Predicting preterm labor by combined testing of cytokines and cell-free DNA
Objective. To investigate the prognostic value of combined testing of total and fetal cell-free DNA and cytokines in preterm labor.Krasnyi A.M., Kan N.E., Tyutyunnik V.L., Sadekova A.A., Saribekova A.G., Kokoeva D.N., Salpagarova Z.Kh., Medzhidova M.K., Vtorushina V.V., Krechetova L.V.
Material and methods. The study comprised 80 patients, including 47 women with spontaneous preterm labor (study group) who were divided based on gestational ages as follows: 22–27 weeks and 6 days (10), 28–33 weeks and 6 days (13) and 34–36 weeks and 6 days of gestation (24). Thirty-three women with normal pregnancies and full-term labor were enrolled in a control group. The levels total and fetal cell-free DNA in blood plasma was estimated by quantitative PCR by determining the concentration of the promoter of the RASSF1A gene and its hypermethylated portion. The analysis included measurements of IL-2, IL-4, IL-6, IL-10, GM-CSF, IFNγ and TNFα concentrations using the multiplex method. The predictive value was determined by logistic regression.
Results. Patients in the study group had increased levels of IL-6 at 22–27 week and 6 days gestation, and in 60% (p = 0.003) of cases, there were signs of intrauterine infection. The level of IL-8 was significantly increased in the study group at 28–33 weeks and 6 days and 34–36 weeks and 6 days gestation. There were no differences between groups in the rates of intrauterine infection at 28–33 week and 6 days. In the study group, intrauterine infection was diagnosed in 37.5% of cases (p = 0.01) at 34–36 weeks and 6 days. The concentration of fetal cell-free DNA was increased at 28–33 week and 6 days gestation. The most predictive of preterm labor at 22–27 weeks and 6 days, 28–33 weeks and 6 days, and 34–36 weeks and 6 days was combined testing of IL-6 and cfDNA (AUC 0.925; 95% CI, 0.83–1), IL-8 and cffDNA (AUC 0.92; 95% CI, 0.85–1), and IL-8, cfDNA, and cffDNA (AUС 0.92; 95% CI, 0.85–1), respectively.
Conclusion. Combined testing of cell-free DNA and cytokines is a promising approach for predicting preterm labor.
Keywords
Despite the persistently high incidence of preterm labor (PL), to date, there is no unified concept of the etiology of this pregnancy complication. In recent years, studies addressing this issue have been increasingly focused on the role of pro-inflammatory cytokines in the activation of the systemic inflammatory response that has been linked to the onset of preterm labor. For example, it has been shown that interleukin (IL) -8, which activates neutrophil migration and the release of proteinases, may play a key role in the development of PL [1–5]. As is known, there is a correlation between PL that is not associated with infection and elevated plasma concentrations of IL-8, IL-1, and IL-6. At the same time, elevated concentrations of tumor necrosis factor (TNF) α, IL-1, IL-6 were detected in infection-associated PL [6, 7]. Besides, some studies have reported increased concentrations of IL-6 interleukin 6 (IL-6) in the amniotic fluid of patients with PL, who had intrauterine infection [8, 9]. Currently, accumulated scientific evidence shows that plasma total cell-free DNA (cfDNA) can induce an inflammatory response that is associated with PL. S. Illanes et al. [10] and M.S. Quezada [11] reported that circulating cell-free fetal DNA (cffDNA) causes an inflammatory response that leads to the development of spontaneous labor. At the same time, activation of Toll-like receptor-9 (TLR-9) and an increase in the level of IL-6 were observed. There has been a growing amount of literature indicating a relationship between high levels of cffDNA and the risk of PL [12, 13]. The main target for cfDNA is considered to be TLR-9, which is activated by unmethylated CpG sequences. It is also known that neutrophil TLR-9 can interact with bacterial DNA, followed by the secretion of IL-8 and, to a lesser extent, IL-6 and TNFα [14]. As noted above, women with a high risk of PL have elevated plasma levels of these cytokines [6]. CffDNA constitutes 0.3–40% of cfDNA [15]; however, the ability of non-fetal cfDNA to induce PL remains unexplored.
