Association between new coronavirus infection and fetal growth restriction
Objective: To investigate the laboratory and instrumental characteristics of fetal growth restriction (FGR) secondary to novel coronavirus infection (NCI) to identify pathogenetically relevant predictive markers.Lipatov I.S., Tezikov Yu.V., Kalinkina O.B., Tyutyunnik V.L., Kan N.E., Majorova M.O., Yakovleva M.A.
Materials and methods: During the epidemic activity of the NCI Delta strain, 140 high-risk pregnant women were tested at 18–21 weeks and 26–34 weeks of gestation. Retrospectively, taking into account the fact of NCI disease and the exclusion of severe somatic and obstetric comorbidities, 2 groups were formed. Group 1 (n=32) included pregnant women with FGR, without a history of NCI. Group 2 (n=41) included pregnant women with FGR who recovered from NCI by the end of the second and third trimesters. Thirty healthy pregnant women served as the controls. In addition to ultrasound assessment of the fetal placental unit, patients underwent testing for markers of inflammation, endothelial hemostasis dysfunction, decidualization, placental angiogenesis, and pathological insulin resistance.
Results: Pregnant women with a history of NCI had a higher incidence of FGR (1.3 times; OR 2.41 [95% CI 1.12–5.17]), more severe forms of FGR (2 times; OR 3.27 [95% CI 1.22–8.76]), more severe fetal-placental blood flow abnormalities (3.5-fold; OR 11.07 [95% CI 3.68-33.27]), and oligohydramnios (4.5-fold; OR 8.94 [95% CI 3.65–30.17]). The impact of NCI on the formation of placental insufficiency was expressed by an increase in systemic changes (thrombopoiesis, apoptosis), modulation of local processes (decidualization, placental angiogenesis), and the development of pathological insulin resistance and hyperinsulinemia, an immunopathological process of endotheliocytes. The identification of the most informative markers of FGR due to NCI allowed the development of a predictive index.
Conclusion: An in-depth study of the impact of NCI on the formation of FGR has important scientific and practical implications for the optimization of FGR prediction, which may help identify appropriate patient management strategies for high-risk pregnant women.
Authors’ contributions: Lipatov I.S., Tezikov Y.V. – analysis of relevant literature, manuscript drafting; Kalinkina O.B.,
Majorova М.О., Yakovleva М.А. – material collection and processing; Tyutyunnik V.L., Kan N.Е. – conception and design of the study, manuscript editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the Samara State Medical University, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Lipatov I.S., Tezikov Yu.V., Kalinkina O.B., Tyutyunnik V.L., Kan N.E.,
Majorova M.O., Yakovleva M.A. Association between new coronavirus infection and fetal growth restriction.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (2): 53-62 (in Russian)
https://dx.doi.org/10.18565/aig.2022.260
Keywords
The scientific and public health community worldwide is gradually accumulating reliable information on the impact of novel coronavirus infection (NCI) on pregnancy, fetal-placental unit (FPU) development and functioning, vertical transmission of SARS-CoV-2, mechanisms of progression and features of clinical manifestations during gestation and puerperium, and effectiveness of methods for prevention, treatment and rehabilitation [1–3]. Currently, available evidence suggests that pregnant women are more susceptible to COVID-19 than the general population [4, 5]. There is a substantial risk of adverse pregnancy and neonatal outcomes in pregnant women with COVID-19, including increase in the risk of early reproductive loss (up to 20%), preterm birth (up to 25%), preterm rupture of membranes (up to 23%), pre-eclampsia (up to 12% – to differentiate with the "pseudo preeclampsia" syndrome), placental abruption (2–6 times), fetal growth restriction (FGR) (25–30%), fetal distress (up to 30%), operative vaginal delivery (3–5 times), caesarean section (over 40%), obstetric hemorrhage (over 50%), low birth weight (up to 30%). More than 40% of newborns require admission to an intensive care unit (ICU) [6–9]. Pregnant women may have a mild and nonspecific clinical manifestation, or NCI can progress to severe and extremely severe forms, developing a critical condition requiring treatment in the ICU. This can be explained, on the one hand, by the increasing proportion of older pregnant women and non-obstetric comorbidities [10–12], and, on the other hand, by the effect of hormones and proteins of pregnancy, which have immunosuppressive properties, reduction in the number and activity of natural killers, dendritic cells, interferon synthesis, simulation of pyroptosis, leading to weakening of antiviral protection and rapid development of a systemic inflammatory response with the cytotoxic and prothrombotic "storm" [13–16].
A considerable amount of literature has been published on NCI and pregnancy. However, analysis of Scopus, eLibrary.ru, PubMed, Cochrane, Medline, Hinari databases showed no articles revealing the mechanisms of placental insufficiency (PI) due to NCI, proposing predictive criteria for placenta-related fetal disorders.
The present study aimed to investigate the laboratory and instrumental characteristics of fetal growth restriction secondary to the novel coronavirus infection to identify pathogenetically relevant predictive markers.
Materials and methods
The main phase of the study was conducted in 2020 during the epidemic activity of the NCI Delta strain. To assess the impact of NCI on FPU status, a high-risk group for FGR was formed using the FGR risk scale according to the Delphi (2016) consensus for a definition of fetal growth restriction [17]. Inclusion criteria were the presence of 1 risk factor for FGR with OR >2.0 or 3 or more risk factors at OR<2.0. Non-inclusion criteria were multiple pregnancy, nonobstetric and infectious diseases (except NCI), congenital malformations, and inherited fetal pathology. Exclusion criteria were extremely severe NCI, non-compliance with the examination protocol. The observation group comprised 140 pregnant women at high risk of FGR who were tested at weeks 18–21 and 26–34 for blood levels of inflammatory markers, IM (tumor necrosis factor (TNF)α, C-reactive protein (CRP)); stromal cell decidualization, DM (PAMG – placental α-1-microglobulin); apoptosis, AM (lymphocytes with CD95+ phenotype – apoptosis initiation receptor FasR-FasL (LCD95+)) [18, 19]; cell transformation in placenta (PGR – placental growth factor); prothrombotic state, PSM (FN – fibronectin, universal marker of hemostasis, functional state of endothelium, MPV – mean platelet volume); endothelial dysfunction, EDM (AEA CI, cytotoxicity index of anti-endothelial antibodies [20], total IgE, HOMA-IR – insulin resistance index, IR) [21].
