Prediction of ART outcomes and treatment of choice in PCOS patients
Objective. To study the clinical and anamnestic factors for prediction of Assisted Reproductive Technology (ART) outcomes and determine the treatment of choice in polycystic ovary syndrome (PCOS) patients.Koloda Yu.A., Podzolkova N.M., Petrichenko Yu.G.
Materials and methods. A retrospective study included 311 patients with PCOS who underwent fertility treatment using ART. Embryo transfer was performed in stimulated cycle in 59 patients (group I). «Freeze all» approach was performed in 98 patients (group II). Frozen embryo transfer (FET) was performed in 154 patients (group III), 35 of them had PGT-A with NGS.
Results. The clinical pregnancy rate was significantly higher in FET group (70.6%) compared to the transfer in the stimulated cycle (52.5%, p=0.013). Transfer of euploid embryo resulted in highest clinical pregnancy rate (82.9%). Miscarriage rate was lowest in FET after PGT-A (OR 0.428; 95% CI 0.19–0.97). Hyperandrogenism, high AMH levels and overweight/obesity turned out to have negative effect on embryological and clinical ART outcomes.
Conclusion. FET is accompanied by a higher pregnancy rate and a lower risk of pregnancy loss in PCOS patients. PGT-A helps to maximize the effectiveness of ART programs with a minimize the risk of miscarriage.
Keywords
Endocrine infertility accounts for 40% of infertile marriage; the main cause of endocrine infertility is polycystic ovary syndrome (PCOS) [1]. The relevance of PCOS is due to both high prevalence of this disease (16–20% in the population) and large number of controversial issues related to its pathogenesis, diagnosis and treatment. According to Hart R. et al. PCOS constitutes 18–25% in the structure of infertile marriage [2]. In assisted reproductive technology (ART) programs, PCOS affects both folliculogenesis and oocyte maturation processes (prolonged stimulation, excessive or suboptimal response, a large percentage of immature oocytes, a low percentage of fertilization and blastocyst formation) and implantation processes, which may be associated with a higher frequency of endometrial pathology, concomitant insulin resistance and hypercoagulation [3, 4]. Improving the effectiveness of ART programs through the development of an individual patient management remains an urgent issue of reproductive medicine.
The aim of this study was to study clinical and anamnestic factors that affect the outcomes of ART programs in PCOS and to determine the optimal management tactics for such patients.
Materials and methods
Retrospective study included 311 patients with PCOS who applied for infertility treatment using ART programs at the Russian Medical Academy of Continuing Professional Education of the Ministry of Health of the Russian Federation from 2017 to 2019. In 59 patients (group I), the embryo transfer was performed in an induced cycle, and in 98 patients (group II), due to the risk of ovarian hyperstimulation syndrome (OHSS), the transfer was cancelled and all good-quality embryos were cryopreserved. Group III consisted of 154 patients who underwent the transfer of thawed embryos obtained earlier in previous ART programs; 35 of them underwent preimplantation genetic testing for chromosomal abnormalities (PGT-A) on 46 chromosomes by the NGS method (subgroup IIIa) in order to select an embryo for selective transfer. In the remaining patients, PGT-A was not performed (subgroup IIIb, n=120).
The criteria for inclusion in the study were the age of women from 20 to 42 years, PCOS, indications for treatment with ART methods.
The exclusion criteria were age younger than 20 years and older than 42 years, contraindications to ART treatment, severe male infertility factor (azoospermia), stage III–IV external genital endometriosis, and uterine malformations.
For ovarian stimulation from the 2nd–3rd day of the menstrual cycle, gonadotropin preparations (recombinant FSH, human menopausal gonadotropin, or a combination of these two) were used. Gonadotropin releasing hormone (GnRH) antagonists were mainly used to suppress the premature peak of LH. Recombinant chorionic gonadotropin at a dose of 250 mcg or GnRH agonists (triptorelin) at a dose of 0.2 mg were used as a trigger for the final maturation of oocytes, depending on the risk of OHSS. 35–36 hours after the introduction of the trigger of the final maturation of oocytes, transvaginal puncture of the follicles and aspiration of oocytes were performed. Fertilization was performed by IVF and / or intracytoplasmic sperm injection. The quality of the embryos was evaluated on the basis of generally accepted criteria. From the day of the puncture, micronized progesterone preparations (90 mg/day vaginal gel or 600 mg/day vaginal capsules or 30 mg/day didrogesterone) were prescribed to support the luteal phase of the cycle. Embryo transfer was performed at the blastocyst stage on the 5th day of cultivation under the control of ultrasound examination. One or two embryos were transferred. At the risk of developing OHSS (retrieval of more than 15 oocytes), the transfer in the induced cycle was cancelled and cryopreservation of all good quality embryos was performed. Preparation for the transfer of thawed embryos was performed in the natural cycle (in patients with ovulatory PCOS, phenotype D) or on the background of hormone replacement therapy. One or two embryos were transferred. To support the luteal phase of the cycle, micronized progesterone preparations were used in the form of 600 mg/day vaginal capsules or 90 mg/day gel and/or didrogesterone at a dose of 20–30 mg / day.
