Production of reactive oxygen species by leukocytes stimulated with zymosan and lipopolysaccharide in women with miscarriage in early pregnancy
Objective: To study the production of reactive oxygen species by polymorphonuclear leukocytes in peripheral blood stimulated with zymosan and lipopolysaccharide, which are recognized by Toll-like receptors in women with miscarriage associated with infection in early pregnancy compared to healthy pregnant women.Zhambalova B.A., Osipov A.N., Vladimirov Yu.A., Dobrokhotova Yu.E., Gankovskaya L.V., Teselkin Yu.O.
Materials and methods: 75 women aged 18–42 years were tested. They were stratified into 3 groups: group 1 – healthy nonpregnant women (n=25); group 2 – healthy pregnant women (n=25); group 3 – women with miscarriage associated with infection (n=25). In all women, the term of pregnancy was the first trimester of gestation (5–12 weeks). The functional activity of polymorphonuclear leukocytes in peripheral blood was determined by luminol-dependent chemiluminescence.
Results: It was found that the production of reactive oxygen species by polymorphonuclear leukocytes stimulated with zymosan and lipopolysaccharide increased from 5 to 100 µg/mL, and the production of reactive oxygen species by the cells stimulated with lipopolysaccharide decreased from 50 to 100 µg/mL compared to healthy pregnant women.
Conclusion: In women with miscarriage associated with infections and in healthy pregnant women on the background of oxidative stress in the first trimester of pregnancy, the production of reactive oxygen species by polymorphonuclear leukocytes in peripheral blood stimulated with zymosan or lipopolysaccharide, which are recognized by Toll-like receptors, may depend on the expression of these receptors on the membrane of cells.
Keywords
Currently, the role of oxidative stress in the pathogenesis of pregnancy miscarriage is being discussed in publications [1, 2]. According to some data, already in the first trimester of pregnancy the development of oxidative stress is observed in healthy pregnant women and in women with pregnancy complications [3, 4]. Phagocytes representing the cellular effectors of innate immunity can act as one of the initiators of free radical reactions in miscarriage, in particular, of infectious genesis. Activation of fagocytes [6] begins as a result of interaction of phagocytes with highly conserved structures of pathogenic microorganisms – the so-called pathogen-associated molecular patterns, which include the components of the fungal and bacterial cell wall, such as zymosan, lipopolysaccharide (LPS), peptidoglycan, lipoproteins, glycolipids and other structures [5]. The key role in recognition of these structures by phagocytes belongs to Toll-like receptors (TLRs) [7]. Pro-inflammatory factors, biologically active compounds, including reactive oxygen species (ROS) are secreted, when TLRs interact with pathogen-associated molecular patterns (PAMPs) of microorganisms [8, 9]. Under pathological conditions, ROS produced by phagocytes can damage various maternal and embryonic tissues and organs, disrupt the processes of implantation and placentation [1], and possibly resulting in miscarriage. However, the triggering mechanisms leading to miscarriage remain poorly studied. At the same time, the first trimester of gestation is most important for research, since it determines the further course of pregnancy.
Based on the above, the aim of our research was to study the production of reactive oxygen species by peripheral blood polymorphonuclear leukocytes stimulated by TLRs in women with miscarriage of infectious genesis in early pregnancy versus healthy pregnant women.
Material and methods
The study included 75 women aged 1842 years (the average age was 30.2 (5.2) years), who underwent examination in the clinic of Medical Faculty of N.I. Pirogov Russian National Research Medical University of the Ministry of Health of Russia. Before examination, all patients have signed informed consent for diagnostic manipulations.
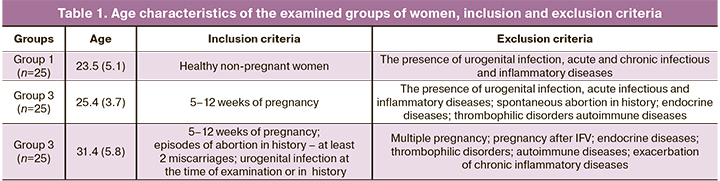
All women were stratified into 3 groups: group 1 – healthy non-pregnant women (n=25); group 2 – healthy pregnant women (n=25); group 3 – women with miscarriage of infectious genesis (n=25). Inclusion criteria in group 3 were women at 5–12 weeks of pregnancy, who had episodes of abortion in medical history – at least 2 miscarriages, and urogenital infection at the time of examination or in history. Exclusion criteria were multiple pregnancy, pregnancy after IVF, endocrine diseases, thrombophilic disorders, autoimmune diseases, exacerbation of chronic inflammatory diseases.