Based on a retrospective analysis of pregnancy outcomes, 140 pregnant women at high risk of FGR in the observation group were divided into group 1 (n=68) who did not have NCI during follow-up, and group 2 (n=72) who suffered from NCI in the late second and third trimesters of pregnancy. In groups 1 and 2, FGR was diagnosed in 63.2% (43/68) and 80.6% (58/72) of the patients, respectively, χ2=4.39, p=0.03. Depending on the occurrence of FGR and the presence of somatic and obstetric comorbidities, each group was divided into two subgroups. The 1A subgroup was represented by 32 pregnant women with isolated FGR, 1B by 11 pregnant women with FGR superimposed on preeclampsia, preterm labor, and non-obstetric comorbidities (chronic pyelonephritis, chronic bronchitis, diabetes mellitus, neurocirculatory dystonia, and hypertension). The 2A subgroup consisted of 41 pregnant women with FGR; 2B included 17 pregnant women with pathology FGR concurrently with similar to the 1B pathology.
To assess the association between NCI and FGR, two comparison groups were formed. Group 1 comprised 32 pregnant women with FGR without concomitant obstetric and somatic pathology; group 2 consisted of 41 pregnant women with FGR with a history of NCI (delta strain) of varying severity in the late second and third trimesters (from 24 to 34 weeks). The control group consisted of 30 healthy women with a healthy pregnancy. NCI infection is possible at any gestational age and is associated with the vague clinical presentation of FGR as early or late phenotypes, making it difficult to clearly identify the true early FGR phenotype due to impaired uteroplacental vascular remodeling and placentation. Taking this into account, only the incidence of FGR before and after 32 weeks of gestation is presented in the Results section, without distinguishing between phenotypes.
AEAs were determined by the serum monocyte and endothelial cell antibody cross-testing technique. The cytotoxic effect of serum was calculated using AEA CI by the following criteria: negative for cells destroyed up to 10%, negative from 10 to 20%, positive from 20 to 50%, and strongly positive from 50 to 100% [20]. LCD95+ detection was performed by enzyme-linked immunosorbent assay (ELISA) using monoclonal antibodies on a Becton Dickinson FACS Calibur flow cytometer. Serum concentrations of PAF, TNFα, CRP, PAMG, insulin, total IgE, and plasma levels of PH were determined by ELISA with an appropriate reagent kit. MPV, as an indicator of platelet activation, was determined on a Sysmex XN-1000 automatic hematology analyzer (Sysmex Corporation, Japan). Diagnosis of PI was based on an examination algorithm, including ultrasound (US), Doppler ultrasound of FPU, cardiotocography, and clinical examination [19]. The diagnosis of PI was verified by morphological examination of the placenta [22]. The diagnosis of NCI and FGR was in accordance with current clinical guidelines [17, 23].
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics version 25. The distribution of continuous variables was tested for normality using the Shapiro–Wilk and Kolmogorov–Smirnov tests. Quantitative variables were expressed as median (Me) with interquartile range (Q1; Q3). The Kruskal–Wallis test was used to compare the numerical data between three or more groups followed by the Mann–Whitney U test with Bonferroni correction (critical level p<0.017). Categorical variables were compared using Pearson's χ2 test with the Yates correction using 2×2 contingency tables. The results were considered statistically significant at p<0.05. To confirm the information value (quality) of the individual parameters and indices, we used ROC-curve analysis to plot the ROC curve and determine the area under the curve (AUC). The AUC of 0.9–1.0 indicates excellent quality; AUC of 0.8–0.9 indicates very good quality; AUC of 0.7–0.8 indicates good; AUC of 0.6–0.7 indicates average; AUC of 0.5–0.6 indicates unsatisfactory quality. The odds ratio (OR) with 95% confidence interval (CI) was calculated to assess the influence of factors on the formation of fetal abnormalities. The predictive significance of the predictive index (PI) was characterized by the most important diagnostic test accuracy measures, sensitivity (Se), and specificity (Sp) [24, 25].
Results and discussion
Among pregnant women in groups 1 and 2, 31.2% (10/32) and 34.1% (14/41) were under 35 years of age, and 68.8% (22/32) and 55.9% (27/41) were over 35 years, respectively (χ2=0.000, p=0.99). Analysis of the clinical course of COVID-19 in group 2 revealed that 92.7% (38/41) and 7.3% (3/41) of the patients were diagnosed with moderate to severe NCI. Chest computed tomography (CT) showed lung tissue damage in all pregnant women in group 2: CT1 pattern was diagnosed in 31.7% (13/41) of women with mild NCI, CT2 pattern was diagnosed in 14.6% (6/41) of women with moderate NCI, CT3-4 pattern was diagnosed in 7.3% (3/41) of those with severe NCI. Consequently, one in two pregnant women with placenta-associated fetal disorders (53.6%) infected with SARS-CoV-2 had lung damage. NCI was confirmed in 22% (9/41) of pregnant women in the late second trimester and in 78% (32/41) in the third trimester of gestation.