The following characteristics of ART protocols were evaluated: duration of ovarian stimulation, total dose of gonadotropins, the number of oocyte-cumulus complexes obtained; the number and percentage of mature (MII) oocytes, fertilization rate, the dose of gonadotropins per 1 oocyte (ovarian sensitivity index), the rate of blastocyst formation, thickness of endometrium at the time of embryo transfer, implantation rate, biochemical and clinical pregnancy rates, miscarriage and OHSS rates. In the transfer cycles of thawed embryos, the endometrial thickness, implantation, biochemical and clinical pregnancy, and miscarriage rates were also evaluated. A correlation analysis was also performed to assess the impact of clinical and anamnestic characteristics (age, body mass index (BMI), PCOS phenotype, hormonal therapy, surgery history, hormones levels) on the embryological and clinical outcomes of ART programs.
Statistical analysis
Statistical processing of the obtained data was performed using the IBM SPSS program, version 23.0. Descriptive statistics of the study results are presented for qualitative values as percentages, for absolute values – as arithmetic averages (M) and standard deviations (SD) – M(SD). In cases where there were no normal distribution of features, the median (Me) and quartiles (Q25, Q75) were used in descriptive statistics. To assess the significance of statistical differences between the study groups, the Student's t-test was used for normally distributed features, provided that their variance in the compared groups was similar according to the Lieven criterion. In other cases, the nonparametric Mann-Whitney test was used. The significance of the differences in qualitative characteristics was assessed using the Pearson criterion χ2. When the expected frequency of occurrence of the trait was 5 or less, the exact Fisher criterion was used in the "2x2" tables. The differences were considered statistically significant at p<0.05. For multiple comparisons in three observation groups, taking into account the Bonferroni correction, the level of statistical significance of differences p<0.02 was used. To determine the correlations between the variables, we used the calculation of the Pearson correlation coefficients (for quantitative normally distributed variables) and Spearman correlation coefficients (for quantitative features that do not have a normal distribution, and categorical variables).
To assess the degree of influence of factorial signs on the probability of pregnancy or its termination, we used the calculation of the odds ratio (OR). The chances for pregnancy and miscarriage after the transfer of thawed embryos were evaluated in relation to the transfers in the induced cycle, as well as separately in the group of patients with the transfer of thawed embryos, provided that PGT-A was performed. OR was considered statistically significant if both confidence interval bounds were greater than or less than 1.
Results
The initial clinical and anamnestic characteristics of the groups are presented in Table 1. The patients of group II were slightly older, the patients of group I had a higher BMI and a lower level of 25OH of vitamin D; no other differences were found between the groups.
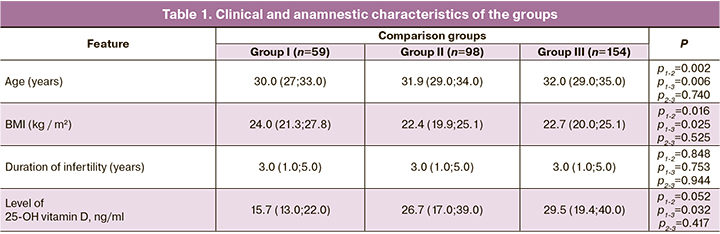
Distribution of patients in different groups depending on the PCOS phenotype is presented in table 2
In group I, phenotype B (ovulatory dysfunction in combination with clinical and/or biochemical hyperandrogenism without polycystic morphology) was significantly more common, which led to a smaller number of oocytes, a lower risk of OHSS, and allowed the transfer in an induced cycle.
The parameters of ovarian stimulation are presented in Table 3.
