Peripheral blood NK-cells in women with unsuccessful attempts of assisted reproduction: quantity, subpopulation composition and activation markers
Objective: To study the subpopulation composition of lymphocytes, quantity, subpopulation composition and activation markers of peripheral blood natural killer cells (pbNK cells) in women with unsuccessful attempts of assisted reproductive technologies (ART). Materials and methods: The main group consisted of 60 women with the previous history of one or more unsuccessful ART attempts who were subdivided into groups as patients with primary and secondary infertility. The patients with secondary infertility were divided into those who had a history of miscarriage and those who did not have it. The control group included 15 healthy fertile women. The study was carried out in the 2nd phase of the menstrual cycle (day 19–22). Subpopulation composition of peripheral blood lymphocytes, subpopulation composition of pbNK cells, their activated forms expressing receptors CD107a and NKG2D were determined using flow cytofluorimetric method. Results: The patients of the main group in comparison with the control group demonstrated a decline in the relative number of B-lymphocytes (p<0.05), number of CD16+CD107a+ pbNK cells (p<0.01) and lower CD107a receptor expression (MFI) (p<0.01). The patients of the main group showed an inverse correlation between the number of pregnancies and the absolute pbNK cell count (rs=-0.55; p<0.01), as well as a direct correlation with MFI CD16+CD107a+ pbNK cells (rs=0.41; p<0.01). The threshold values associated with the absence of pregnancy were more than 0.312 k/μL for pbNK cells. The patients of the main group with primary infertility in comparison with those with secondary infertility had a decrease in the relative number of T-helpers (p<0.05), an increase in the absolute number of pbNK cells, as well as the number of NKG2D+CD56dimCD16bright and NKG2D+CD56brightCD16dim of pbNK cells (p<0.05). The patients who had secondary infertility and a history of miscarriage showed a decrease in the number of CD56dimCD16bright and CD56brightCD16dim of pbNK cells (p<0.05) and an increase in the number of CD56dimCD16dim of pbNK cells (p<0.05) compared to the patients who had no history of miscarriage. Conclusion: Depending on primary or secondary infertility as well as the presence of miscarriage in the patient’s history, the changes in pbNK cell parameters of the patients with unsuccessful ART attempts may reflect the immunological characteristics of the impaired mechanisms of implantation or termination of pregnancy in the early stages.Zagaynova V.A., Kogan I.Yu., Selkov S.A., Bespalova O.N., Krikheli I.O., Mikhailova V.A., Davydova A.A., Milyutina Yu.P., Sokolov D.I.
Keywords
Nowadays, the cause of infertility cannot be identified in 20–30% of infertile couples using generally accepted methods of examination, and when repeated episodes of reproductive failures occur, this indicator increases to 50% [1, 2]. In this regard, the study of the immunological causes of reproductive losses is of great scientific and practical interest as these causes play a significant role in the pregnancy failure, as well as in the termination of pregnancy in this cohort of patients.
Various examination methods are used to assess immunological abnormalities in reproductive disorders. The most accessible material for the analysis is peripheral blood. It is possible to determine the subpopulation composition of lymphocytes, the level of regulatory T-lymphocytes (Tregs), autoantibodies, cytokines, the number and activity of natural killer cells of peripheral blood (pbNK cells) and other parameters.
On the basis of the assessment of the surface receptor profile of pbNK cells, their subpopulation affiliation and functional activity can be determined [3]. The main subpopulation of pbNK cells with the CD56dimCD16bright phenotype (=90% of the total number of pbNK cells) is described as cytotoxic. A minor subpopulation of pbNK cells with the CD56brightCD16dim phenotype (=10% of the total number of pbNK cells) has a regulatory function. The cytotoxic activity of the CD56dimCD16bright pbNK cell subpopulation is due to the content of a large number of granules with perforin, granzymes and other cytolytic substances, rapid contact with target cells [4]. CD56brightCD16dim pbNK cells express chemokine and adhesion receptors that contribute to their transendothelial migration from the bloodstream into tissues, including the organs of the reproductive system, the endometrium and the decidual membrane [5]. The cells of the regulatory subpopulation actively produce cytokines, such as interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), granulocyte colony-stimulating factor (G-CSF), interleukin-10 and interleukin-13 (IL-10, IL-13); they affect the functions of immune cells and contribute to the formation of adaptive immune response [6]. Although CD56bright pbNK cells have low cytotoxicity, this effect is enhanced in inflammatory conditions after their activation [7].