All women with pulmonary involvement were over 35 years of age and had NCI in the third trimester. This association of the severity of NCI with age and gestational age is supported by several studies [11] and can be explained by changes in immunoreactivity and the nature of adaptive processes in the respiratory and coagulation systems in the third trimester [13, 26], as well as increased expression of angiotensin-converting enzyme 2 (ACE-2), which, along with transmembrane serine protease 2 (TMPRSS 2) and transmembrane glycoprotein CD147 (basigin), is an entry gateway for SARS-CoV-2 entry into host cells, in placenta and amniotic fluid in late pregnancy [27].
Assessment of FPU status in pregnancy with differential growth rates of estimated fetal weight (EFW) and abdominal circumference (AC) showed the following results. The incidence of FGR, taking into account the degree of slowing of FW growth, AC <10th percentile in combination with impaired blood flow in FPU, was 71.9% (23/32) in group 1 and 43.9% (18/41) in group 2 (χ2=4.63, p=0.03). Diagnosis of marked FGR, (EFW <3rd percentile) was observed in group 1 in 28.1% (9/32) and in group 2 in 56.1% (23/41) – χ2=4.63, p=0.03. FGR before and after 32 weeks of gestation manifested in 25% (8/32) and 75% (24/32) of the observations in group 1 and in 24.4% (10/41) and 75.6% (31/41) in group 2, respectively (gestational age ratio 1:3; χ2=0.05, p=0.83). These figures clearly depend both on individual differences and the timing of SARS-CoV-2 infection and the severity of the FPU virus alteration [3, 7, 9]. The first ultrasound FGR criteria were diagnosed 3-6 weeks after SARS-CoV-2 infection, which justifies the development of prognostic laboratory markers. Doppler characterization of FPU blood flow in pregnant women without NCI and those with NCI showed differences in the degree of hemodynamic abnormalities. The following abnormalities were observed in groups 1 and 2, respectively: stage IA abnormalities were diagnosed in 78.1% (25/32) and 24.4% (10/41) of pregnant women, χ2=18.70, p<0.001; stage IB abnormalities in 9.4% (3/32), p<0.001, stage I in 9.4% (3/32) and 31.7% (13/41) – χ2=4.01, p=0.04; stage II in 6.3% (2/32) and 26.8% (11/41) – χ2=3.89, p=0.04; stage III in 6.3% (2/32) and 17.1% (7/41) – χ2=1.08, p=0.30. The analysis showed that more severe blood flow abnormalities (stages II and III) were statistically significantly more prevalent in pregnant women with NCI, 43.9% (18/41) versus 12.5% (4/32) – χ2=6.20, p<0.01. The groups also differed significantly by fetal blood flow abnormalities (stages IB, II, III) with the prevalence of abnormalities in the fetal circulation with the predominance of abnormalities in pregnant women with NCI being 75.6% (31/41) versus 21.9% (7/32) in group 1, χ2=18.69, p<0.001. Oligohydramnios of varying severity was detected in 12.5% (4/32) and 56.1% (23/41) in groups 1 and 2 – χ2=12.85, p<0.001, confirming a statistically significant predominance of this pathology in FGR due to NCI. All patients with oligohydramnios in both groups had severe FGR.
The current recommended classification of FGR, according to current clinical guidelines (17), is 'FGR' and’severe FGR': EFW/AC in the ninth to third percentile combined with abnormal blood flow in the FPU is 'FGR'; EFW <3rd percentile is severe FGR. Previously, the classification of FGR was applied according to the gestational age of the main fetometric characteristics (in weeks) (19): stage I by 1–2 weeks, stage II by 3–4 weeks, and stage III by more than 4 weeks. A diagnostic parallel between the two classifications was performed in groups 1 and 2 of pregnant women with FGR (73 observations in total): the FGR grades were compared by fetometric data (stages I, II, III) with FGR according to the EFW percentiles. The following results were obtained: Stage I FGR occurred in 52.1% (38/73) of pregnant women, Stage II FGR in 24.7% (18/73), Stage III in 23.2% (17/73); "FGR" (EFW 9th to 3rd percentile with impaired blood flow) was 56.2% (41/73), "severe FGR" (EFW <3rd percentile) 43.8% (32/73). Comparing the severity of FGR according to fetometric lag (in weeks) with the EFW (percentile scale) showed the following pattern: 91.4% of FGR in stages II and III (in weeks) met criteria for "severe FGR" (EFW percentiles) while 100% of FGR in stage I and 8.6% of FGR in stage II met criteria for "FGR" (EFW 9th to 3rd percentile with impaired blood flow in FPU). Similar data, without statistical differences, were obtained in separate comparisons in group 1 without NCI and group 2 with NCI, confirming the universality of the diagnostic criteria for FGR, regardless of its etiological factor.
Caesarean delivery due to the severity of fetal condition associated with PI was performed in 40.6% (13/32) in group 1 and in 78% (32/41) in group 2 – χ2=9.12, p<0.01. Pregnant women with NCI were more likely to undergo emergency abdominal delivery (43.9% (18/41) versus 12.5% (4/32) in Group 1, χ2=6.20, p<0.01).
Thus, clinically, high-risk pregnant women with FGR showed a negative effect of NCI on the course and outcome of pregnancy, compared to those without NCI, as suggested by the statistically significantly higher overall incidence of FGR (OR 2, 41 [95% CI 1.12–5.17]), an increased incidence of severe FGR (OR 3.27 [95% CI 1.22–8.76]), oligohydramnios (OR 8.94 [95% CI 3.65–30.17]), and more severe maternal-placental-fetal bleeding disorders (OR 11.07 [95% CI 3.68–33.27]), and higher rates of operative delivery, mostly for critical fetal status. The rationale for this adverse effect of NCI on fetal growth and development was sought by investigating the impact of the infection on the mechanisms of PI and FGR, as assessed by laboratory monitoring of pregnant women.
