Preterm birth: causes, pathogenesis, and management
Premature labor (PL) remains one of the most important obstetric problems, because perinatal morbidity and mortality are very high among premature babies. However, the pathogenesis of spontaneous PL remains unclear so far. Objective. To study the role of toll-like receptors (TLR2, TLR4, and TLR9) in the pathogenesis of spontaneous PL of unknown etiology at 25-33 weeks’ gestation. Subjects and methods. The investigation enrolled 165 pregnant women with a singleton pregnancy and threatened spontaneous PL at 25-33 weeks’ gestation; pregnant women with preeclampsia, placental insufficiency, infection, placenta previa, diabetes mellitus, fetal malformations or premature amniorrhea were excluded. After excluding the presence of an infectious agent in the cervix and vagina, the investigators selected 42 patients with the clinical presentations of threatened PL and 32 patients with uncomplicated pregnancy. The expression of TLR by the cervical canal epithelial cells was investigated by polymerase chain reaction. Results. The women with threatened PL had a history of high infection rates (71.4 and 21.9%; p <0.001). TLR2 expression by cervical canal epithelial cells in pregnant women with threatened PL was 3.65 times higher than in those with uncomplicated pregnancy (174.9 (132.3; 1996.3) and 48.0 (33.7; 109.1); p = 0.027), TLR4 expression was also higher (70.9 (28.3; 116.9) and 53.2 (17.7; 111.3); p = 0.072). At the same time, the expression of intracellular TLR9 was slightly lower in pregnant women with threatened PL (58.7 (17.1; 100.4) and 76.2 (57.0; 112.6)); p = 0.12). Conclusion. The findings may suggest that even with infectious agent exclusion and in the absence of an obvious inflammatory process in the vagina and cervix, the inflammatory process that results from TLR activation underlies the genesis of spontaneous PL.Belousova V.S., Strizhakov A.N. , Svitich O.A., Timokhina E.V., Kukina P.I., Bogomazova I.M., Pitskhelauri E.G.
Keywords
Preterm birth has been a matter of keen interest to researchers around the world due to incomplete understanding of its pathogenesis. Besides, the high morbidity and mortality of preterm neonates motivate intense research efforts seeking to develop new pathogenetic methods to reduce the risk of preterm birth. That is why an understanding of the pathogenesis of spontaneous preterm onset of labor is very important.
Pregnancy is the development of a semi-allogeneic fetus with father’s antigens in the uterus. The emerging fetal–placental unit is a unique immunological interface between the mother and the fetus that allows immunologically foreign fetal tissue to be normally accepted by the mother’s immune system.
What is currently known about changes in the immune system during pregnancy:
- in the human placenta, the syncytiotrophoblast cover of the villi is directly exposed to maternal blood, but it does not express HLA class I antigens on its surface and therefore is not “visible” to the maternal immune system;
- in the trophoblast, active substances are produced that activate and stimulate the apoptosis of maternal T-lymphocytes (for example, protein B7H1, IDO);
- trophoblast cells produce regulatory proteins for the compliment system (CD46, CD55, CD59), and this helps to protect embryonic tissues from cytotoxic maternal antibodies;
- in the uterus, a subpopulation of immune cells undergoes significant changes resulting in a release of a large number of decidual macrophages with anti-inflammatory activity (M2 phenotype), which in turn secrete immunosuppressive factors, which reduces inflammatory reactions in the fetal–placental unit. Preterm birth, preeclampsia, and placental insufficiency, on the contrary, are associated with macrophage activation;
- progesterone stimulates the production of anti inflammatory cytokines (for example, interleukin (IL-10) from placental cells; besides, it suppresses the maternal immune response and alters the Th1\Th2 helper balance towards the anti-inflammatory state of Th2, and blocks the synthesis of tumor necrosis factor (TNF).
It should be noted that during pregnancy, all immune changes occur only locally without generalized immunosuppression in pregnant women [1].
Many immunologists consider pregnancy as an anti-inflammatory condition, while spontaneous miscarriages and other pregnancy complications are associated with an increase in cytokine levels. So the increase in IL-6 in the cervical-vaginal secretion and the amniotic fluid is associated with preterm birth [2]; high levels of IL-1β and TNF also were reported to exert elevated risk for preterm birth [3].