This study aimed to investigate the prognostic value of combined testing of total and fetal cell-free DNA and cytokines in PL.
Material and methods
The study comprised 80 patients, including 47 women with PL (Group I, study group) and 33 women who had full-term labor (Group II, comparison group). The study was approved by the ethical committee of the V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology, and Perinatology of Minzdrav of Russia. All patients signed informed consent to participate in this study. Blood samples from women with PL were obtained at the onset of the first stage of labor and were divided into groups based on gestational ages according to WHO recommendations: 22–27 weeks and 6 days, 28–33 weeks and 6 days, and 34–36 weeks and 6 days of gestation. For comparison, blood obtained at the same gestational ages from women with normal pregnancies and full-term labor was examined.
The analysis included measurements of IL-2, IL-4, IL-6, IL-8, IL-10, granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon (IFN) γ, and TNFα concentrations in peripheral blood plasma by multiplex method using Standard Bio-Plex Pro Human Cytokine 8-plex Assay (Bio-Rad, USA) on a flow-based laser Bio-Plex 200 system (Bio-Rad, USA). The results were processed using the Bio-Plex Manager software version 6.0 (Bio-Rad, USA). IL concentrations are reported as pg/ ml.
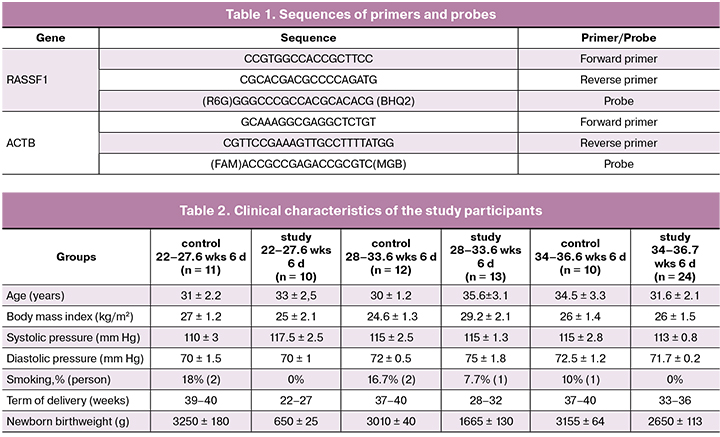
Maternal venous whole blood (5 ml) was drawn into EDTA vacuum blood collection tubes and processed within an hour after collection. The plasma was separated by centrifugation. Blood samples were centrifuged at 4°C in two stages: at 200 g for 10 min and then at 4500 g for 10 min. Plasma samples were stored at -80°C. In this study, the level of plasma cfDNA was estimated by quantitative polymerase chain reaction (PCR) by determining the concentration of the promoter of the RASSF1A gene. The assessment of cffDNA is based on the data that the promoter of the RASSF1A gene in the fetal genome is hypermethylated [16], and, accordingly, the concentration of its hypermethylated portion in the maternal blood will correspond to the number of cffDNA genomic units. The procedure for isolating the hypermethylated portion of RASSF1A was described in detail in the work of A.M. Krasnyi et al. [17]. PCR was performed with a real-time CFX96 amplifier (Bio-Rad, USA). Thermocycling conditions for PCR were as follows: 95°C for 5 min; 40 cycles: 95°C for 10 s., 60°C for 30 s. Primers and probes sequences are presented in Table 1.
Statistical analysis was performed using the XLstat statistical software (Addinsoft, USA). Differences in values between groups were assessed using the 2-tailed Mann-Whitney test. Data are presented as medians (M), 1 and 3 quartiles (25%, 75%). Differences were considered statistically significant at p < 0.05. A logistic regression model was constructed to estimate the probability of PL and the term of delivery.
Results and discussion
There were no statistically significant between-group differences regarding the somatic and obstetric history of pregnant women and their health status. Also, the groups of the study did not differ significantly in age, body mass index, blood pressure, and smoking status. Clinical characteristics of pregnant women, categorized by gestational age are presented in Table 2.
