The diagnostic value of α-1-microglobulin in the development of preterm labor
Objective. To investigate the components of the connective tissue α-1-microglobulin and lumican, and compounds involved in their metabolic activation during preterm labor.Drukker N.A., Durnitsyna O.A., Nikashina A.A., Selyutina S.N.
Material and methods. The material for the study was blood serum of 89 women in the first period of labor, of whom 42 experienced preterm labor and 47 had full-term delivery. The laboratory investigations included testing for lumican, α-1-microglobulin, Tlr-4 (toll-like receptor-4), insulin receptor, and TNF-α using an enzyme immunoassay.
Results. Changes in the structure of the connective tissue as a result of an increase in the level of α-1-microglobulin and its induration due to lumican modification, as well as thickening (in the case of blood), underlie the impairment of the trophoblast invasion and the decrease in fetal uterine blood flow.
Conclusion. Increased serum concentration of α-1-microglobulin in women at 34 weeks’ gestation can serve as a predictor of preterm labor.
Keywords
The adverse demographic trends in our country are aggravated by an increase in infant mortality due to an increased rate of low birth weight preterm deliveries that have reached 20% in recent decades [1-3]. Preterm birth is known to be associated with not a single, but a group of factors leading to a premature uterine activity. The cumulative effect of many bioactive components [4] triggers this process, and an increase in stimulants or a decrease in the production of any of its inhibitors may result in preterm labor [5]. Of major interest is impaired production of bioactive substances that are components of the connective tissue, due to its importance to the human body which is more than 50% connective tissue. One of its particular functions is regulatory (influence on the activity of other tissues through its biologically active substances). First of all, it is α-1-microglobulin and lumican. Increased production of α-1-microglobulin prevents cytotrophoblast from passing into the spongy layer of the endometrium thus impairing the formation of a healthy trophoblast [6]. Another component of connective tissue, lumican, is significantly associated with its density.
Preterm labor is known to modify the inflammatory response resulting in a dysregulation of toll-like receptor 4 (Tlr-4), which is an essential component of innate immunity. By binding to membrane lipopolysaccharides of the infectious agent, Tlr-4 leads to the formation of complexes inhibiting the glucose transporter type 4 (GLUT-4). A modification of the Tlr-4 system leads to increased production of TNF-α (tumor necrosis factor-alpha), which is an important indicator of the inflammatory process. At the same time, the insulin receptor during gestation plays a significant role in biochemical processes, providing the metabolic linkage of insulin with GLUT-4.
This study aimed to investigate serum levels of connective tissue components α-1-microglobulin, lumican, and compounds involved in their metabolic activation in women with preterm labor.
Materials and methods
The material for the study was blood serum of women in the first period of labor, which was tested for the levels of α-1-microglobulin, TLR4, insulin receptor, lumican, and TNF-α.
Clinical evaluation was carried out by the current order: Order of the Ministry of Health of the Russian Federation dated November 12, 2012, № 572 n «On approval of the procedure for rendering medical care in obstetrics and gynecology, except the use of assisted reproductive technologies.» All parturient women underwent ultrasound placentography by ultrasound color Doppler mapping using a 3.5–6.5 MHZ convex probe. The diagnosis of preterm labor was refined by measuring cervical length and dilatation of the internal cervical os by a 5 MHZ transvaginal probe (Aloka 1400, TOSHIBA (Eccocee)) SSA – 340A (Japan).
Biochemical parameters (α-1-microglobulin, lumican, insulin receptor, Tlr-4, TNF-α) were determined using enzyme immunoassay based on a sensitive sandwich assay. Lumican and Tlr-4 were tested using USCN Life Science Inc. Elisa Kits; α-1-microglobulin was measured using ImmunDiagnostik kits; insulin receptors were determined using BCM Diagnostics kits. Levels of TNF-α were measured by the enzyme-linked immunosorbent assay. Quantitative evaluation of all studied compounds was carried out with the kits of the indicated firms on a multifunctional programmable counter for immunological studies with a computer and Multilabel Counter Victor-2 1420 software (Finland), TECAN Sunrise immunoassay (Austria), and an Immulite 2000xp automatic analyzer (Germany).