The role of NK cells in infertility and habitual miscarriage remains debatable and understudied [8–10]. According to the results of meta-analysis, the number of pbNK cells in patients with these conditions is higher than in healthy women [11], however, some studies demonstrated a decrease or even the absence of differences in their level [12, 13]. Some authors report the prognostic significance of the number and activity of pbNK cells for assessing the outcomes of pregnancies that occurred after in vitro fertilization (IVF) or were spontaneous, but the data remain contradictory [14, 15].
Changes in the expression of various groups of pbNK cell receptors were shown in habitual miscarriage, spontaneous miscarriages, and infertility [16, 17]. One of the main receptors characterizing the activation of NK cells is the transmembrane lectin-like receptor NKG2D. Another marker reflecting the cytotoxic activity of pbNK cells is the expression of glycoprotein CD107a, which appears on the plasma membrane during the fusion of the lysosomal and cytoplasmic cell membranes during degranulation [18].
In recent years, the assessment of the functional activity of pbNK cells, as well as the number, has become particularly important. Variable changes in the receptor profile and cytotoxicity of pbNK cells in reproductive losses are shown. Thus, there is a change in the subpopulation composition towards the predominance of the cytotoxic phenotype in case of habitual miscarriage [14]. According to a number of authors, infertile patients have a lower expression of activating receptors on the surface of pbNK cells than healthy women; no similar differences were found in other studies [15, 16]. At the same time, comprehensive data on the subpopulation composition of lymphocytes and pbNK cells, their functional activity in infertile patients and IVF failures are few.
There is a lack of data on the role of Treg in repeated implantation failures in assisted reproductive technology (ART). One of the immunoregulatory functions of Treg is the regulation of the phenotype and activity of endometrial NK cells [19, 20]. A decrease in the amount of Treg is associated with miscarriage and a number of obstetric complications, such as preeclampsia and fetal growth retardation syndrome [21].
The aim of the study was to determine the quantity, subpopulation composition and phenotypic markers of pbNK cell activation, as well as subpopulation composition of lymphocytes in peripheral blood in women with a previous history of failed ART attempts.
Materials and methods
The main group consisted of 60 women with the previous history of one or more unsuccessful ART attempts. There were the following inclusion criteria: one or more ineffective ART attempts with the transfer of good quality embryos (≥3ВВ according to the classification of Gardner D.K., 1999) in the history, normal karyotype of partners. The patients of the main group were divided into subgroups with primary (Infertility I, n=25) and secondary infertility (Infertility II, n=35). The patients with secondary infertility were subdivided into groups of patients who had a miscarriage and those who did not have one (miscarriage (+) and miscarriage (-)). The control group consisted of 15 healthy fertile women.
There were the following criteria for inclusion in the control group: one or more term births in the history, absence of reproductive losses, regular menstrual cycle. There were the following exclusion criteria for all groups: age less than 20 or more than 40 years, body mass index (BMI)≥30 kg/m2, genital abnormalities, uterine fibroids (type 0–3, FIGO 2011, node diameter ≥3 cm), external genital endometriosis, II–IV degree hyperplasia, endometrial polyp, thin endometrium (M-echo≤7 mm), diabetes mellitus, infectious diseases, administration of antibacterial, antiviral drugs, as well as hormonal contraception less than 3 months before the study. The study was approved by the local Ethics Committee of D.O. Ott Research Institute of Obstetrics, Gynecology and Reproduction (protocol No. 100, dated 19.12.2019). The informed consent was obtained from all the patients who participated in the study, they also provided an informed consent for the publication of their data.
Peripheral blood samples were obtained from the patients in phase II of the menstrual cycle (on days 19–22) on an empty stomach; blood was collected in test tubes with the anticoagulant EDTA-2Na.
Identification of the subpopulation composition of peripheral blood lymphocytes
To determine the subpopulation composition of peripheral blood lymphocytes, a commercial Multitest 6-Color TBNK kit (BD, USA) was used. The percentage and absolute content of T-lymphocytes (CD3+), B-lymphocytes (CD3+CD19+), T-helper cells (CD3+CD4+), cytotoxic T-lymphocytes (T-killers, CD3+CD8+), pbNK cells (CD56+CD16+) were determined. The study was conducted using flow cytometry (FACSCanto II, BD, USA).