The total dose of gonadotropins and the duration of stimulation did not differ between group I and II, but the total number of oocytes obtained was higher in group II compared to group I, and this determined the management tactics. Fertilization rate was higher in group I (73.6% vs. 64.4%, p=0.014), while the rate of blastocyst formation was comparable in both groups (60.05% and 65.81%, p=0.16). The dose of gonadotropins per 1 oocyte was significantly higher in group I (137.5 (95.19; 277.40) versus 115.0 (70.23; 177.67), p=0.014. In group III, ovarian stimulation was not performed.
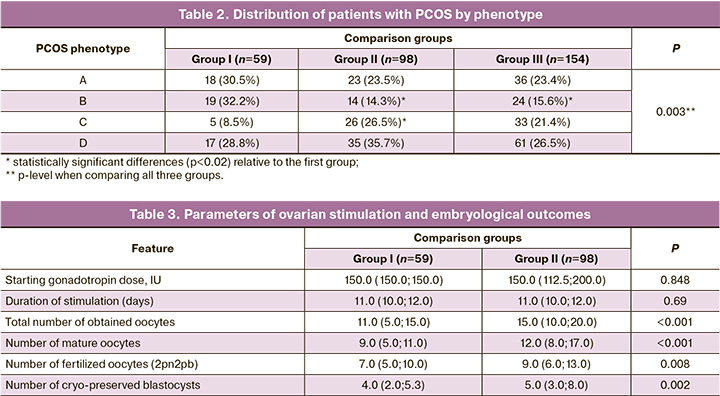
Selective single embryo transfer was performed in 67.8% of group I patients and in 69.7% of group III patients (p>0.05). In group II, embryo transfer was not performed. Due to hypercoagulation disorders during embryo transfer in the induced cycle, anticoagulant therapy had to be added much more often, compared with the transfer of unfrozen embryos (in 61% and 38.7%, respectively, p=0.12). No cases of OHSS were observed during the transfer in the induced cycle. During cryopreservation of all embryos, due to the use of a GnRH agonist as an ovulation trigger, OHSS was also avoided.
The implantation rate in group I was 37.2%, and in group III – 50.7%, p<0.05. In subgroup IIIa (after PGT-A), the implantation rate was the highest – 63.4%. The frequency of clinical pregnancy was significantly higher in group III (70.6%), compared to group I (52.5%, p=0.013). In group II, embryo transfer was not performed. The OR for the onset of pregnancy during the transfer of thawed embryos, compared with the transfer in the induced cycle, was 2.168 (95% CI 1.168–4.022). Subgroup analysis showed that after performing PGT-A and euploid embryo transfer, the frequency of clinical pregnancy was 82.9%, and without PGT-A – 66.9% (p=0.07). The OR for the onset of clinical pregnancy after PGT-A was 2.386 (95% CI 0.91–6.23).
There was a positive significant correlation between the level of LH and AMH (r=0.188; p=0.035); the level of LH and testosterone (r=0.213, p=0.017); age and dose of gonadotropins (r=0.253, p=0.001); percentage of mature oocytes and the fertilization rate (r=0.607, p<0.001); fertilization rate and time of the introduction of the trigger before the puncture of follicles (r=0.181, p=0.023); ovarian surgery history and duration of infertility (r=0.205, p=0.010); percentage of blastocyst formation and the clinical pregnancy rate (r=0.284; p=0.031); endometrium thickness on the day of embryo transfer and implantation rate (r=0.275, p=0.035); as well as the BMI and miscarriage rate after thawed embryo transfer (r=0.203, p=0.011). Also a negative significant correlation between the concentration of testosterone and 25HE vitamin D (r=-0.419, p=0.012); past ovarian surgery and LH levels (r=-0.210, p=0.018); the level of 17-Oh-progesterone and frequency of blastocysts formation (r=-0.224, p=0.013); the level of AMH and pregnancy rate in the induced cycle (r=-0.315, p=0.015) were detected. No association was found between the use of combined oral contraceptives or progestogens prior to stimulation and the embryological and clinical outcomes of ART programs in patients with PCOS.
When transferring thawed embryos, the risk of miscarriage was lower than when transferring in the induced cycle (OR 0.381, 95% CI 0.20–0.72). The probability of miscarriage during PGT-A decreased by 2 times (OR 0.428; 95% CI 0.19–0.97).
