии ароматазы в клетках гранулезы и блокирует превращение андрогенов в эстрогены [30]. Сравнение между группами пациенток, разделенных по наличию (группа 1) или отсутствию ИР (группа 2), показало более высокую частоту ожирения (82,4%) и биохимической гиперандрогении (88,2%) в первой группе в сравнении со второй (29,4 и 47,1% соответственно). Это является дополнительным подтверждением взаимного негативного влияния ожирения и гиперандрогении друг на друга.
Мы не обнаружили отличий в динамике гормональных показателей после терапии инсулинсенситайзерами между группами женщин с выявленной ИР и без нее. Данный факт, по нашему мнению, свидетельствует не столько об эффективности инозитола у женщин без ИР, сколько о более широком распространении нарушений углеводного обмена среди включенных в исследование пациенток и недостаточной информативности использованных тестов выявления ИР.
Ограничения исследования
Настоящий анализ был ограничен 12-недельным наблюдением, что исключало долгосрочную оценку эффектов комбинации МИ и ДХИ. Также этого периода было недостаточно для достижения и анализа клинических изменений, таких как нормализация менструального цикла, восстановление репродуктивной функции, клинических проявлений гиперандрогении. Необходимы дальнейшие исследования с использованием большего размера выборки и более чувствительных тестов выявления ИР.
Заключение
Классический фенотип СПКЯ ассоциирован с высокой частотой метаболических нарушений, около 80% обследованных нами женщин имели ИР и/или семейный анамнез сахарного диабета 2 типа. Назначение комбинации МИ и ДХИ 40:1 в количестве 2255 мг в сутки в течение 3 месяцев оказало статистически значимое влияние на метаболические параметры у пациенток с СПКЯ – снизился ИМТ, уровень инсулина в крови, индекс ИР (НОМА)). Улучшились маркеры яичникового стероидогенеза – снизился сывороточный уровень общего и свободного тестостерона, достоверно выросли концентрации ГСПГ и эстрадиола. Секреция ЛГ значительно снизилась через 3 месяца терапии, но оставалась повышенной в отношении ФСГ в начале менструального цикла, что может свидетельствовать о целесообразности более длительного приема инсулинсенситайзеров. Кроме того, следует учитывать недостаточную чувствительность индекса ИР (НОМА) и в ряде случаев рекомендовать проведение перорального глюкозотолерантного теста с определением инсулина в крови для выявления пациенток на более ранних этапах развития ИР, что позволит расширить когорту комплаентных пациенток с СПКЯ к назначению препаратов инозитола.