Among the mechanisms of PI formation, many researchers [13, 19] identify proinflammatory state as a factor of destabilization of immunobiological regulation, metabolism, integration of hormones, functional reactions of organs and systems, including the functional system "mother-placenta-fetus". For NCI, inflammation is the leading damaging link in the pathogenetic chain of complex interactions between SARS-CoV-2 and the body [13, 14, 16]. The results of the IM studies in the second and third trimesters of pregnancy (Tables 1, 2) showed that in patients at high risk for FGR, there was a statistically significant increase in TNFα and CRP levels at 18-21 weeks (before NCI in group 2), indicating the preclinical formation of the pro-inflammatory status in this pregnancy cohort. Group 2 patients who suffered from NCI showed a significant increase in IM, which differed from the control (p<0.001) by a factor of 3.2 for TNFα and 5.5 for CRP, and from group 1 with FGR without NCI (p<0.001) by a factor of 1.8 for TNFα and 3.1 for CRP.
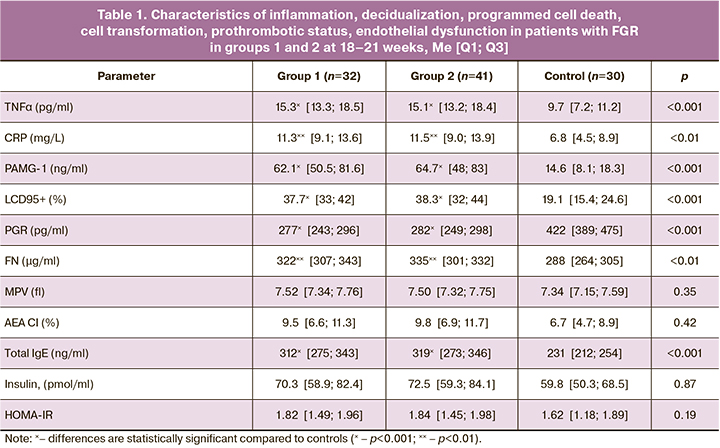
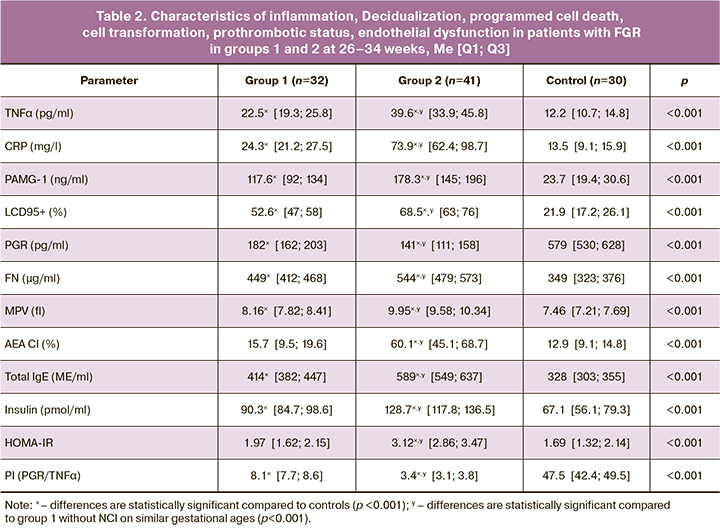
In the complicated course of pregnancy, there is a known association between inflammation and prothrombotic changes [13], leading to impaired hemodynamics in the placental-fetal system, microcirculation, and tissue ischemia. Thrombopoiesis is a particularly important link in the genesis of C19 and is associated with both disease progression and poor prognosis (26). The evaluation of PSM in gestational dynamics (Tables 1, 2) showed an increase in the universal hemostasis marker FN in pregnant women in the high-risk FGR group (from 322 µg/mL to 449 µg/mL in group 1 and from 335 µg/mL to 544 µg/mL in group 2, p<0.001), with a significant 1.2-fold predominance in the third trimester in patients with NCI. MPV, which is indicative of platelet hemostasis activation [28], was slightly different: There were no statistical differences between the control and high-risk groups at 18-21 weeks, with a significant difference in the third trimester with both groups (p<0.001), a 1.3 times excess of MPV in group 2 with NCI versus group 1 (p<0.001).
Analysis of the clinical course of NCI and its outcomes has shown that one of the most adverse pathological changes, characteristic of severe course of the infection, is the formation of pathological IR [21]. This dysregulation of all types of metabolism and cellular energy supply is associated with fatal outcomes in NCI in women with obesity, hypertension, metabolic syndrome, and diabetes mellitus [11]. During gestation, the development of IR is the 'norm of pregnancy' and is associated with an evolutionary function for fetal energy and nutrient supply [29]. However, when compensatory-adaptive processes are disrupted, transformation into pathological IR occurs with increasing hyperinsulinemia (HI). Examining pregnant women at high risk of FGR revealed interesting data on HOMA-IR, which was statistically significantly elevated only in the third trimester in pregnant women with FGR who suffered from NCI (3.12 [2.86; 347] versus 1.97 [1.62; 2.15] in group 1 (p<0.001) and versus 1.69 [1.32; 2.14] in controls (p<0.001)). Insulin levels in study groups increased significantly in the third trimester in women with FGR, with the highest hormone 1.4-fold increase in pregnant women to NCI, compared with group 1, and 1.9-fold, compared with controls. The increase in HOMA-IR in patients with NCI is consistent with the generally accepted understanding of the protective role of IR in acute infections, which is associated with the need to protect host cells from the virus through intracellular oxidative stress and inflammation [20]. The identified feature of the effect of NCI on FPU in the form of pathological IR and HI is that disruption of the ratio of IR levels in the pregnant woman, in the placenta, and in the fetus, which normally differ by 2–3 orders of magnitude [21, 29], leads to a decrease in the fetal assimilation of energy and nutrients and contributes to the development of FGR.