Discussion
The effectiveness of ART programs is determined both by the initial clinical and anamnestic data of patients (age, BMIpast surgeries, hormone levels, etc.), and by the features of ovarian stimulation in the ART program. Transfer tactics (in the induced cycle, or cryopreservation of all obtained embryos and delayed transfer) can also be of great importance. The effectiveness and indications for PGT-A in ART programs is still discussed.
We conducted a correlation analysis in order to identify predictive factors of embryological and clinical outcomes in ART programs for PCOS, to assess the impact of different approaches, and to choose the most optimal tactics.
We found no negative correlation between age and the effectiveness of ART programs. The number of patients of older reproductive age (35 years and older) in group I was only 17%; the most advanced age was 38 years. In 2016, an age-dependent study of the effectiveness of ART programs in PCOS was conducted [5]. Treatment outcomes were evaluated in different age groups (30–32 years; 33–35 years; 36–38 years; 39–41 years). The effectiveness of ART programs in PCOS was higher than in tubal-peritoneal factor at the age of 33–35 years (41.3% vs. 28.6%, p=0.038) and at the age of 36–38 years (40.4% vs. 15.1%, p=0.002). The authors concluded that, despite the fact that in general population, the effectiveness of ART programs decreases as early as 35 years, in PCOS it remains high up to and including 38 years. In a 2019 study involving 3.502 patients with PCOS and 18,596 women with tubal-peritoneal factor infertility, a slower age-related decline in the effectiveness of ART programs in PCOS was also demonstrated. Even in patients over 40 years of age, there were fairly high rates of implantation (27.8% vs. 15.7%, p<0.05), clinical pregnancy (51.4% vs. 26.1%, p<0.05), live birth rate (42.3% vs. 18.2%, p<0.05), and cumulative live birth rate (50.0% vs. 21.5%, p<0.05) [6]. We faced the need to use higher doses of gonadotropins in patients with PCOS of more advanced reproductive age, which may be due to a decrease in ovarian reserve and a lower risk of OHSS in these patients.
When receiving a large number of mature oocytes, the fertilization rate remained high, and, with an increase in the time from trigger management to follicle puncture, more mature oocytes were obtained. This may be due to the fact that GnRH agonists were used as the ovulation trigger in the vast majority of cycles. It has been shown that in empty follicle syndrome, an increase in the time from the introduction of a GnRH agonist to 40 hours, as well as the use of a double trigger, increases the number of oocytes obtained during puncture [7]. According to our data, the high frequency of blastocyst formation can be considered a positive prognostic factor for the onset of clinical pregnancy in patients with PCOS.
We noted an adverse effect of hyperandrogenism on the frequency of blastocyst formation and implantation, as well as an increased risk of miscarriage in such patients during embryo transfer in the induced cycle, which is also confirmed by other authors. Thus, Yang in 2018 showed that patients with PCOS and hyperandrogenism had a bigger number of retrieved oocytes, total dose of gonadotropins was smaller, but the incidence of miscarriage was higher and live birth rates were lower than in patients with PCOS without hyperandrogenism in women with tubal-peritoneal factor infertility [8]. In such situations, the authors recommend cancelling the transfer in the induced cycle, cryopreserving all the resulting embryos, and performing a delayed embryo transfer to avoid the negative impact of hyperandrogenism on the outcomes of ART programs. Previous studies have also demonstrated a negative effect of hyperandrogenism on follicular growth and embryogenesis [9].
Comparative evaluation of the effectiveness of ART programs in embryo transfer in induced cycles and during the transfer of thawed embryos was studied in a multicenter randomized placebo-controlled study involving 1508 patients with PCOS [10]. It was shown that the live birth rate after the transfer of thawed embryos was significantly higher than after the transfer of an embryo in the induced cycle (49.3% vs. 42.0%, p=0.004); the risk of OHSS significantly decreased (1.3% vs. 7.1%, p<0.001), as well as the miscarriage rate (22.0% vs. 32.7%, p<0.001). A systematic review and meta-analysis in 2019 confirmed a higher rate of live births during the transfer of unfrozen embryos, compared to the transfer in the induced cycle in patients with an excessive response to ovarian stimulation [11].