All women were examined in the first trimester of pregnancy (512 weeks).
Age characteristics of the examined groups, inclusion and exclusion criteria are shown in Table 1.
Among women with miscarriage of infectious genesis in group 3, urogenital infections of viral genesis (CMV, Herpes simplex virus II) and bacterial genesis (Chlamydia trachomatis, Ureaplasma urealyticum, Gardnerella vaginalis, Mycoplasma hominis) were detected by PCR. Ureaplasma urealyticum was found most often (72%). CMV and Herpes simplex virus II was found in 64 and 52% of cases, respectively.
For cellular activation, zymosan A (Sacchoromyces cerevisiae) (Sigma, США) and lipopolysaccharide (LPS) (E. Coli 0127:B8) (Sigma, USD) were used, which are recognized by TLR2 and TLR4, respectively [10, 11].
To measure ROS production, luminol-dependent chemiluminescence (LDCL) assay of peripheral blood polymorphonuclear leukocytes (PMNLs) [3] was performed using chemiluminometer Lum-5773 (LLC «DiSoft, Mocow).
LDCL intensity of peripheral blood PMNLs was quantified as the difference between the maximum intensity of induced and spontaneous chemiluminescence (CL).
Statistical analysis
The STATISTICA Software System 6.0 (Stat Soft Inc., Tulsa, OK, USA) was used for statistical analysis. The data obtained in the study followed the normal distribution. The Shapiro–Wilk W-test was used to assess the normality of distribution. The parametric Student's t-test was used to compare the mean values in the groups. The difference between the groups was considered statistically significant at p<0.05. The significance in difference between the mean values of LDCL intensity of peripheral blood PMNLs in healthy pregnant women and in women with miscarriage of infectious genesis was assessed using analysis of variance (ANOVA). The data were presented as median values (М) and standard deviation (SD) – М (SD).
Results and discussion
The first step of the research was to identify the maximal effective concentration of ligands in blood samples from healthy non-pregnant women. We studied the dependence of LDCL intensity of peripheral blood PMNLs on the concentration of the added ligand with a constant number of cells. The researchers use different doses of zymosan and LPS to study the functional activity of cells by luminol-dependent chemiluminescence assay. For example, Makni-Maalej K. et al. [12] activated neutrophils with zymosan at a concentration of 10-500 μg /mL in a reaction medium containing 5×105 cells/L and 10 μM luminol. Braga P. et al. [13] used LDCL assay to identify the functional activity of peripheral blood PNMLs at a concentration of 50 μg /mL. Wagner D.R. [14] used LPS at a concentration of 2,5 μg /mL in a reaction medium containing 5×106/mL of PNMLs and 100 μM luminol. А Remer K. et al. [15] stimulated the cells by LPS at a concentration of 4 μg /mL. The results obtained in our study are shown in Figure 1.
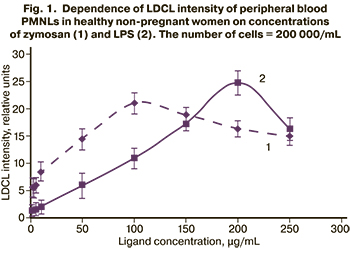
A dose-dependent change was found in LDCL intensity of peripheral blood PMNLs. The maximum intensity was observed at a concentration of zymosan equal to 100 μg/mL, and LPS = 200 μg/mL.
According to the published data [12], as well our experimental results, it is known that, when low ligand concentrations are used, the appropriate cellular response to stimulation can be skipped. Conversely, when the cells are stimulated with excessively high concentrations, this can lead to inhibition of the cellular response to stimulation. Therefore, in our study it was necessary to determine the exact concentration range of ligand, in which the cells respond to ligand stimulation. Figure 1 shows that the increase in zymosan concentration from 1 to 100 μg / mL, and LPS concentration from 1 to 200 μg /mL increased LDCL intensity of peripheral blood PMNLs in healthy non-pregnant women. A further increase in ligands concentration up to 250 μg/mL led to slightly decreased intensity of LPS. This can be explained by the absence of binding sites with cellular receptors. In case of LPS, a sharp drop in luminescence intensity was observed possibly due to the toxic effect of high ligand concentrations, and consequently due to inhibition of the cellular response to stimulation.