Analysis of perinatal outcomes showed that at 22–27 weeks and 6 days gestation 60% of the newborn infants in the study group had a congenital infection (p = 0.004), while there were no significant differences at 28–32 week and 6 days gestation (p = 0.34); at 33–36 weeks and 6 days gestation, 37.5% of pregnant women in the study group were diagnosed with intrauterine infection (p = 0.01). These findings, on the one hand, confirm the relationship of PL with the intrauterine infection, on the other hand, they indicate an important role of infection in the occurrence of PL in the early pregnancy. It should be noted that the correlation analysis established the relationship between the incidence of newborn intrauterine infection and the presence of colpitis in pregnant women, including candidal infection, herpes viral infection, positive bacterial culture from the cervix uteri, which can be considered as a risk factor for developing PL and intrauterine infection.
The plasma levels of cfDNA, cffDNA, as well as the cytokines IL-2, IL-4, IL-6, IL-10, GM-CSF, IFNγ, and TNFα in pregnant women are presented in table 3.
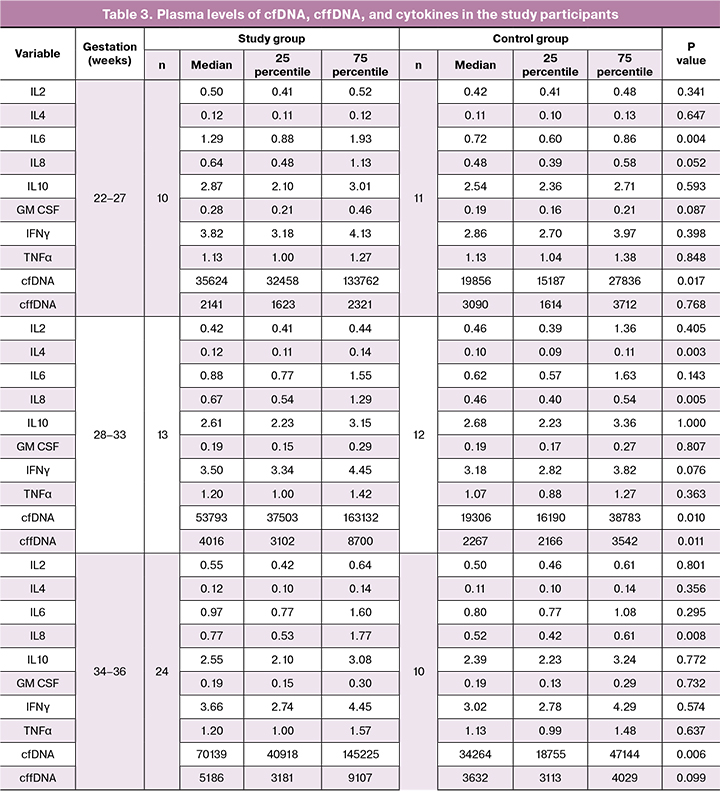
There was a significant 1.7-fold increase in IL-6 levels in the patients with PL at 22–27 weeks and 6 days gestation compared to the control group (p = 0.004), although no such an IL-6 rise was observed at 28–33 weeks 6 days and 34–36 weeks and 6 days gestation.
At the same time, the levels of IL-8 did not differ significantly at 22–27 weeks and 6 days gestation, but significantly increased 1.5 times at 28–33 weeks and 6 days (p = 0.005) and at 34–36 weeks 6 days (p = 0.008). Compared to the control subjects, in PL patients the level of cfDNA showed a significant 2.3, 2.8, and 2-fold increase at 22–27 weeks and 6 days (p = 0.017), 28–33 weeks and 6 days (p = 0.01) and at 34–36 weeks and 6 days (p = 0.006), respectively. It should be noted that the level of cffDNA in the PL patients was significantly higher than in the control group only at 28–33 week and 6 days gestation (p = 0.011).