Recently, the indications for PGT-A in a non-selective population of patients with infertility and in certain groups of patients have been discussed [12]. The feasibility of performing PGT-A in PCOS is due not only to the need to select the most promising one from the many embryos obtained, which will shorten the awaiting pregnancy interval, but also to data on the possible higher risk of chromosomal abnormalities in the fetus. Thus, the study of chorionic villi after aborted pregnancies in ART programs showed that in PCOS chromosomal abnormalities were more common (61.3% vs. 47.8%) [13]. Univariate and multivariate analyses have shown that PCOS is an independent risk factor for chromosomal abnormalities in an embryo/fetus. At the same time, other studies have shown that the levels of testosterone and dehydroepiandrosterone sulfate in serum do not affect the ploidy of embryos in ART programs [14]. The study of the role of PGT-A in ART programs in young patients with PCOS requires further study. According to our data, this approach provides the highest possible rate of clinical pregnancy and reduces the risk of miscarriage. However, there is an evidence [15] that even after euploid embryo transfer, the risk of early fetal loss in PCOS and normal body weight is higher than in patients without PCOS, suggesting the presence of additional risk factors for miscarriage. In PCOS and obesity, the risks of miscarriage after the transfer of thawed embryos increase, which is confirmed by our data and the results of other studies [16].
Receptivity of endometrium plays an important role in the transfer of euploid embryos. When analyzing the expression of endometrial genes in patients with PCOS, progesterone resistance genes, which may hinder endometrial transformation and embryo implantation were identified [17]. We observed the increased possibility of implantation with increasing thickness of the endometrium; however, in case of thickness 15 mm or more, the transfer was cancelled, and histological evaluation was performed to rule out endometrial hyperplastic processes. Data on possible progesterone resistance in PCOS require a review of approaches to progestagen support. The issues of the most optimal luteal support in the transfer cycles of thawed embryos and the role of progesterone level assessment are the subject for discussion [18].
Conclusion
The risk factors for adverse embryological and clinical outcomes in ART programs for PCOS are hyperandrogenism, high AMH levels, and overweight/ obesity. Late reproductive age, surgical interventions on the ovaries in the anamnesis, and phenotype B in patients with PCOS in ART programs are accompanied by a lower number of oocytes, a lower risk of OHSS, and allow for transfer in an induced cycle. However, the transfer of thawed embryos is accompanied by a higher pregnancy rate and a lower risk of miscarriage, compared to a fresh transfer in the induced cycle. Performing PGT-A in PCOS allows to achieve maximum effectiveness of ART programs with minimal risk of miscarriage.
References
- Mohammad M.B., Seghinsara A.M. Polycystic ovary syndrome (PCOS), diagnostic criteria, and AMH. Asian Pac. J. Cancer Prev. 2017; 18(1): 17-21. https://dx.doi.org/10.22034/APJCP.2017.18.1.17.
- Hart R., Doherty D.A. The potential implications of a PCOS diagnosis on a woman’s long-term health using data linkage. J. Clin. Endocrinol. Metab. 2015; 100(3): 911-9. https://dx.doi.org/10.1210/jc.2014-3886.
- Thakre N., Homburg R. A review of IVF in PCOS patients at risk of ovarian hyperstimulation syndrome. Expert Rev. Endocrinol. Metab. 2019; 14(5): 315-9. https://dx.doi.org/10.1080/17446651.2019.1631797.
- Jabara S., Coutifaris C. In vitro fertilization in the PCOS patient: clinical considerations. Semin. Reprod. Med. 2003; 21(3): 317-24. https://dx.doi.org/10.1055/s-2003-43310.
- Hwang Y.I., Cha S.W., Song I.O., Yang K.M., Min E.G., Kim H.O. Fertility of patients with polycystic ovary syndrome undergoing in vitro fertilization by age. Int. J. Gynaecol. Obstet. 2016; 135(1): 91-5. https://dx.doi.org/10.1016/j.ijgo.2016.03.033.
- Li J., Liu X., Hu L., Zhang F., Wang F., Kong H. et al. A slower age-related decline in treatment outcomes after the first ovarian stimulation for in vitro fertilization in women with polycystic ovary syndrome. Front. Endocrinol. (Lausanne). 2019; 10: 834. https://dx.doi.org/10.3389/fendo.2019.00834.
- Orvieto R. Triggering final follicular maturation - hCG, GnRH-agonist or both, when and to whom? J. Ovarian Res. 2015; 8: 60. https://dx.doi.org/10.1186/s13048-015-0187-6.
- Yang W., Yang R., Yang S., Li J., Tu B., Gao C. et al. Infertile polycystic ovary syndrome patients undergoing in vitro fertilization with the gonadotropin-releasing hormone-antagonist protocol: role of hyperandrogenism. Gynecol. Endocrinol. 2018; 34(8): 715-8. https://dx.doi.org/10.1080/09513590.2018.1431773.