Based on the obtained results, zymosan and LPS at concentrations of 5, 50 and 100 μg/mL were selected to identify the difference in cellular responses in the examined groups of patients.
Further, the effects of these ligands on LDCL intensity of peripheral blood PNMLs in healthy pregnant women and in women with miscarriage of infectious genesis was studied (Fig. 2).
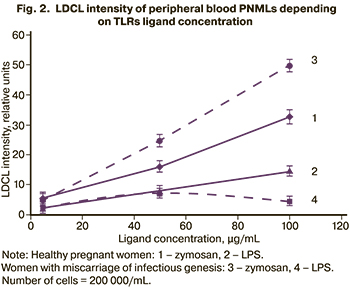
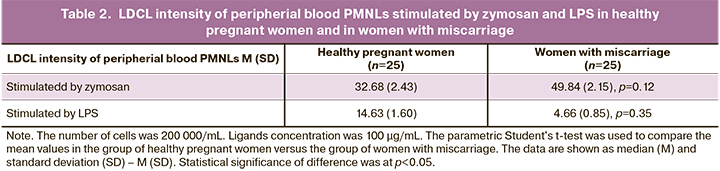
As is shown in Figure 2, LDCL intensity of peripheral blood PNMLs increased in both groups of patients with increasing zymosan concentration from 5 to 100 μg/mL. Moreover, LDCL intensity of cells stimulated by zymosan at a concentration of 100 μg/mL was higher in women with miscarriage of infectious genesis compared to healthy pregnant women (р=0,12). Quantitative data are shown in Table 2.
When the cells were stimulated by LPS with increasing ligand concentration in the studied groups of patients, a pronounced difference in cellular response was observed. An increase in LDCL intensity of peripheral blood PMNLs with increasing LPS concentrations from 5 to 100 μg/mL was observed only in healthy pregnant women. An increase in LDCL intensity of peripheral blood PMNLs was observed in women with miscarriage of infectious genesis only at LPS concentrations of 5–50 μg/mL, and further increase up to 100 μg/mL resulted in reduced LDCL intensity of peripheral blood PMNLs. At the same time, LDCL intensity of peripheral blood PMNLs stimulated by LPS at a concentration of 100 μg/mL in women with miscarriage of infectious origin was low versus healthy pregnant women (p=0.35). The pronounced inhibition of ROS production in the latter case may be associated with reduction in the expression of cell membrane receptors.
We have shown previously, that oxidative stress in the first trimester was observed in healthy pregnant women and women with complications of pregnancy. At the same time, the presence of urogenital infection in pregnant women contributes to a greater imbalance between the intensity of free radical reactions and the activity of the antioxidant system towards increased production of prooxidants and activation of free-radical reactions [3]. Some authors [16] showed a clear shift in favor of oxidative reactions and ROS production, as well as a significant decrease in the ratio of antioxidants (restoration of glutathione levels)/oxidants (oxidized glutathione) in whole blood and placental tissue in patients with miscarriage versus healthy pregnant women. Healthy women and women with miscarriage had 12.6 (2.8) weeks pregnancy length. Al-Sheikh Y.A. et al. [17] found increased levels of oxidative stress markers (malondialdehyde, superoxide anions, and hydrogen peroxide) both in plasma and placental tissues during miscarriage at the end of the first trimester and at the beginning of the second trimester versus the level of these markers in healthy pregnant women. Some researchers [18] studied the production of reactive oxygen species by blood granulocytes in the blood of women with habitual abortions (2–3 spontaneous abortions in the first trimester and missed abortions). The level of spontaneous LDCL intensity in unfractionated peripheral blood was high in this group of patients compared to the group of women with normal reproductive function. This indicated a predisposition to oxidative stress and poor cytotoxic function of granulocytes in women with recurrent miscarriage.
This study found development of oxidative stress in the first trimester in women with pregnancy complications. LDCL intensity of peripheral blood PMNLs stimulated by zymosan was higher in women with miscarriage of infectious genesis compared to healthy pregnant women.