Toll-like receptors (TLRs) are important components of the innate immune system. These receptors are located on the surface and in the cytoplasmic granules of various body cells. Most TLRs are expressed by monocytes and macrophages. It has been found that there are10 types of TLRs in humans. All types of TLRs have their ligands, including microbial components such as lipopolysaccharides, lipoteichoic acid, flagellin, etc., as well as bacterial and viral nucleic acids. Following the recognition of ligands, TLRs activation leads to the induction of innate immune responses by producing inflammatory cytokines (TNF, IL) resulting in the development of the inflammatory process. The severity of the inflammatory process may also depend on the types and amount of TLRs on the cell surface of a given structure or tissue.
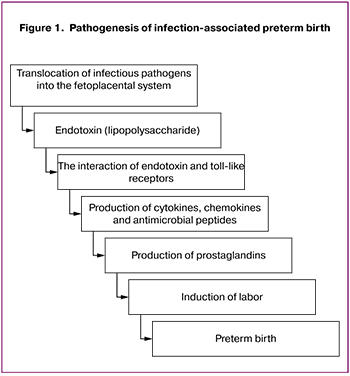 Currently, there is plenty of research evidence to support the role of TLRs in the pathogenesis of preterm births that are associated with the presence of an infectious agent [4]. There is a known pathogenetic pathway of the fetal egg rejection through the activation of TLRs by endotoxins of various bacteria, which leads to preterm pregnancy termination (Fig. 1) [5].
Currently, there is plenty of research evidence to support the role of TLRs in the pathogenesis of preterm births that are associated with the presence of an infectious agent [4]. There is a known pathogenetic pathway of the fetal egg rejection through the activation of TLRs by endotoxins of various bacteria, which leads to preterm pregnancy termination (Fig. 1) [5].
However, in spontaneous preterm births that constitute about a third of all preterm births, no cause is identified [6] and their management is based on symptomatic rather than pathogenetic therapy.
This study was aimed to identify a possible pathogenetic mechanism of spontaneous preterm birth mediated by activation of TLRs. To this end, pregnant women with threatened preterm birth were examined for the expression of TLR2, TLR4, and TLR9 in cervical epithelial cells and levels of pro-inflammatory cytokines (TNF, IL-6) in cervical mucus.
Materials and methods
The study comprised 165 singleton pregnant women with threatened preterm birth at 25–33 weeks’ gestation. The diagnosis of threatened preterm birth was based on patients’ complaints of pulling lower abdominal pain and was confirmed by an increased uterine tone and a cervical shortening of less than 20 mm. At the selection stage, women with cervical and vaginal infections confirmed by culture were excluded, as well as patients with any other pregnancy complications (preeclampsia, placental insufficiency, infections, placenta previa, diabetes mellitus, fetal malformations, and preterm rupture of membranes) –119 (72.1%) of women.
Besides, we investigated the level of pro-inflammatory cytokines (TNF and IL-6) in the cervical-vaginal secretion. Four patients had pro-inflammatory cytokines in cervical secretions without bacterial or viral agents. They were also excluded from the study. Therefore, only 42 patients with threatened preterm birth and without cervical and vaginal infections were included in the study, which allowed them to be considered as having threatened spontaneous preterm birth. The control group consisted of 32 women with an uncomplicated pregnancy.
To examine the expression of TLRs in cervical epithelial cells after mucus removal, cervical scrape samples were collected by type D model 1 cytobrush and placed into separate sterile Eppendorf tubes. At the first stage, RNA was isolated from epithelial cells by affinity sorption on silica gel particles using the AmpliPrim RIBO-sorb kit of InterLabService (the RF) strictly following the protocol. Next, a reverse transcription reaction was performed using the Reverse Transcription Kit (Synthol, RF) strictly following the protocol. Primers for the sequences of the studied mRNAs were selected using the Vector NTI 8.0 software and synthesized by Syntol. The primers and probes for real-time polymerase chain reaction (RT PCR) were modeled in the Vector NTI 8.0 software following the mRNA sequences of the genes under study (mRNA sequences were taken from the GenBank database) and synthesized by Syntol (the RF). PCR was performed in the presence of the SYBR Green I dye (Syntol, the RF) and used strictly following the protocol. After the preparation of the reaction mixtures, the tubes were placed in a RT PCR DT-96 amplifier (DNA - technology, RF). The reaction mode was 40 cycles (95°C for 20 min, 60°C for 40 min).