- Qiao J., Feng H.L. Extra- and intra-ovarian factors in polycystic ovary syndrome: impact on oocyte maturation and embryo developmental competence. Hum. Reprod. Update. 2011; 17(1): 17-33. https://dx.doi.org/10.1093/humupd/dmq032.
- Chen Z.J., Shi Y., Sun Y., Zhang B., Liang X., Cao Y. et al. Fresh versus frozen embryos for infertility in the polycystic ovary syndrome. N. Engl. J. Med. 2016; 375(6): 523-33. https://dx.doi.org/10.1056/NEJMoa1513873.
- Roque M., Haahr T., Geber S., Esteves S.C., Humaidan P. Fresh versus elective frozen embryo transfer in IVF/ICSI cycles: a systematic review and meta-analysis of reproductive outcomes. Hum. Reprod. Update. 2019; 25(1): 2-14. https://dx.doi.org/10.1093/humupd/dmy033.
- Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. The use of preimplantation genetic testing for aneuploidy (PGT-A): a committee opinion. Fertil. Steril. 2018; 109(3): 429-36. https://dx.doi.org/10.1016/j.fertnstert.2018.01.002.
- Li Y., Wang L., Xu J., Niu W., Shi H., Hu L. et al. Higher chromosomal aberration rate in miscarried conceptus from polycystic ovary syndrome women undergoing assisted reproductive treatment. Fertil. Steril. 2019; 111(5): 936-43.e2. https://dx.doi.org/10.1016/j.fertnstert.2019.01.026.
- Kunicki M., Skowrońska P., Pastuszek E., Jakiel G., Smolarczyk R., Łukaszuk K. Do serum androgens influence blastocysts ploidy in karyotypically normal women? Syst. Biol. Reprod. Med. 2019; 65(4): 281-7. https://dx.doi.org/10.1080/19396368.2019.1601295.
- Luo L., Gu F., Jie H., Ding C., Zhao Q., Wang Q. et al. Early miscarriage rate in lean polycystic ovary syndrome women after euploid embryo transfer - a matched-pair study. Reprod. Biomed. Online. 2017; 35(5): 576-82. https://dx.doi.org/ 10.1016/j.rbmo.2017.07.010.
- Qiu M., Tao Y., Kuang Y., Wang Y. Effect of body mass index on pregnancy outcomes with the freeze-all strategy in women with polycystic ovarian syndrome. Fertil. Steril. 2019; 112(6): 1172-9. https://dx.doi.org/10.1016/j.fertnstert.2019.08.009.
- Savaris R.F., Groll J.M., Young S.L., DeMayo F.J., Jeong J.W., Hamilton A.E. et al. Progesterone resistance in PCOS endometrium: a microarray analysis in clomiphene citrate-treated and artificial menstrual cycles. J. Clin. Endocrinol. Metab. 2011; 96(6): 1737-46. https://dx.doi.org/10.1210/jc.2010-2600.
- Bosch E., De Vos M., Humaidan P. The future of cryopreservation in assisted reproductive technologies. Front. Endocrinol. (Lausanne). 2020; 11: 67. https://dx.doi.org/10.3389/fendo.2020.00067.
Received 12.08.2020
Accepted 09.11.2020
About the Authors
Yulia А. Koloda, Russian Medical Academy of Postgraduate Education, Ministry of Health of Russia. E-mail: julkol@yandex.ru. ORCID: 0000-0003-2502-575X.125993, Russia, Moscow, Barrikadnaya str., 2/1-1.
Natalia М. Podzolkova, Russian Medical Academy of Postgraduate Education, Ministry of Health of Russia. Tel.: +7(499)748-15-30. ORCID: 0000-0001-8991-1369.
125993, Russia, Moscow, Barrikadnaya str., 2/1-1.
Yulia G. Petrichenko, Russian Medical Academy of Postgraduate Education, Ministry of Health of Russia. ORCID: 0000-0002-5488-5460.
125993, Russia, Moscow, Barrikadnaya str., 2/1-1.
For citation: Koloda Yu.A., Podzolkova N.M., Petrichenko Yu.G. Prediction of ART outcomes and treatment of choice in PCOS patients.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2021; 2: 84-89 (in Russian)
https://dx.doi.org/10.18565/aig.2021.2.84-89