Statistical analysis was performed using the Statistica v. 5.1.software package (StatSoft. Inc.) and Excel 2002. Statistical significance was tested using the Student’s test (t-test) and the non-parametric Mann – Whitney test (I-test). Differences were considered significant at p < 0.05.
Results and discussion
The study comprised 89 women aged 20–39 years at 34–40 weeks’ gestation, who were divided into 2 groups. Group 1 included 42 patients with preterm labor at 34–37 weeks’ gestation; of them, 47.1%, 30.9%, and 22% were aged 25–29, 30–34, and 35–39 years, respectively. Forty-seven women aged 25–29 years (31%) and 30–34 years (31.9%) with physiological pregnancy and childbirth were enrolled in the control group (Group 2). Therefore, the age of participants did not differ between the groups. Women included in Group 1 and 2 constituted 60.8% and 39.2% of the total cohort, respectively.
Women in Group I reported a history of early fetal demise twice more often than women in the control group. Women with preterm labor at 33–36 weeks’ gestation more often had a history gynecological disease of the pelvic organs co-occurring with vaginitis and dysbiosis.
Thirty seven women in Group 1 were found to have extragenital diseases, including endocrine disorders (87.4%), congenital adrenocortical dysfunction (CAD) (4.7%), hypocorticism (2.3%), polycystic ovary syndrome (PCOS) (23.8%), diabetes (14.2%), thyroid diseases (14,2%). And the most prevalent comorbidities were PCOS and thyroid diseases.
The most common pregnancy complication was threatened miscarriage observed in 57.1% of women, followed by FPI (42.8%), anemia (19%), polyhydramnios (16.6%), and preeclampsia (6, 2%).
A comparative analysis of labor complications showed that the most common of them was premature rupture of membranes, which occurred in 59.5% of patients in Group 1. Two of the women in each group underwent a Cesarean section (4.25% in Group 1and 4.76% in Group 2).
In Group 2, 97.8% of newborns were full-term infants born with an Apgar score of 8–10 and birthweight 2970-3590 g. Five (11.9%) infants in Group 1 had morphofunctional immaturity and birthweight ranging from 2050 to 2510 g.
The results of biochemical testing are presented in the table.
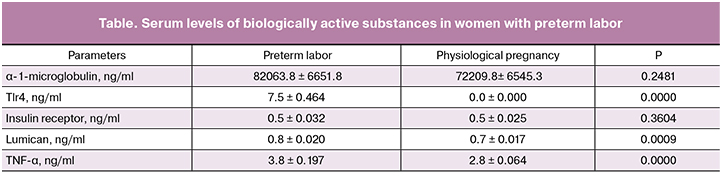
Women with preterm labor had 7.5-fold higher levels of TlR-4 compared to the women in the control group (p = 0.0000). Of note is the co-occurring 1.1-fold change in the levels of lumican and α-1-microglobulin (p = 0.0009). Levels of TNF-α, which is the most important predictor of the inflammatory process [7] was 1.36 times higher (p = 0.0000) than in women with physiological pregnancy and timely delivery. Values of the insulin receptor did not differ significantly between the groups. Interaction of insulin with its receptor located on the cell membrane results in the formation of an insulin-receptor complex, which provides a metabolic link with GLUT-4. The preservation of the receptor’s nativity suggests that in the gestation process such a situation is of a compensatory nature, which allows the maintenance of energy metabolism at the required level by transporting glucose to all organs and tissues.
The findings on TlR-4 (high level) suggests that, in this case, the metabolic relationship between TlR-4 and TNF-α (TlR-4 activates the synthesis of TNF-α) [8] is reflected in the increase in TNF-α synthesis. Elevated levels of TNF-α are proven to plays a significant diagnostic role in the presence of the inflammatory process. These observations lead to the conclusion that women with preterm labor are predisposed to a systemic inflammatory process.