The cytokines IL-6 and TNF in n the cervical mucosa were determined using enzyme immunoassays (according to the relevant regulations) for the quantitative determination of IL-6 and TNF: Human IL-6 Platinum ELISA and Human TNF alpha Platinum ELISA from Thermo Fisher Scientific (USA).
Statistical analysis was performed using BioStat software and MS Excel. Quantitative variables showing normal distribution were expressed as means (M) and standard deviation (SD) and presented as M (SD); otherwise, the median (Me) and the quartiles Q1 and Q3 in the Me (Q1; Q3) format were reported. Qualitative variables were summarized as counts and percentages. Categorical variables were compared by the χ2 test with the Yates correction. The expression of TLRs was evaluated in relative units (RU). For each sample, the logarithm of the number of copies of the studied gene and the number of copies of the β-actin gene were obtained to normalize the results. The number of copies of the determined gene was subsequently recounted relative to 1 million copies of the β-actin gene. RU is the ratio of the number of copies for the studied gene (in our case TLR2, TLR4 and TLR 9) to the number of copies for the housekeeping gene (β-actin gene). To assess the statistical significance of differences in gene expression in the studied groups, the nonparametric Mann – Whitney test was used. Differences between the groups were considered statistically significant at p < 0.05.
Results
The gestational age in both study groups did not differ (28 weeks) and ranged from 25 to 33 weeks. In both groups, there were twice as many primiparous as multiparous women. Among women at risk of preterm birth, 14.3% had a history of preterm birth. Women with an uncomplicated pregnancy had no history of preterm birth (table).
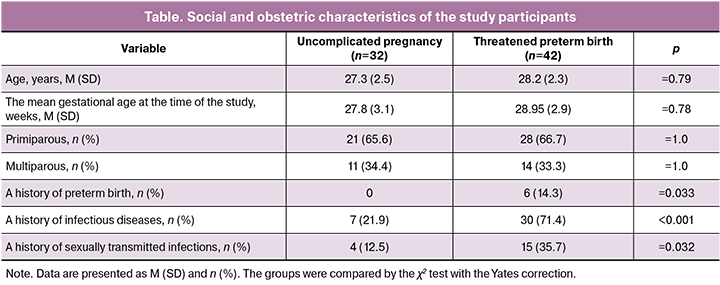
Women with threatened preterm birth 3.26 times more often reported a history of infectious diseases (chronic pyelonephritis, tonsillitis, cystitis) than women in the control group (71.4 and 21.9%; p < 0.001). Also, these women were almost 3 times more likely to have a history of sexually transmitted infections (35.7 and 12.5%; p = 0.032).
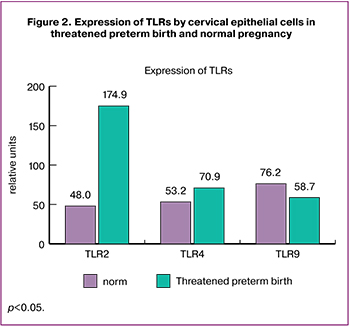 The findings regarding TLRs expression in cervical epithelial cells are presented in Fig. 2. We found that patients with threatened preterm birth had 3.65-fold higher levels of TLR2 expression compared with the control group [(174.9 (132.3; 1996.3) versus 48.0 (33.7; 109.1)], p = 0.027. TLR4 expression was also 1.33 times higher in patients with threatened preterm birth [(70.9 (28.3; 116.9) and 53.2 (17.7; 111.3)], p = 0.072. However, TLR9 expression was 1.6 times lower in patients with threatened preterm birth compared with women in the control group [(58.7 (17.1; 100.4) and 76.2 (57.0; 112.6)], p = 0.12 (Fig. 2).