Research evidence [13, 29] suggests a direct link between IM, PSM, pathological IR, and HI and the functional state of the vascular endothelium and the development of endothelial dysfunction (ED). Several studies [8, 15, 19] reported an important role of ED in the mechanisms of PI formation, in particular, FGR; at the same time, there is no information on the immune alteration of endotheliocytes in pregnant women with PI. We obtained data on AEAT levels in the dynamics of healthy and FGR complicated pregnancies. The cytotoxic effect of AEAT in the form of a positive and sharply positive reaction was recorded in the third trimester of gestation in the group of pregnant women with FGR and NCI (Table 2). In group 1 and among controls, AEAT was recorded as negative or weakly positive in both the second and third trimesters of pregnancy. This indicates an additional damaging effect of SARS-CoV-2 on endotheliocytes with the development of an autoimmune process in pregnant women, which is an unfavorable factor of C19 progression on the one hand, and an important mechanism of vascular disorders in FPU with the formation of PI. Monitoring of total IgE, which is both a marker of ED and an indicator of the immunopathological process in pregnant women with C19 [19], showed a 1.3-fold increase of in IgE levels in groups 1 and 2 in the second trimester compared to controls. The highest levels of total IgE were observed in group 2 in the third trimester, which were 1.5 times higher compared to group 1 (p<0.001). This observation can be explained by an increase in immunopathological reactions and an increase in ED during NCI.
Markers of decidualization, programmed cell death, cell proliferation, and transformation characterizing local FPU status, in particular the maternal (PAMG) and fetal (LCD95+) parts of the placenta. Placental angiogenesis (PAG) showed multidirectional changes in parameters, which differed statistically significantly from controls (Tables 1, 2). PAMG levels, which controls stromal cell decidualization and regulate the biological activity of insulin-like growth factors, in pregnant women with high-risk FGR differed from the reference values in the second (p<0.001) and third (p<0.001) trimesters of gestation, with the highest 1.5-fold deviation recorded in women with NCI at 26–34 weeks, compared to group 1, and 7.7-fold deviation compared with control. While the pattern of changes in the decidua can be objectified by the concentration of serum levels of PAMG, the state of syncytiotrophoblast (fetal part of the placenta), which induces lymphocyte apoptosis, can be assessed by the level of lymphocytes expressing Fas/APO-1/CD95 receptor, which is associated with a protective function of the trophoblast and is aimed at reducing immunopathological responses in FPU [18]. There were differences in LCD95+ content between healthy pregnant women and those at high risk for the FGR groups (p<0.001), with an increase in apoptosis ready immunocytes in the 3rd trimester in pregnant women with NCI (68.5% versus 52.6% in group 1 (p<0.001)). An important marker of cell proliferation, development and stability of the placental vasculature is FPU, which was consistently decreased in the 1st and 2nd high-risk FGR groups with the maximum deviation in the 3rd trimester in women with FGR and NCI. This finding is understandable, both as a direct effect of SARS-CoV-2 on FPU through specific receptors and as an indirect effect of systemic and local pathological mechanisms of FGR and NCI.
Thresholds were determined for the most informative parameters with respect to predicting FGR in pregnant women with NCI in the late second and third trimesters: FGR<160 ng/ml [AUC=0.781, OR 0.89 (95% CI 0.72–0.96), p<0.01, Se 72.7%, Sp 66.9%]; TNFα>30 ng/ml [AUC=0.706, OR 1.85 (95% CI 1.49–2.39), p<0.01, Se 70.1%, Sp 76.3%]; PAMG>140 ng/mL [AUC=0.693, OR 1.68 (95% CI 1.23–2.07), p<0.01, Se 67.7%, Sp 72.8%]; HOMA-IR>2.4 [AUC=0.687, OR 1.52 (95% CI 1.16–1.98), p=0.01, Se 73.3%, Sp 65.7%].
According to the ROC analysis, monitoring of the FPU markers showed only an "average" and "good" prediction of FGR in patients with NCI (figure) for the individual parameters. To overcome the lack of information about individual parameters, a PI was proposed that incorporates the integral characteristics of the key pathogenetic mechanisms of the formation of FGR in pregnant women with NCI was proposed. The PI for FGR in pregnant women with NCI in the late second and third trimesters was proposed to be the PI=PGR/TNFα, whose parameters characterize the severity of inflammation, control of cell proliferation and transformation, and placental angiogenesis (PGR, TNFα) and have 'good' informative value, in contrast to other individual indicators (PAMG, HOMA-IR), which have 'moderate' informative value. The PI values are presented in Table 2.
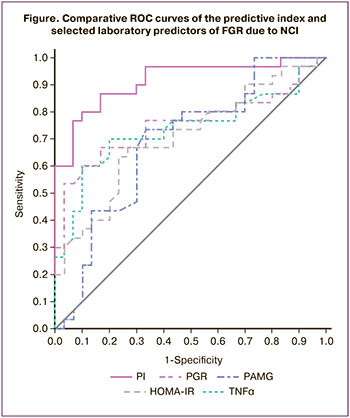
The analysis of PI values showed statistically significant differences both with controls and between group 1 and group 2, suggesting that they should be considered in the treatment of pregnant women with NCI. ROC analysis confirmed the higher informative value of the PIs compared to individual measures (Figure): AUC=0.906, OR 3.65 (95% CI 2.97–4.28), p<0.001, Se 86.7%, Sp 84.3%, characterizing the "excellent" quality of predicting FGR due to NCI.
Conclusion
Regardless of the course of NCI, pregnant women are a vulnerable population during the SARS-CoV-2 pandemic, due to gestational immunosuppression and extensive representation in the FPU of surface receptors for intracellular penetration of the SARS-CoV-2 virus. This comparative cohort study in the second and third trimesters of pregnancy during the most aggressive NCI delta strain circulation as an example, has highlighted the mechanisms of FGR formation in pregnant women with NCI and evaluated the laboratory and instrumental characteristics of FGR due to NCI, with the identification of highly informative pathogenetically relevant prognostic markers of FGR.