Of note is a high level of serum TlR-4 in these women, which, as is well known, along with the activation of the synthesis of TNF-α is a powerful inducer of intracellular signaling pathways [9] and antibacterial protection [10]. Thereby, it seems that an increase in serum TlR-4 concentration in parturient women in this group can also be considered a compensatory factor.
Worthy of considerable attention is the change in the levels of connective tissue components α-1-microglobulin and lumican, which play a crucial role in the development of preterm labor. Thus, an increase in α-1-microglobulin plays a pathogenic role in the development of preterm labor [6, 11] because increased production of α-1-microglobulin prevents cytotrophoblast from passing into the spongy layer of the endometrium thus limiting the growth of the trophoblast. [11].
Under these conditions, lumican, another component of the connective tissue undergoes a modification. In this case, it can be assumed that the increased level of keratin sulfate proteoglycan had a significant effect on the structure of the connective tissue, increasing its density, including blood, which is also connective tissue. In the latter case, it leads to thickening of the blood.
Changes in the structure of the connective tissue as a result of an increase in the level of α-1-microglobulin and its induration due to lumican modification, as well as thickening (in the case of blood), undoubtedly underlie the metabolic changes of this tissue in the uterine muscle layer, impairing trophoblast invasion and decreasing fetal – uterine blood flow. These changes in the bioactive components of the connective tissue suggest a certain role of impaired α-1-microglobulin production in the development of placental insufficiency, which is one of the indicators of this pregnancy complication, a diagnostic criterion of which is an increase in α-1-microglobulin [12, 13]. In this case, N.V. Starodubtsev [14] emphasizes that the impairment of trophoblast invasion plays a major role in the development of preeclampsia, combined with abnormal fetal-uterine blood flow. Under these conditions, the most frequent pregnancy complications were threatened miscarriage and FPI, which occurred in 86% and 42.2% of parturient women, respectively; FPI was observed 22 times more often than in control patients.
Of interest is the fact that the precursor of α-1-microglobulin is bikunin, which during preterm labor is secreted into the amniotic fluid without prior splitting, thereby causing a significant increase in α-1-microglobulin [11].
At the same time, the distribution of α-1-microglobulin in various tissues, including blood, is associated with protective and anti-inflammatory functions. Particularly noteworthy is the change in α-1-microglobulin, which as a protein capable of disrupting fetal-uterine circulation due to modification of the placenta formation.
Conclusion
Summarizing the study’s findings, we can conclude that modification of α-1-microglobulin and lumican production, which is reflected in an increase in serum levels of these connective tissue components in pregnant women, results in an impairment of trophoblast invasion into the spongy layer of the endometrium and disturbances of fetal uterine circulation.
The identified activation of the metabolic interrelation of TlR-4 and TNF-α indicates the presence of systemic inflammatory process causing modification of the connective tissue components (α-1-microglobulin and lumican), leading to an impairment of trophoblast invasion into the spongy layer of the endometrium and the development of fetoplacental insufficiency.
Increased serum concentration of α-1-microglobulin in parturient women can serve as a predictor of preterm labor.
The established serum level of the insulin receptor in women with preterm labor indicates the preservation of the body energy level and is compensatory in nature.
Anamnestic data indicate that most women under observation had a history of early fetal demise, diseases of the pelvic organs, and extragenital diseases, most often endocrine disorders – PCOS and thyroid diseases. The latter dictates the need for timely management of co-existing conditions.