Identification of expression of CD107a as an activation marker by pbNK cells
Peripheral blood mononuclear cells were isolated by Ficoll density gradient (ρ=1.077 g/µl, Sigma, USA) using the standard method. Then part of the cells was incubated in a culture medium RPMI-1640 (Sigma, USA) with the addition of a standard reagent for activating leukocytes containing a mixture of phorbol-myristate acetate and ionomycin (BD, USA) for 3 hours at 37°С with 5% CO2 content, the second part of the cells were incubated without the addition of an activator. After incubation, the cells were washed with Hanks solution (Biolot, Russia) and treated with monoclonal antibodies to CD16, CD56, CD3, CD45, CD107a (BD, USA) following the manufacturer’s recommendations. The study was conducted using flow cytometry (FACSCanto II, BD, USA). The following parameters were determined: the number of spontaneously and induced activated pbNK cells, the number of CD16+CD107a+ and CD16-CD107a+ pbNK cells.
Identification of subpopulations of pbNK cells and their expression of the NKG2D receptor
In order to divide pbNK cells into separate subpopulations in detail, we evaluated the expression of CD56 and CD16 surface receptors in the obtained blood samples. After isolating peripheral blood mononuclear cells using a standard method on Ficoll density gradient (ρ=1.077 g/µl, Sigma, USA), cells were washed with CellWash solution (BD, USA) by centrifugation at 200g, 22°C for 10 minutes. Further, the cells were treated with a reagent blocking Fc receptors (MACS, Germany) to prevent nonspecific binding of antibodies. Then the cells were treated with monoclonal antibodies to CD45, CD3, CD14, CD16, CD56, NKG2D according to the manufacturer’s recommendations. Isotypic antibodies (BD, USA) were used as a control of nonspecific binding of antibodies. Subpopulations CD56dimCD16bright, CD56dimCD16dim, CD56brightCD16dim, CD56brightCD16dim were isolated from CD45+CD3-CD14-CD56+CD16+ pbNK cells and their expression of the NKG2D receptor was evaluated. The study was conducted using flow cytometry (FACSCanto II, BD, USA).
Assessment of the amount of Treg in peripheral blood
The amount of Treg (CD3+CD4+CD25+CD127low/-) in peripheral blood was determined using a commercial kit Human Regulatory T Cell Cocktail (BD, USA) according to the manufacturer’s instructions; the study was conducted using flow cytometry (FACSCanto II, BD, USA).
Statistical analysis
Statistical processing of the results was carried out using Statistica 10 (StatSoft, Inc.) software package. The normality of the data distribution was checked using the Shapiro-Wilk test. The nonparametric Mann–Whitney U test was used for paired comparison of the parameters. The Kruskal–Wallis test and the Bonferroni correction were used for multiple comparisons. In this case, the maximum number of compared samples was 3, and therefore, the critical level was statistically significant; it was calculated using the formula m=n(n-1)/2, where n is the number of samples. Continuous variables are presented as medians and interquartile interval Me (Q1;Q3). Correlation analysis was performed using the estimation of Spearman’s rank correlation (rs). Linear regression was used to analyze the relationship between variables; verification of basic assumptions (analysis of variance and normality of the distribution of the residuals) was performed using Statistica 10 and SPSS software packages. The normality of the distribution of the residuals was analyzed using the Shapiro–Wilk test of standardized residuals. The outlier analysis was performed by estimating standardized predicted values, standardized residuals, and Cook’s distance. The significance of the coefficient of determination is verified using the F-criterion. When comparing the parameters measured in the nominal scale, the Pearson χ2 test was used. The value of p<0.05 was accepted as statistically significant.