These findings suggest that NCI contributes to important links in the pathogenesis of PI, such as inflammation and prothrombotic status, programmed cell death, and, as a result, destabilization of the vascular endothelium with an increase in endothelial dysfunction. Along with systemic changes, the effect of NCI on FPU is also exerted through modulation of local processes in the fetal and maternal parts of the placenta, such as decidualization and placental angiogenesis. This study highlights new mechanisms for FGR formation in pregnant women with C19. The development of abnormal IR and HI in the late second and third trimesters of pregnancy against the background of NCI can affect IR compensation in FPU and therefore reduce the uptake of energy and nutrients by both the placenta and fetus. In addition, the involvement of endotheliocytes altered by SARS-CoV-2 in the immunopathological process contributes to an increase in endothelial hemostasis, thrombopoiesis, and circulatory and metabolic disorders, including FPU.
Differences in the dynamics of changes in FPU markers between pregnant women at high risk of FGR without NCI and those with NCI, by applying statistical and mathematical methods, allowed selection of a PI for this fetal pathology associated with PI, based on the severity of inflammation and features of placental angiogenesis, and recommended inclusion of the proposed PI in the complex of dynamic examination of pregnant women with NCI.
An in-depth study and identification of the pathogenetic mechanisms underlying the formation of fetal pathology in PI due to diseases is of important medical and social significance and promising for determining the priority areas of medical strategies.
References
- Никифоров В.В., Суранова Т.Г., Чернобровкина Т.Я., Янковская Я.Д., Бурова С.В. Новая коронавирусная инфекция (COVID-19): клинико-эпидемиологические аспекты. Архивъ внутренней медицины. 2020; 10(2): 87-93. [Nikiforov V.V., Suranova T.G., Chernobrovkina T.Ya., Yankovskaya Ya.D., Burova SV. Novel coronavirus infection (COVID-19): clinical and epidemiological aspects. Internal Medicine Archive. 2020; 10(2): 87-93. (in Russian)]. https://dx.doi.org/10.20514/2226-6704-2020-10-2-87-93.
- Jafari M., Pormohammad A., Sheikh Neshin S.A., Ghorbani S., Bose D. Clinical characteristics and outcomes of pregnant women with COVID-19 and comparison with control patients: A systematic review and meta-analysis. Rev. Med. Virol. 2021; 31(5): 1-16. https://dx.doi.org/10.1002/rmv.2208.
- Diriba K., Awulachew E., Getu E. The effect of coronavirus infection (SARSCoV-2, MERS-CoV, and SARS-CoV) during pregnancy and the possibility of vertical maternal-fetal transmission: a systematic review and meta- analysis. Eur. J. Med. Res.. 2020; 25: 39. https://dx.doi.org/10.1186/s40001-020-00439-w.
- Subbaraman N. Pregnancy and COVID: what the data say. Nature. 2021; 591(7849:)): 193-5. https://dx.doi.org/10.1038/d41586-021-00578-y.
- Papapanou M., Papaioannou M., Petta A., Routsi E., Farmaki M., Vlahos N., Siristatidis C. Maternal and neonatal characteristics and outcomes of COVID19 in pregnancy: an overview of systematic reviews. Int. J. Environ. Res. Public Health. 2021; 18(2): 596. https://dx. doi.org/10.3390/ijerph18020596.
- Parums D.V. Editorial: Maternal SARS-CoV-2 infection and pregnancy outcomes from current global study data. Med. Sci. Monit. 2021; 27: e933831. https://dx.doi.org/10.12659/MSM.933/831.
- Кудрявцева Е.В., Мартиросян С.В., Утамурадова С.У. COVID-19 и плаценто-ассоциированные осложнения беременности: есть ли связь? Уральский медицинский журнал. 2022; 21(4): 78-84. [Kudryavtseva E.V., Martirosyan S.V., Utamuradova S.U. COVID-19 and placenta-associated pregnancy complications: is there any connection? Ural Medical Journal. 2022; 21(4): 78-84. (in Russian)]. https://dx.doi.org/10.52420/2071-5943-2022-21-4-78-84.
- Nayak A.H., Kapote D.S., Fonseca M., Chavan N., Mayekar R.,Sarmalkar M., Bawa A. Impact of the coronavirus infection in pregnancy: a preliminary study of 141 patients. J. Obstet. Gynaecol. India. 2020; 70(4):256-61. https://dx.doi.org/10.1007/s13224-020-01335-3.
- Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020; 395(10226): 809-15. https://dx.doi.org/10.1016/S0140-6736(20)30360-3.
- Петрова У.Л., Шмаков Р.Г. Новая коронавирусная инфекция 2019 и беременность: что мы знаем? Акушерство и гинекология. 2022; 2: 4-11. [Petrova U.L., Shmakov R.G. New coronavirus infection 2019 and pregnancy: what do we know? Obstetrics and Gynecology. 2022; (2): 4-11. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.2.4-11.
- Allotey J., Stallings E., Bonet M. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. Obstetric Anesthesia Digest. 2021; 41(2): 81-2. https://dx. doi.org/10.1097/01.aoa.0000744128.44930.48.
- Ackerman C.M., Nguyen J.L., Ambati S., Reimbaeva M., Emir B., Cabrera J. et al. Clinical and pregnancy outcomes of coronavirus disease 2019 among hospitalized pregnant women in the United States. Open Forum Infect. Dis. 2021; 9(2): ofab429. https://dx.doi.org/10.1093/ofid/ofab429.