References
- Селихова М.С., Кузнецова О.А., Вдовин С.В., Дмитриенко Г.В. Роль эндоксемии и механизмов врожденного иммунитета в патогенезе неразвивающейся беременности. Вестник Волгоградского государственного медицинского университета. 2012; 1: 17-20. [Selikhova M.S., Kuznetsova O.A., Vdovin S.V., Dmitrienko G.V. The role of endoxemia and the mechanisms of innate immunity in the pathogenesis of non-developing pregnancy. Bulletin of the Volgograd State Medical University. 2012; (1): 17-20. (in Russian)]
- Погорелова Т.Н., Гунько В.О., Линде В.А. Протеомные маркёры программирования преждевременных родов. Российский вестник акушера-гинеколога. 2016; 16(2): 18-22. [Pogorelova T.N., Gunko V.O., Linde V.A. Proteomic markers of preterm labor programming. Russian Bulletin of the obstetrician-gynecologist. 2016; 16 (2): 18-22. (in Russian)]
- Ковалёв В.В., Цывьян П.Б., Миляева Н.М., Лукин О.Н., Проценко Ю.Л. Физиологические основы регуляции сократительной активности матки. Акушерство и гинекология. 2010; 3: 10-3. [Kovalev V.V., Tsyvyan P. B., Milyaeva N.M., Lukin O.N., Protsenko Yu.L. Physiological basis for the regulation of uterine contractile activity. Obstetrics and gynecology. 2010; (3): 10-3. (in Russian)]
- Дегтярёва А.С., Никашина А.А., Крукиер И.И., Кухта О.И. Дисбаланс в системе цитокинов плаценты и плодных оболочек при самопроизвольном прерывании беременности в ранние сроки и преждевременных родах. Российский иммунологический журнал. 2015; 9(1-1): 49-50. [Degtyareva A.S., Nikashina A.A., Krukier I.I., Kukhta O.I. An imbalance in the cytokine system of the placenta and fetal membranes with spontaneous abortion in early pregnancy and preterm labor. Russian immunological journal. 2015; 9 (1-1): 49-50. (in Russian)]
- Погорелова Т.Н., Куценко И.И., Бутова О.А., Гунько В.О., Никашина А.А., Аллилуев И.А. Белки-маркеры метаболических и функциональных нарушений при осложнённой гестации. Современные проблемы науки и образования. 2016; 6: 42. [Pogorelova T.N., Kutsenko I.I., Butova O.A., Gun’ko V.O., Nikashina A.A., Alliluyev I.A. Protein markers of metabolic and functional impairments in complicated gestation. Modern problems of science and education. 2016; (6): 42. (in Russian)]
- Pogorelova T.N., Gunko V.O., Linde V.A., Krukier I.I., Nikashina A.A., Drukker N.A., Alliluev I.A. Regulation of redox status in placenta in case of physiological pregnancy and complicated pregnancy. FEBS J. 2015; 282 (Suppl.1: 40th FEBS congress - The biochemical basis of life. Berlin, Germany July 4-9, 2015): 120.
- Мкртумян А.М., Бирюкова Е.В., Морозова И.А. Эффективность и безопасность ситаглиптина: доказательная база для клинического применения и перспективы. Поликлиника. 2015; 1-2: 63-70. [Mkrtumyan A.M., Biryukova E.V., Morozova I.A. Efficacy and safety of sitagliptin: an evidence base for clinical use and prospects. Polyclinic. 2015; 1-2: 63-70. (in Russian)]
- Нефёдова Д.Д., Линде В.А., Левкович М.А. Иммунологические аспекты беременности (обзор литературы). Медицинский вестник Юга России. 2013; 4: 16-21. [Nefedova D.D., Linde V.A., Levkovich M.A. Immunological aspects of pregnancy (literature review). Medical Bulletin of the South of Russia. 2013; (4): 16-21. (in Russian)]
- Verhelst K., Verstrepen L., Carpentier I., Beyaert R. Linear ubiquitination in NF-Kb signaling and inflammation: What we do understand and what we do not. Biochem. Pharmacol. 2011; 82(9): 1057-65.