The findings regarding TLRs expression in cervical epithelial cells are presented in Fig. 2. We found that patients with threatened preterm birth had 3.65-fold higher levels of TLR2 expression compared with the control group [(174.9 (132.3; 1996.3) versus 48.0 (33.7; 109.1)], p = 0.027. TLR4 expression was also 1.33 times higher in patients with threatened preterm birth [(70.9 (28.3; 116.9) and 53.2 (17.7; 111.3)], p = 0.072. However, TLR9 expression was 1.6 times lower in patients with threatened preterm birth compared with women in the control group [(58.7 (17.1; 100.4) and 76.2 (57.0; 112.6)], p = 0.12 (Fig. 2).
Discussion
There is plenty of research evidence demonstrating the role of TLRs in the pathogenesis of preterm birth in the presence of an infectious factor. The objective of the present study was to assess the involvement and role of TLRs in the pathogenesis of preterm birth in the absence of infection and any other causes for the development of spontaneous preterm birth. That is why women with other complications of pregnancy, such as preeclampsia, placental insufficiency, infections, placenta previa, diabetes mellitus, fetal malformations, and preterm rupture of membranes were excluded from the study.
For the study, we selected three subspecies of TLRs: TLR2, TLR4, and TLR9. TLR2 and TLR4 are receptors located on the surface of cells that recognize the bacterial component. Also, TLR9, an intracellular receptor that can be activated by nucleic acids, was selected for the study.
There is an abundance of literature suggesting the role of TLR2 and TLR4 in the pathogenesis of preterm birth associated with infection [7–9]. That is why we considered that a careful selection of patients was essential to exclude the infectious nature of inflammation. At the selection stage, 72.1% of women with threatened preterm birth were found to have cervical and vaginal infections confirmed by culture results suggesting that the main cause of most preterm birth is infection. Among women with a threatened preterm birth, 14.3% reported a history of preterm birth, which is a risk factor for this complication [10].
Patients with spontaneous preterm birth had higher expression of TLRs in cervical epithelial cells than women with an uncomplicated pregnancy. So, TLR2 expression was increased 3.65 fold [(174.9 (132.3; 1996.3) versus 48.0 (33.7; 109.1)], p = 0.027; TLR4 expression was increased 1.33 fold [(70.9 (28.3; 116.9) versus 53.2 (17.7; 111.3)], p = 0.072. These findings suggest that even with the exclusion of an infectious factor and the absence of an obvious inflammatory process in the cervix and vagina, the inflammatory process caused by TLRs activation induces spontaneous preterm birth.
Unfortunately, it is yet unclear what activates TLRs in spontaneous preterm birth. Our findings revealed a higher incidence of infectious diseases (chronic pyelonephritis, tonsillitis, cystitis) (71.4 and 21.9%; p < 0.001), including sexually transmitted infections (35.7 and 12, 5%; p = 0.032) in patients with threatened preterm birth. And even though these infections were treated, which is confirmed by subsequent bacteriological and virological investigations, it is possible that mobilization of the immune system in the past leads to its faster activation during pregnancy. Since TLR2 and TLR4 are receptors that react to bacterial components, it is possible that an increase in their expression is associated with the presence of some latent infectious agent that was not detected by bacteriological investigations. At the same time, TLRs can recognize not only the exogenous structures of bacteria, but also respond to molecules released during tissue damage: fibronectin [11], fatty acids, heme, and others [12].
In patients with threatened preterm birth, a cervical and vaginal infection should be excluded, which, according to this study findings, was detected in 72.1% of pregnant women with this pregnancy complication. Treatment for these threatened preterm births should include antibiotic therapy.
Patients with threatened preterm birth, who have no cervical or vaginal infection should receive anti-inflammatory medications since our study showed the role of the inflammatory process in the pathogenesis of spontaneous preterm birth. Besides, studies are currently underway to use the TLR4 antagonist to prevent and treat spontaneous preterm birth [13]. Perhaps soon, this approach will allow for pathogenetic therapy of threatened spontaneous preterm births and reduce their incidence.