When the cells were stimulated by LPS at a concentration of 100 μg/mL, LDCL intensity of peripheral blood PMNLs in women with miscarriage of infectious genesis was low compared to healthy pregnant women. According to literature [12] it can be assumed, that one of the reasons for reduced ROS generation by PMNLs stimulated by high concentrations of LPS in women with miscarriage of infectious genesis versus healthy pregnant women is reduced cell membrane TLR4 expression. Due to chronic infection, persistent stimulation of receptors by infectious agents, may cause inhibition of TLR4, reduced expression of this receptor, and in the subsequent stimulus with the same ligands, inhibition of cellular response and decreased ROS production is observed As a result, there is a decreased bactericidal activity of PMNLs in women with miscarriage of infectious genesis, which may be one of the mechanisms underlying miscarriage.
Yaroustovsky M. et al. [19] also discussed the fact that in the presence of infection as a result of large doses of LPS penetrating into the bloodstream, neutrophils cannot interact with immune complexes, since the receptors are occupied or cells are depleted by long-term activity with a subsequent decrease in receptor expression.
The comparative analysis of the effect of TLR2 and TLR4 ligands on ROS production showed the following difference (Table 3): in healthy pregnant women, LDCL maximum intensity value of peripheral blood PMNLs stimulated by LPS at a concentration of 100 μg/mL was lower versus stimulation by zymosan (p=0.17).
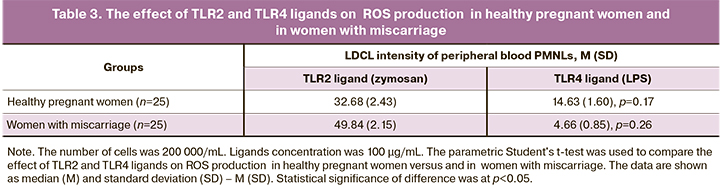
In women with miscarriage of infectious genesis, the difference was significant: LDCL intensity of peripheral blood PMNLs stimulated by LPS at a concentration of 100 μg/mL was significantly lower compared to cell stimulation by zymosan at the same concentration (p=0.26). It is not unthinkable that that TLR2-mediated ROS production can make a significant contribution to the total ROS production by phagocytes in women with miscarriage of infectious genesis.
Conclusion
The obtained results showed that in women with miscarriage of infectious genesis and in healthy pregnant women on the background of oxidative stress in the first trimester of pregnancy, ROS production by peripheral blood PMNLs stimulated by zymosan or LPS may depend on the expression of cell membrane receptors. This may be considered to be one of the mechanisms underlying miscarriage.
References
- Schoots M.H., Gordijn S.J., Scherjon S.A., van Goor H., Hillebrands J.-L. Oxidative stress in placental pathology. Placenta. 2018; 69: 153-61. https://dx.doi.org/10.1016/j.placenta.2018.03.003.
- Sultana Z., Maiti K., Aitken J., Morris J., Dedman L., Smith R. Oxidative stress, placental ageing-related pathologies and adverse pregnancy outcomes. Am. J. Reprod. Immunol. 2017; 77(5). https://dx.doi.org/10.1111/aji.12653.
- Жамбалова Б.А., Носикова И.Н., Малушенко С.В., Максина А.Г., Ганковская Л.В., Доброхотова Ю.Э., Осипов А.Н., Теселкин Ю.О. Показатели оксидативного стресса у здоровых беременных женщин и беременных женщин с урогенитальной инфекцией на ранних сроках гестации. Акушерство и гинекология. 2017; 9: 40-6. [Zhambalova B.A., Nosikova I.N., Malushenko S.V., Maksina A.G., Gankovskaya L.V., Dobrokhotova Yu.E., Osipov A.N., Tesyolkin Yu.O. Oxidative stress indicators in pregnant women, those who are healthy and those who have urogenital infection, in the early stages of gestation. Obstetrics and Gynecology. 2017; 9: 40-6. (in Russian)]. https://dx.doi.org/10.18565/aig.2017.9.40-6.
- Yiyenoglu O.B., Ugur M.G., Ozcan H.C., Can G., Ozturk E., Balat O. et al. Assessment of oxidative stress markers in recurrent pregnancy loss: a prospective study. Arch. Gynecol. Obstet. 2014; 289(6): 1337-40. https://dx.doi.org/10.1007/s00404-013-3113-4.