Predictive value was determined by constructing a logistic regression model. At the final analysis, the most predictive of PL at 22–27 weeks and 6 days, 28–33 weeks and 6 days, and 34–36 weeks and 6 days was combined testing of IL-6 and cfDNA (AUC 0.925; 95% CI, 0.83–1), IL-8 and cffDNA (AUC 0.92; 95% CI, 0.85–1), and IL-8, cfDNA, and cffDNA (AUС 0.92; 95% CI, 0.85–1), respectively.
An increase in IL-6 in women with PL has been reported in a previous study [18]. We also established an elevated IL-6 level in PL at 22–27 weeks and 6 days gestation, while no IL-6 rise was observed in PL occurring at later gestational ages. Taking into account the high incidence of intrauterine infection (60%) among PL patients at 22–27 weeks and 6 days gestation, the infectious origin of PL can be assumed in these gestational ages, which is realized by increasing plasma level of IL-6. The above findings are consistent with the data reported by R. Romero et al. [9], indicating the infectious origin of early PL. This trend was not noted at later gestational ages. This observation may explain that in our study, among patients with PL there was an increase only in the level of IL-8 at 28–33 weeks 6 days and 34–36 weeks of 6 days of gestation. Analysis of cell-free DNA showed that patients with PL had significantly elevated concentrations of cfDNA at all gestational ages, whereas increased levels of cffDNA were observed only at 28–33 weeks and 6 days gestation. Other studies have emphasized the role of cffDNA in the onset of PL [13, 19]. Given that both groups had similar rates of intrauterine infection at a gestational age of 28–33 weeks and 6 days, it can be assumed that cffDNA play an important role in the development of PL only in this group. At other gestational ages, patients in the study group had increased levels of cfDNA and a significantly higher rate of intrauterine infection.
The literature has reported some diagnostic models for predicting PL based on maternal blood cytokines or cell-free DNA, but they did not have sufficient predictive value [17]. The developed combined use of cfDNA, cffDNA and cytokines allows construction of a model with significantly higher predictive value. Besides, using logistic regression based simultaneously on cytokines and cell-free DNA enabled the development of predictive models for PL at each gestational age.
Conclusion
The study findings show that the combined testing of cell-free DNA and cytokines is a promising approach for predicting PL.
References
- Сухих Г.Т., Серов В.Н., Адамян Л.В., Филиппов О.С., Баев О.Р., Клименченко Н.И., Тетруашвили Н.К., Тютюнник В.Л., Ходжаева З.С., Холин А.М.Преждевременные роды. Клинические рекомендации (протокол). М.: Министерство здравоохранения Российской Федерации; 2014. 35с. [Sukhikh G.T., Serov V.N., Adamyan L.V., Filippov O.S., Baev O.R., Klimenchenko N.I., Tetruashvili N.K., Tyutyunnik V.L., Khojaev Z.S., Kholin A.M. Premature birth. Clinical recommendations (protocol). M .: Ministry of Health of the Russian Federation; 2014. 35c. (in Russian)]
- Hadži-Lega M., Markova A.D., Stefanovic M., Tanturovski M. Interleukin 6 and fetal fibronectin as a predictors of preterm delivery in symptomatic patients. Bosn. J. Basic Med. Sci. 2015; 15(1): 51-6.
- Ковальчук Л.В., Игнатьева Г.А., Ганковская Л.В., ред. Иммунология. Практикум. Учебное пособие. М.: ГЭОТАР-Медиа; 2012. 176с. [Kovalchuk L.V., Ignatieva G.A., Gankovskaya L.V., ed. Immunology. Workshop. Tutorial. M .: GEOTAR-Media; 2012. 176p. (in Russian)]
- Suryanegara K., Kawilarang S. Difference of maternal serum interleukin-8 in preterm labor and normal pregnancy. Med. J. Obstet. Gynecol. 2017; 5(4): 1108.