- Макацария А.Д., Слуханчук Е.В., Бицадзе В.О., Хизроева Д.Х., Третьякова М.В., Макацария Н.А., Акиньшина С.В., Шкода А.С., Панкратьева Л.Л., Ди Ренцо Д.К., Риццо Д., Григорьева К.Н., Цибизова В.И., Гри Ж.К., Элалами И. Внеклеточные ловушки нейтрофилов участие в процессах воспаления и дизрегуляции гемостаза, в том числе у пациентов с COVID-19 и тяжелой акушерской патологией. Акушерство, гинекология и pепродукция. 2021; 15(4): 335-50. [Makatsariya A.D., Slukhanchuk E.V., Bitsadze V.O., Khizroeva D.Kh., Tretyakova M.V., Makatsariya N.A., Akinshina S.V., Skoda A.S., Pankratieva L.L., Di Renzo D.K., Rizzo D., Grigoryeva K.N., Tsibizova V.I., Gris J.K., Elalami I. Neutrophil extracellular traps: a role in inflammation and dysregulated hemostasis as well as in patients with COVID-19 and severe obstetric pathology.Obstetrics, Gynecology and Reproduction. 2021; 15(4): 335-50. (in Russian)]. https://dx.doi.org/10.17749/2313-7347/ob.gyn.rep.2021.238.
- Сухих Г.Т., Долгушина Н.В., Шмаков Р.Г., Климов В.А., Яроцкая Е.Л., Петрова У.Л. Исходы беременности у пациенток, вакцинированных от COVID-19 во время беременности: предварительные данные. Акушерство и гинекология. 2021; 11: 5-8. [Sukhikh G.T., Dolgushina N.V.,Shmakov R.G., Klimov V.A., Yarotskaya E.L., Petrova U.L. Pregnancy outcomes after maternal COVID-19 vaccination during pregnancy: preliminary data. Obstetrics and Gynecology. 2021; (11): 5-8. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.11.5-8.
- Favre G., Mazzetti S., Gengler C., Bertelli C., Schneider J., Laubscher B. et al. Decreased fetal movements: a sign of placental SARS-CoV-2 infection with perinatal brain injury. Viruses. 2021; 13 (12): 2517. https://dx.doi.org/10.3390/v13122517.
- Щеголев А.И., Туманова У.Н., Серов В.Н. Поражения плаценты у беременных с SARS-CoV-2-инфекцией. Акушерство и гинекология. 2020; 12: 44-52. [Shchegolev A.I., Tumanova U.N., Serov V.N. Placental lesions in pregnant women with SARS-CoV-2 infection. Obstetrics and Gynecology. 2020; (12): 44-52. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.12.44-52.
- Министерство здравоохранения Российской Федерации. Недостаточный рост плода, требующий предоставления медицинской помощи матери (задержка роста плода). Клинические рекомендации. М.; 2022. [Ministry of Health of Russian Federation. Insufficient growth the fetus, reguiring the provision of medical attention to the mother. Clinical guidelines. Moscow; 2022. (in Russian)].
- Талаев В.Ю., Бабайкина О.Н., Ломунова М.А., Заиченко И.Е., Никонова М.Ф., Лебедева И.Е., Талаева Е.Б., Кропотов В.С. Влияние цито- и синтициотрофобласта плаценты человека на апоптоз лимфоцитов. Иммунология. 2005; 26(3): 132-8. [Tаlaev V.Yu., Babaijkina O.N., Lomunova M.A., Zaichenko I.E., Nikonova M.F., Lebedeva I.E., Talaeva E.B., Kropotov V.S. The influence of human cyto- and syncytiotrophoblast on lymphocyte apoptosis. Immunology. 2005; 26(3): 132-8. (in Russian)].
- Стрижаков А.Н., Липатов И.С., Тезиков Ю.В. Плацентарная недостаточность. Самара: Офорт; 2014. 239с. [Strizhakov A.N., Lipatov I.S.,Tezikov Yu.V. Placental insufficiency. Samara: Ofort; 2014. 239p.(in Russian)].
- Halperin R., Peller S., Rotschild M., Bukovsky I., Schneider D. Placental apoptosis in normal and abnormal pregnancies. Gynecol. Obstet. Invest. 2000; 50(2): 84-7. https://dx.doi.org/10.1159/000010287.
- Bano S., Agrawal A., Asnani M., Das V., Singh R., Pandey A. Correlation of insulin resistance in pregnancy with obstetric outcome. J. Obstet. Gynaecol. India. 2021; 71(5): 495-500. https://dx.doi.org/10.1007/s13224-021-01426-9.
- Юсенко С.Р., Траль Т.Г., Толибова Г.Х., Коган И.Ю. Морфологические особенности плацент при хронической плацентарной недостаточности и задержке роста плода. Вопросы гинекологии, акушерства и перинатологии. 2022; 21(3): 95-101. [Yusenko S.R., Tral T.G., Tolibova G.Kh., Kogan I.Yu. Placental morphology in chronic placental insufficiency and fetal growth restriction. Gynecology, Obstetrics, Perinatology. 2022; 21(3): 95-101. (in Russian)]. https://dx.doi.org/10.20953/1726-1678-2022-3-95-101.
- Министерство здравоохранения Российской Федерации. Организация оказания медицинской помощи беременным, роженицам, родильницам и новорожденным при новой коронавирусной инфекции COVID-19. Методические рекомендации. Версия 5. 28.12.2021. 135 c. [Ministry of Health of the Russian Federation. Organization of medical care for pregnant women, women in labor, maternity hospitals and newborns with a new coronavirus infection COVID-19. Methodological recommendations. Version 5. 12.2021. 135 p. (in Russian)].
- Котельников Г.П., Шпигель А.С. Доказательная медицина. Научно обоснованная медицинская практика: монография. 2-е изд. М.:ГЭОТАР-Медиа; 2012. 239с. [Kotelnikov G.P., Shpigel A.S. Evidence-based medicine. Evidence-based medical practice: monograph. 2nd ed. Moscow: GEOTAR-Media; 2012. 239p. (in Russian)].