- Di Gioia M., Spreafico R., Springstead J.R., Mendelson M.M., Joehanes R., Levy D. et al. Endogenous oxidized phospholipids reprogram cellular metabolism and boost hyperinflammation. Nat. Immunol. 2020; 21(1): 42-53. https://dx.doi.org/10.1038/s41590-019-0539-2.
- Bosl K., Giambelluca M., Haug M., Bugge M., Espevik T., Kandasamy R.K. et al. Coactivation of TLR2 and TLR8 in primary human monocytes triggers a distinct inflammatory signaling response. Front. Physiol. 2018; 9: 618. https://dx.doi.org/10.3389/fphys.2018.00618.
- Afkham A., Eghbal-Fard S., Heydarlou H., Azizi R., Aghebati-Maleki L., Yousefi M. Toll-like receptors signaling network in pre-eclampsia: an updated review. J. Cell. Physiol. 2019; 234(3): 2229-40. https://dx.doi.org/10.1002/jcp.27189.
- Bryant A.H., Spencer-Harty S., Owens S.-E., Jones R.H., Thornton C.A. Interleukin 4 and interleukin 13 downregulate the lipopolysaccharide-mediated inflammatory response by human gestation-associated tissues. Biol. Reprod. 2017; 96(3): 576-86. https://dx.doi.org/10.1095/biolreprod.116.145680.
- Akhter N., Madhoun A., Arefanian H., Wilson A., Kochumon S., Thomas R. et al. Oxidative stress induces expression of the Toll-Like Receptors (TLRs) 2 and 4 in the human peripheral blood mononuclear cells: implications for metabolic inflammation. Cell. Physiol. Biochem. 2019; 53(1): 1-18. https://dx.doi.org/10.33594/000000117.
- Nohmi K., Tokuhara D., Tachibana D., Saito M., Sakashita Y., Nakano A. et al. Zymosan induces immune responses comparable with those of adults in monocytes, dendritic cells, and monocyte-derived dendritic cells from cord blood. J. Pediatr. 2015; 167(1): 155-62.e1-2. https://dx.doi.org/10.1016/j.jpeds.2015.03.035.
- Mazgaeen L., Gurung P. Recent advances in lipopolysaccharide recognition systems. Int. J. Mol. Sci. 2020; 21(2): 379. https://dx.doi.org/10.3390/ijms21020379.
- Makni-Maalej K., Chiandotto M., Hurtado-Nedelec M., Bedouhene S., Gougerot-Pocidalo M.A., Dang P.M. et al. Zymosan induces NADPH oxidase activation in human neutrophils by inducing the phosphorylation of p47phox and the activation of Rac2: involvement of protein tyrosine kinases, PI3Kinase, PKC, ERK1/2 and p38MAPkinase. Biochem. Pharmacol. 2013; 85(1): 92-100. https://dx.doi.org/10.1016/j.bcp.2012.10.010.
- Braga P., Sasso M., Bovio C., Sgaragli G.P. Effect of butylated hydroxyanisole and some of its derivatives on human neutrophil oxidative burst: chemiluminescence evaluation. Pharmacology. 2003; 68(1): 9-16. https://dx.doi.org/10.1159/000068726.
- Wagner D.R., Heinrich D. Influence of polyclonal immunoglobulins on the polymorphonuclear leukocyte response to lipopolysaccharide of Salmonella enteritidis as measured with luminol-enhanced chemiluminescence. Infect. Immun. 1994; 62(10): 4320-4. https://dx.doi.org/10.1128/iai.62.10.4320-4324.1994.
- Remer K., Brcic M., Jungi T.W. Toll-like receptor-4 is involved in eliciting an LPS-induced oxidative burst in neutrophils. Immunol. Lett. 2003; 85(1): 75-80. https://dx.doi.org/10.1016/s0165-2478(02)00210-9.
- Ghneim H.K., Alshebly M.M. Biochemical markers of oxidative stress in Saudi women with recurrent miscarriage. J. Korean Med. Sci. 2016; 31(1): 98-105. https://dx.doi.org/10.3346/jkms.2016.31.1.98.