- Савельева Г.М., Шалина Р.И., Курцер М.А., Клименко П.А., Сичинава Л.Г., Панина О.Б., Плеханова Е.Р., Выхристюк Ю.В., Лебедев Е.В. Преждевременные роды как важнейшая проблема современного акушерства. Акушерство и гинекология. 2012; 8-2: 4-10. [Savel’eva G.M., Shalina R.I., Kurcer M.A., Klimenko P.A., Sichinava L.G., Panina O.B., Plekhanova E.R., Vyhristyuk Yu.V., Lebedev E.V. Preterm birth as the most important problem of modern obstetrics. Obstetrics and gynecology/Akusherstvo i ginekologiya. 2012;(8):4-10. (in Russia)]
- Bogavac M., Brkić S., Celić D., Simin N., Matijasević J., Ilić T. Interferon gamma, interleukin 8 and interleukin 10 in serum of patients with the cervical infection and symptoms of the imminent preterm delivery. Srp. Arh. Celok. Lek. 2013; 141(9-10): 623-8.
- Szpecht D., Gadzinowski J., Nowak I., Cygan D., Seremak-Mrozikiewicz A., Kurzawińska G. et al. The significance of IL-1β +3953C>T, IL-6 -174G>C and -596G>A, TNF-α -308G>A gene polymorphisms and 86 bp variable number tandem repeat polymorphism of IL-1RN in bronchopulmonary dysplasia in infants born before 32 weeks of gestation. Cent. Eur. J. Immunol. 2017; 42(3): 287-93.
- Chaemsaithong P., Romero R., Korzeniewski S.J., Martinez-Varea A., Dong Z., Yoon B.H. et al. A rapid interleukin-6 bedside test for the identification of intra-amniotic inflammation in preterm labor with intact membranes. J. Matern. Fetal Neonatal Med. 2016; 29(3): 349-59.
- Romero R., Grivel J.C., Tarca A.L., Chaemsaithong P., Xu Z., Fitzgerald W. et al. Evidence of perturbations of the cytokine network in preterm labor. Am. J. Obstet. Gynecol. 2015; 213(6): 836. e1-836. e18.
- Illanes S., Gomez R., Fornes R., Figueroa-Diesel H., Schepeler M., Searovic P. et al. Free fetal DNA levels in patients at risk of preterm labour. Prenat. Diagn. 2011; 31(11): 1082-5.
- Quezada M.S., Francisco C., Dumitrascu-Biris D., Nicolaides K.H., Poon L.C. Fetal fraction of cell-free DNA in maternal plasma in the prediction of spontaneous preterm delivery. Ultrasound Obstet. Gynecol. 2015; 45(1): 101-5.
- Jakobsen T.R., Clausen F.B., Rode L., Dziegiel M.H., Tabor A. High levels of fetal DNA are associated with increased risk of spontaneous preterm delivery. Prenat. Diagn. 2012; 32(9): 840-5.
- Dugoff L., Barberio A., Whittaker P.G., Schwartz N., Sehdev H., Bastek J.A. Cell-free DNA fetal fraction and preterm birth. Am. J. Obstet. Gynecol. 2016; 215(2): 231. e1-7.
- József L., Khreiss T., El Kebir D., Filep J.G. Activation of TLR-9 induces IL-8 secretion through peroxynitrite signaling in human neutrophils. J. Immunol. 2006; 176(2): 1195-202.
- Dhallan R., Au W.C., Mattagajasingh S., Emche S., Bayliss P., Damewood M. et al. Methods to increase the percentage of free fetal DNA recovered from the maternal circulation. JAMA. 2004; 291(9): 1114-9.
- Chan K.C., Ding C., Gerovassili A., Yeung S.W., Chiu R.W., Leung T.N. et al. Hypermethylated RASSF1A in maternal plasma: A universal fetal DNA marker that improves the reliability of noninvasive prenatal diagnosis. Clin. Chem. 2006; 52(12): 2211-8.
- Красный А.М., Грачева М.И., Садекова А.А., Вторушина В.В., Балашов И.С., Кан Н.Е., Боровиков П.И., Кречетова Л.В., Тютюнник В.Л. Комбинированное исследование общей, фетальной ДНК, цитокинов в плазме крови матери при преэклампсии. Бюллетень экспериментальной биологии и медицины. 2017; 164(12): 686-91. [Krasnyy A.M., Gracheva M.I., Sadekova A.A., Vtorushina V.V.,Balashov I.S., Kan N.E., Borovikov P.I., Krechetova L.V., Tyutyunnik V.L. Combined study of total, fetal DNA, cytokines in the mother’s blood plasma with preeclampsia. Bulletin of experimental biology and medicine. 2017; 164 (12): 686-91. (in Russian)]
- Arababadi M.K., Aminzadeh F., Hassanshahi G., Khorramdelazad H., Norouzi M., Zarandi E.R. et al. Cytokines in preterm delivery. Lab. Med. 2012; 43(4): 27-30.