- Ланг Т., Альтман Д. Основы описания статистического анализа в статьях, публикуемых в биомедицинских журналах. Руководство «Статистический анализ и методы в публикуемой литературе (САМПЛ)». Медицинские технологии. Оценка и выбор. 2014; 1: 11-6. [Lang T., Altman D. Basic description of statistical analysis in articles published in biomedical journals. The leadership of the «Statistical analyses and methods in the published literature (SAMPL)». Medical technologies. Evaluation and selection. 2014; (1): 11-6. (in Russian)].
- Макацария А.Д., Григорьева К.Н., Мингалимов М.А., Бицадзе В.О., Хизроева Д.Х., Третьякова М.В., Элалами И., Шкода А.С., Немировский В.Б., Блинов Д.В., Митрюк Д.В. Коронавирусная инфекция (COVID-19) и синдром диссеминированного внутрисосудистого свертывания. Акушерство, гинекология и репродукция. 2020; 14(2): 123-31. [Makatsaria A.D., Grigorieva K.N., Mingalimov M.A., Bitsadze V.O., Khizroeva D.Kh., Tretyakova M.V., Elalami I., Shkoda A.S., Nemirovskii V. .B., Blinov D.V., Mitryuk D. Coronavirus disease (COVID-19) and disseminated intravascular coagulation syndrome. Obstetrics, Gynecology and Reproduction. 2020; 14(2): 123-31. (in Russian)]. https://dx.doi.org/10.17749/2313-7347.132.
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S. SARS-CoV-2 Cell entry depends on ACE2 and TMPRSS2 and iis blocked by a clinically proven protease inhibitor. Cell. 2020; 181(2): 271-80.e8.https://dx.doi.org/10.1016/j.cell.2020.02.052.
- Кузнецова Н.Б., Буштырева И.О., Чернавский В.В., Дмитриева М.П., Баранов А.П. Новый гематологический маркер внутриутробного сепсиса при преждевременных родах и преждевременном разрыве плодных оболочек. Акушерство и гинекология. 2021; 4: 84-9. [Kuznetsova N.B., Bushtyreva I.O., Chernavsky V.V., Dmitrieva M.P., Baranov A.P. A new hematological marker for intrauterine sepsis associated with preterm labor and premature rupture of membranes. Obstetrics and Gynecology. 2021; (4): 84-9. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.4.84-89.
- Brennan J.J., Gilmore T.D. Evolutionary origins of toll-like receptor signaling. Evolutionary origins of toll-like receptor signaling. Mol. Biol. Evol. 2018; 35(7): 1576-87. https://dx.doi.org/10.1093/molbev/msy050.
Received 03.11.2022
Accepted 12.01.2023
About the Authors
Igor S. Lipatov, Professor, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology of the Institute of Clinical Medicine, Samara State Medical University, Ministry of Health of the Russian Federation, +7(846)958-24-18, i.lipatoff2012@yandex.ru, Researcher ID: С-5060-2018, SPIN-код: 9625-2947,Author ID: 161371, Scopus Author ID: 6603787595, https://orcid.org/0000-0001-7277-7431, 443099, Russia, Samara, Chapaevskaya str., 89.
Yurii V. Tezikov, Professor, Dr. Med. Sci., Head of the Department of Obstetrics and Gynecology of the Institute of Clinical Medicine, Samara State Medical University, Ministry of Health of the Russian Federation, +7(846)958-24-18, yra.75@inbox.ru, Researcher ID: С-6187-2018, SPIN-код: 2896-6986,
Author ID: 161372, Scopus Author ID: 6603787595, https://orcid.org/0000-0002-8946-501X, 443099, Russia, Samara, Chapaevskaya str., 89.
Olga B. Kalinkina, Associate Professor, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology of the Institute of Clinical Medicine, Samara State Medical University, Ministry of Health of the Russian Federation, +7(846)958-24-18, maiorof@mail.ru, Researher ID: I-3529-2014, SPIN-код: 1260-6181, Author ID: 335830,
https://orcid.org/0000-0002-1828-3008, 443099, Russia, Samara, Chapaevskaya str., 89.
Victor L. Tyutyunnik, Professor, Dr. Med. Sci., Leading Researcher of Research and Development Service, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, +7(903)969-50-41, tioutiounnik@mail.ru, Researcher ID: B-2364-2015,
SPIN-код: 1963-1359, Authors ID: 213217, Scopus Author ID: 56190621500, https://orcid.org/0000-0002-5830-5099, 117997, Russia, Moscow, Ac. Oparina str., 4.
Natalia E. Kan, Professor, Dr. Med. Sci., Deputy Director for Science, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, +7(926)220-86-55, kan-med@mail.ru, Researcher ID: B-2370-2015, SPIN-код: 5378-8437,
Authors ID: 624900, Scopus Author ID: 57008835600, https://orcid.org/0000-0001-5087-5946, 117997, Russia, Moscow, Ac. Oparina str., 4.
Mariya O. Majorova, 5th year student at the Institute of Clinical Medicine, Samara State Medical University, Ministry of Health of the Russian Federation, +7(846)958-24-18, maria002507@mail.ru, https://orcid.org/0000-0002-1844-1838, 443099, Russia, Samara, Chapaevskaya str., 89.
Mariya A. Yakovleva, 6th year student at the Institute of Clinical Medicine, Samara State Medical University, Ministry of Health of the Russian Federation, +7(846)958-24-18, mariayakovleva99@mail.ru, https://orcid.org/0000-0002-0137-5045, 443099, Russia, Samara, Chapaevskaya str., 89.
Corresponding author: Yurii V. Tezikov, yra.75@inbox.ru