Conclusion
In 72.1% of patients, preterm births are associated with an inflammatory process caused by the presence of cervical and vaginal infection.
A history of preterm birth is a risk factor for spontaneous preterm birth.
Women with threatened preterm birth are significantly more likely to have a history of infectious diseases, including sexually transmitted infections.
An inflammatory process triggered by the activation of TLRs, in particular, TLR2 and TLR4 underlie the pathogenesis of spontaneous preterm birth.
Given the activation of the immune system through TLRs in the absence of an infectious agent, it is imperative to remember about other possible causes triggering the inflammatory process, for example, previous (including treated) infections that mobilize and activate the immune system during pregnancy. Besides, a noninfectious inflammatory response can be initiated by damage-associated molecular patterns (DAMPs).
References
- Abrahams V.M., Stiehm R., Lockwood C.J. Immunology of the maternal-fetal interface//UptoDate, 2018. https://www.uptodate.com/contents/immunology-of-the-maternal-fetal-interface?search=Immunology%20of%20the%20maternal-fetal%20interface&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Wei S.Q., Fraser W., Luo Z.C. Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: a systematic review. Obstet Gynecol. 2010; 116(2 Pt 1): 393-401. doi: 10.1097/AOG.0b013e3181e6dbc0.
- Ashford K., Chavan N.R., Wiggins A.T., Sayre M.M., McCubbin A., Critchfield A.S., O’Brien J. Comparison of Serum and Cervical Cytokine Levels throughout Pregnancy between Preterm and Term Births. AJP Rep. 2018; 8(2): e113-e120. doi: 10.1055/s-0038-1656534
- Schatz F., Kayisli U.A., Vatandaslar E., Ocak N., Guller S., Abrahams V.M., Krikun G., Lockwood C.J. Toll-like receptor 4 expression in decidual cells and interstitial trophoblasts across human pregnancy. Am J Reprod Immunol. 2012; 68(2):146–53. doi: 10.1111/j.1600-0897.2012.01148.x.
- Thaxton J.E., Nevers T.A., Sharma S. TLR-mediated preterm birth in response to pathogenic agents. Infect Dis Obstet Gynecol. 2010; 2010: pii: 378472. doi: 10.1155/2010/378472
- Стрижаков А.Н., Белоусова В.С., Свитич О.А. Клиническое значение toll-подобных рецепторов в патогенез преждевременных родов. Вопросы гинекологии, акушерства и перинатологии. 2016; 15(1): 35–41. doi: 10.20953/1726-1678-2016-1-35-40 [Strizhakov A.N., Belousova V.S., Svitich O.A. Klinicheskoe znachenie toll-podobnyh receptorov v patogenez prezhdevremennyh rodov. Voprosy ginekologii, akusherstva i perinatologii. 2016; 15(1): 35-41. (in Russian)]. doi: 10.20953/1726-1678-2016-1-35-40
- Moço N.P., Martin L.F., Pereira A.C., Polettini J., Peraçoli J.C., Coelho K.I., da Silva M.G. Gene expression and protein localization of TLR-1, -2, -4 and -6 in amniochorion membranes of pregnancies complicated by histologic chorioamnionitis. Eur J Obstet Gynecol Reprod Biol. 2013; 171(1): 12–7. doi: 10.1016/j.ejogrb.2013.07.036.
- Cappelletti M., Lawson M.J., Chan C.C., Wilburn A.N., Divanovic S. Differential outcomes of TLR2 engagement in inflammation-induced preterm birth. J Leukoc Biol. 2018; 103(3): 535–43. doi: 10.1002/JLB.3MA0717-274RR.
- Abrahams V.M., Potter J.A., Bhat G., Peltier M.R., Saade G., et al. Bacterial modulation of human fetal membrane Toll-like receptor expression. Am J Reprod Immunol. 2016; 69: 33–40. doi: 10.1111/aji.12016
- Blencowe H., Cousens S., Chou D., Oestergaard M., Say L., Moller A.B., Kinney M., Lawn J.; Born Too Soon Preterm Birth Action Group. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health. 2013; 10 Suppl 1: S2. doi: 10.1186/1742-4755-10-S1-S2.