- Scharfe-Nugent A., Corr S.C., Carpenter S.B., Keogh L., Doyle B., Martin C. et al. TLR9 provokes inflammation in response to fetal DNA: mechanism for fetal loss in preterm birth and preeclampsia. J. Immunol. 2012; 188(11): 5706-12.
Received 13.04.2018
Accepted 20.04.2018
About the Authors
Krasnyi, Aleksey M., PhD, head of the cytology laboratory of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after AcademicianV.I. Kulakov Ministry of Health of Russia.
117997, Russia, Moscow, Ac. Oparina, 4 str. Tel.: +74954382272. E-mail: alexred@list.ru
Kan, Natalia E., MD, head of the obstetric department of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician
V.I. Kulakov Ministry of Health of Russia.
117997, Russia, Moscow, Ac. Oparina, 4 str. Tel.: +79262208655. E-mail: kan-med@mail.ru. Number Researcher ID B-2370-2015. ORCID ID 0000-0001-5087-5946
Tyutyunnik, Victor L., MD, head of the Obstetric Physiological Department of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named
after Academician V.I. Kulakov Ministry of Health of Russia.
117997, Russia, Moscow, Ac. Oparina, 4 str. Tel.: +79039695041. E-mail: tioutiounnik@mail.ru. Number Researcher ID B-2364-2015.ORCID ID 0000-0002-5830-5099
Sadekova, Alsu A., scientific researcher of the cytology laboratory of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named
after Academician V.I. Kulakov Ministry of Health of Russia.
117997, Russia, Moscow, Ac. Oparina, 4 str. Tel.: +74954382272. E-mail: a_sadekova@oparina4.ru
Saribekova, Alena G., the postgraduate student of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia.
117997, Russia, Moscow, Ac. Oparina, 4 str. Tel.: +79265517824. E-mail: arushanovaa@mail.ru
Kokoeva, Diana N., the postgraduate student of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia .
117997, Moscow, Ac. Oparina, 4 str. Tel.: +79372674440. E-mail: dikokoeva@mail.ru
Salpagarova, Zalina Kh., the postgraduate student of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician
V.I. Kulakov Ministry of Health of Russia.
117997, Russia, Moscow, Ac. Oparina, 4 str. Tel.: +79258539622. E-mail: z.salpagarova1990@yandex.ru
Medzhidova, Marzanat K., PhD candidate of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov
Ministry of Health of Russia.
117997, Russia, Moscow, Ac. Oparina, 4 str. Tel.: +79263811710. E-mail: marzhana-m@yandex.ru
Vtorushina, Valentina V., PhD in medical sciences, doctor of laboratory diagnostics in Laboratory of Clinical Immunology of the National Medical Research Center
for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia.
117997, Russia, Moscow, Ac. Oparina, 4 str. Tel.: +74954381183. E-mail: v_vtorushina@oparina4.ru
Krechetova, Lubov V., PhD the head of the immunology laboratory of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named
after Academician V.I. Kulakov Ministry of Health of Russia.
117997, Russia, Moscow, Ac. Oparina, 4 str. Tel.: +74954381183. E-mail: l_krechetova@oparina4.ru
For citation: Krasnyi A.M., Kan N.E., Tyutyunnik V.L., Sadekova A.A., Saribekova A.G., Kokoeva D.N., Salpagarova Z.Kh., Medzhidova M.K., Vtorushina, V.V., Krechetova, L.V. Predicting preterm labor by combined testing of cytokines and cell-free DNA. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; (1): 86-91. (in Russian)
http: // dx.doi.org/10.18565/aig.2019.1.86-91