- Стяжкина С.Н., Черненкова М.Л., Кривенко П.А., Гайлямова Л.И. Течение и исходы беременности у женщин с хроническим пиелонефритом. Современные проблемы науки и образования. 2015; 1-1: 1296. [Styazhkina S.N., Chernenkova M.L., Krivenko P.A., Gailyamova L.I. Course and outcomes of pregnancy in women with chronic pyelonephritis. Modern problems of science and education. 2015; (1-1): 1296. (in Russian)]
- Гунько В.О., Погорелова Т.Н., Линде В.А. Протеомные исследования околоплодных вод – новый подход к поиску маркёров задержки роста плода. Журнал фундаментальной медицины и биологии. 2013; 4: 10-7. [Gunko V.O., Pogorelova TN, Linde V.A. Proteomic studies of amniotic fluid - a new approach to the search for markers of fetal growth retardation. Journal of Fundamental Medicine and Biology. 2013; (4): 10-7. (in Russian)]
- Герасимова Л.И., Васильева Э.Н., Денисова Т.Г., Драндров Г.Л. Комплексная терапия в профилактике первичной плацентарной недостаточности и синдрома задержки роста плода. Практическая медицина. 2011; 6: 42-4. [Gerasimova L.I., Vasilyeva E.N., Denisova T.G., Drandrov G.L. Combined therapy in the prevention of primary placental insufficiency and fetal growth retardation syndrome. Practical medicine. 2011; (6): 42-4. (in Russian)]
- Серченя Т.С., Свиридов О.В. Альфа-1-микроглобулин человека, биомедицинские аспекты. Лечебное дело: научно-практический терапевтический журнал. 2011; 6: 69-78. [Serchenya T.S., Sviridov O.V. Human alpha-1-microglobulin, biomedical aspects. Medical business: scientific and practical therapeutic journal. 2011; (6): 69-78. (in Russian)]
- Стародубцева Н.Л., Бугрова А.Е., Кононихин А.С., Вавина О.В., Широкова В.А., Наумов В.А., Гаранина И.А., Лагутин В.В., Попов И.А., Логинова Н.С., Ходжаева З.С., Франкевич В.Е., Николаев Е.Н., Сухих Г.Т. Возможность прогнозирования и ранней диагностики преэклампсии по пептидному профилю мочи. Акушерство и гинекология. 2015; (6): 46-52. [Starodubtseva NL, Bugrova A.E., Kononikhin A.S., Vavina O.V., Shirokova V.A., Naumov V.A., Garanina I.A., Lagutin V.V., Popov I..A., Loginova N.S., Khodzhaeva Z.S., Frankevich V.E., Nikolaev E.N., Sukhikh G.T. The ability to predict and early diagnosis of preeclampsia on the peptide profile of urine. Obstetrics and gynecology. 2015; 6: 46-52. (in Russian)]
Received 01.02.2018
Accepted 02.03.2018
About the Authors
Drukker, Nina A., Dr.Bio.Sci., associate professor, chief researcher at the Department of Medico-Biological Problems in Obstetrics, Gynecology and Pediatrics, Rostov Research Institute of Obstetrics and Pediatrics, Rostov State Medical University of Minzdrav of Russia.344012, Russia, Rostov-on-don, Mechnikova street 43. Tel.: +78632275077. E-mail: n.drukker@yandex.ru
Durnitsyna, Olga A., junior researcher of the Department of Medico-Biological Problems in Obstetrics, Gynecology and Pediatrics, RRIOP of Minzdrav of Russia.
344012, Russia, Rostov-on-don, Mechnikova street 43. Tel.: +78632275077. E-mail: doa-ozz@yandex.ru
Nikashina, Anastasia A., PhD (Bio.Sci.), researcher at the Department of Medico-Biological Problems in Obstetrics, Gynecology and Pediatrics, RRIOP of Minzdrav of Russia. 344012, Russia, Rostov-on-don, Mechnikova street 43. Tel.: +78632275077. E-mail: laigash@yandex.ru
Selyutina, Svetlana N., PhD (Bio.Sci.), researcher at the Department of Medico-Biological Problems in Obstetrics, Gynecology and Pediatrics, RRIOP of Minzdrav of Russia. 344012, Russia, Rostov-on-don, Mechnikova street 43. Tel.: +78632275077. E-mail: edu.center@rniiap.ru.
For citation: Drukker N.A., Durnitsyna O.A., Nikashina A.A., Selyutina S.N. The diagnostic value of α-1-microglobulin in the development of preterm labor. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; (1): 81-5. (in Russian)
https://dx.doi.org/10.18565/aig.2019.1.81-85