- Al-Sheikh Y.A., Ghneim H.K., Alharbi A.F., Alshebly M.M., Aljaser F.S., Aboul-Soud M.A.M. Molecular and biochemical investigations of key antioxidant/oxidant molecules in Saudi patients with recurrent miscarriage. Exp. Ther. Med. 2019; 18(6): 4450-60. https://dx.doi.org/10.3892/etm.2019.8082.
- Safronova V.G., Matveeva N.K., Avkhacheva N.V., Sidelʹnikova V.M., Vanʹko L.V., Sukhikh G.T. Changes in regulation of oxidase activity of peripheral blood granulocytes in women with habitual abortions. Bull. Exp. Biol. Med. 2003; 136(3): 257-60. https://dx.doi.org/10.1023/b:bebm.0000008977.57795.69.
- Yaroustovsky M., Rogalskaya E., Plyushch M., Klimovich L., Samsonova N., Abramyan M. The level of oxidative neutrophil response when determining endotoxin activity assay: a new biomarker for defining the indications and effectiveness of intensive care in patients with sepsis. Int. J. Inflam. 2017; 2017: 3495293. https://dx.doi.org/10.1155/2017/3495293.
Received 23.04.2021
Accepted 05.06.2021
About the Authors
Bayarma A. Zhambalova, Ph.D., Department of Medical Biophysics, N.I. Pirogov Russian National Research Medical University, Ministry of Health of Russia,+7(985)413-34-27, ZhambalovaBA@inbox.ru, 117997, Russia, Moscow, Ostrovityanov str., 1.
Anatoliy N. Osipov, Dr. Bio. Sci., Professor, Corresponding Member of the RAS, Head of the Department of General and Medical Biophysics of the Faculty of Medicine and Biology; Head of the Department of Medical Biophysics, Research Institute of Translational Medicine, N.I. Pirogov Russian National Research Medical University,
Ministry of Health of Russia, +7(495)434-22-66, ex. 1410, anosipov@yahoo.com, 117997, Russia, Moscow, Ostrovityanov str., 1.
Yuriy A. Vladimirov, Dr. Bio. Sci., Academician of the RAS, Professor, Department of General and Medical Biophysics of the Faculty of Medicine and Biology, N.I. Pirogov Russian National Research Medical University, Ministry of Health of Russia, 117997, Russia, Moscow, Ostrovityanov str., 1.
Yuliya E. Dobrokhotova, Honored Doctor of the Russian Federation, Dr. Med. Sci., Professor, Head of the Department of Obstetrics and Gynecology of the Medical Faculty, N.I. Pirogov Russian National Research Medical University, Ministry of Health of Russia, 117997, Russia, Moscow, Ostrovityanov str., 1.
Lyudmila V. Gankovskaya, Dr. Med. Sci., Professor, Head of the Department of Immunology of the Faculty of Medicine and Biology, N.I. Pirogov Russian National Research Medical University, Ministry of Health of Russia, 117997, Russia, Moscow, Ostrovityanov str., 1.
Yuriy O. Teselkin, Dr. Bio. Sci., Chief Researcher of the Department of Medical Biophysics, Research Institute of Translational Medicine, N.I. Pirogov Russian National Research Medical University, Ministry of Health of Russia, 117997, Russia, Moscow, Ostrovityanov str., 1.
Corresponding author: Bayarma A. Zhambalova, ZhambalovaBA@inbox.ru
Authors’ contributions: Osipov A.N., Teselkiin Yu.O., Zhambalova B.A., Dobrokhotova Yu.E., Gankovskaya L.V. – the concept and design of the study; Zhambalova B.A., Teselkin Yu.O. – material collection and processing; writing the text of the article; Zhambalova B.A. – statistical data processing; Gankovskaya L.V., Osipov A.N., Vladimirov Yu.A. – editing the text of the article.
Conflicts of interest: The authors declare that they have no conflicts of interest.
Funding: The source of financing is N.I. Pirogov Russian National Research Medical University of the Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data (and associated images)
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Zhambalova B.A., Osipov A.N., Vladimirov Yu.A., Dobrokhotova Yu.E., Gankovskaya L.V., Teselkin Yu.O. Production of reactive oxygen species by leukocytes stimulated with zymosan and lipopolysaccharide in women with miscarriage in early pregnancy.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 10: 55-60 (in Russian)
https://dx.doi.org/10.18565/aig.2021.10.55-60