- Okamura Y., Watari M., Jerud E.S., Young D.W., Ishizaka S.T., Rose J., Chow J.C., Strauss J.F. 3rd. The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem. 2001; 276(13): 10229–33. PMID: 11150311 Free Article
- Yu L., Wang L., Chen S. Endogenous toll-like receptor ligands and their biological significance. J Cell Mol Med. 2010; 14(11): 2592–603. doi: 10.1111/j.1582-4934.2010.01127.x.
- Chin P.Y., Dorian C.L., et al. Novel Toll-like receptor-4 antagonist (+)-naloxone protects mice from inflammation-induced preterm birth. Sci Rep. 2016; 6: 36112. doi: 10.1038/srep36112
Received 13.09.2019
Accepted 04.10.2019
About the Authors
Vera S. Belousova, candidate of medical sciences, associate professor of the Department of Obstetrics, Gynecology and Perinatology, Institute of Clinical Medicine, I.M.Sechenov First Moscow State Medical University (Sechenov University). Tel +7 (903) 715-45-02; e-mail: desdemosha@mal.ru; https://orcid.org/0000-0001-8332-7073Address: 119991 Russia, Moscow, ul. B. Pirogovskaya .2, p. 4.
Alexander N. Strizhakov, academician of the Russian Academy of Sciences, MD, professor, head of the Department of Obstetrics, Gynecology and Perinatology, Institute o
f Clinical Medicine, I.M.Sechenov First Moscow State Medical University (Sechenov University). Tel +7 (916) 194-99-91; e-mail: kafedra-agp@mail.ru
Address: 119991 Russia, Moscow, st. B. Pirogovskaya .2, p. 4
Oksana A. Svitich, Corresponding Member of the Russian Academy of Sciences, MD, Professor, Director of the Research Institute of Vaccines and Serums named
after I.I. Mechnikov. Tel.: +7 (495) 917-49-00; e-mail: svitichoa@yandex.ru
Address: 105064, Russia, Moscow, Maly Kazenny lane, 5a
Elena V. Timokhina, MD, professor of the Department of Obstetrics, Gynecology and Perinatology, Institute of Clinical Medicine, I.M.Sechenov First Moscow State Medical University (Sechenov University). Tel +7 (916) 607-45-34; e-mail: elena.timokhina@mail.ru; https://orcid.org/0000-0001-6628-0023
Address: 119991 Russia, Moscow, st. B. Pirogovskaya .2, p. 4
Polina I. Kukina, Clinical Resident at the Institute of Clinical Medicine, I.M.Sechenov First Moscow State Medical University (Sechenov University). Tel +7 (977) 974-41-27; e-mail: renoru47@gmail.com. Address: 119991 Russia, Moscow, st. B. Pirogovskaya .2, p. 4
Irina M. Bogomazova, Candidate of Medical Sciences, Associate Professor of the Department of Obstetrics, Gynecology and Perinatology, Institute of Clinical Medicine,
I.M. Sechenov First Moscow State Medical University (Sechenov University). Tel +7 (926) 305-04-03; e-mail: irinka.bogomazova@mail.ru;
https://orcid.org/0000-0003-1156-7726. Address: 119991 Russia, Moscow, st. B. Pirogovskaya .2, p. 4
Elena G. Pitskhelauri, candidate of medical sciences, associate professor of the Department of Obstetrics, Gynecology and Perinatology, Institute of Clinical Medicine,
I.M. Sechenov First Moscow State Medical University (Sechenov University). Tel +7 (916) 334-00-89; e-mail: elena-doc@rambler.ru; https://orcid.org/0000-0002-9634-1541
Address: 119991 Russia, Moscow, st. B. Pirogovskaya .2, p. 4
For citation: Belousova V.S., Strizhakov A.N., Svitich O.A., Timokhina E.V., Kukina P.I., Bogomazova I.M., Pitskhelauri E.G.Preterm birth: causes, pathogenesis, and management.
Akusherstvo i Ginekologiya/Obstetrics and gynecology. 2020; 2: 82-7.(In Russian).
https://dx.doi.org/10.18565/aig.2020.2.82-87