Results
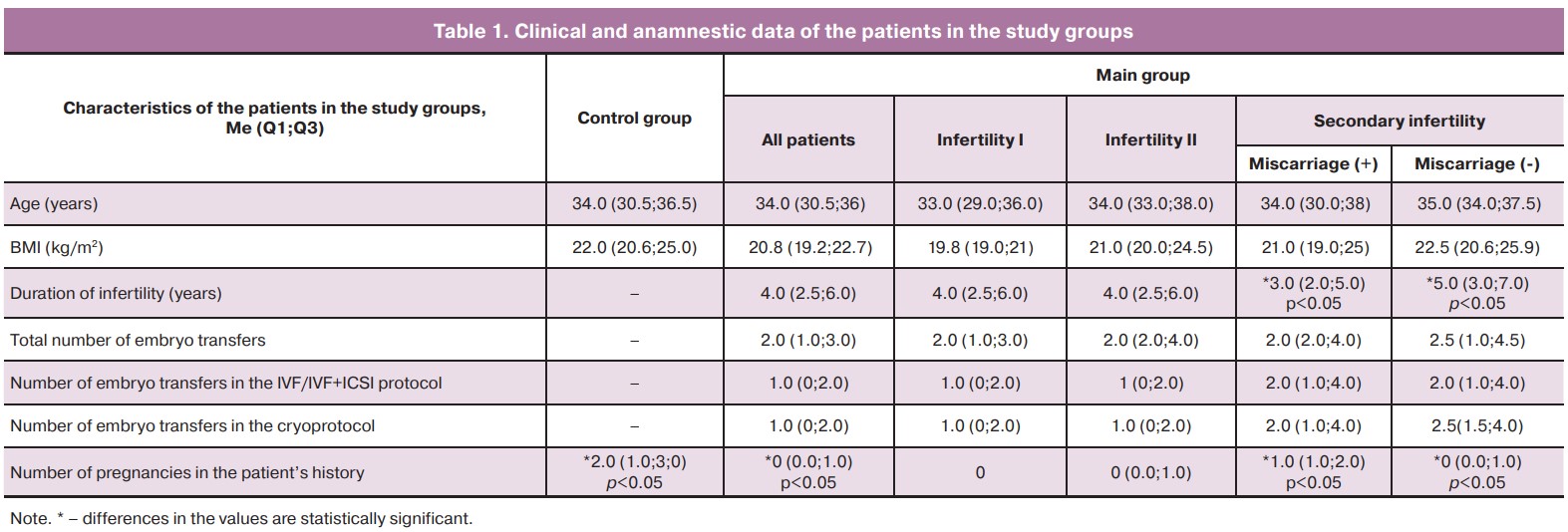
The patients of the study groups were comparable in age and body mass index (BMI). The duration of infertility in the women of the main group varied from 1 to 13 years (median 4.0 (2.5;6.0)); it did not differ between the subgroups of women with primary and secondary infertility, but it was less in patients with a history of miscarriage in comparison with the patients who had no history of miscarriage (p<0.05, Table 1).
The number of ART protocols, as well as embryo transfers in the IVF protocol or in the cryoprotocol, were comparable between the subgroups of the patients (Table 1). At the same time, single embryo transfer was observed in 21.7% (n=13) of patients, double embryo transfer was noted in 33.3% (n=20) and more than three attempts of embryo transfer were made in 45% (n=27) of cases. The number of pregnancies in the patient’s history was statistically significantly higher in the patients of the control group than in the patients of the main group (p<0.05); the same result was observed in patients with the previous history of miscarriage in comparison with ones who had no miscarriage previously (p<0.05).
Infertility was caused by various factors in the patients of the main group: tuboperitoneal in 28.3% (n=17) of women, anovulation in 18.3% (n=11), male factor in 13.3% (n=8), combined forms of infertility in 18.3% (n=11), unexplained infertility in 15% (n=9) of cases, endometriosis-associated infertility in 6.7% (n=4) of patients. Etiology of infertility did not differ between the subgroups of the patients who took part in the study.
When assessing different concomitant gynecological diseases in the patients of the main group, we revealed a higher incidence of pelvic inflammatory diseases, extragenital endometriosis and uterine fibroids in comparison with the patients of the control group (70, 15, 18% and 0, 0, 6.6%, respectively, p<0.05). When comparing subgroups of women with primary and secondary infertility, as well as the patients with secondary infertility and a history of miscarriage or its absence, we did not find any statistically significant differences.
The frequency of pelvic surgery was statistically significantly lower in the control group compared to the patients of the main group and amounted to 20 and 75%, respectively (p<0.05). Three patients of the control group had a history of cesarean section. The patients of the main group had the following interventions: pelvic laparotomy was performed in 13.3% of patients, laparoscopic surgery in 51.6% of cases, hysteroscopy in 58.3% of women. The frequency of surgical interventions did not differ between subgroups of patients with primary and secondary infertility, as well as subgroups with secondary infertility and a history of miscarriage in comparison with the patients who did not have a miscarriage previously. Uterine curettage was performed 2.7 times more often in patients with secondary infertility in comparison with the patients suffering from primary infertility (75 and 27%, respectively, p<0.01). The frequency of this operation was also higher in the patients with secondary infertility and a history of miscarriage in comparison with the patients who had no miscarriage previously (82 and 60%, respectively, p<0.05).
The assessment of the peripheral blood lymphocyte subpopulations showed a decrease in the relative number of B-lymphocytes (CD19%) (9.2 (7.7;11.4) and 11.7 (10.2;14), p<0.05) in patients of the main group compared with the patients of the control group (Fig. 1A). There were no statistically significant differences in the pbNK cell subpopulations and their expression of the NKG2D receptor among the above groups.
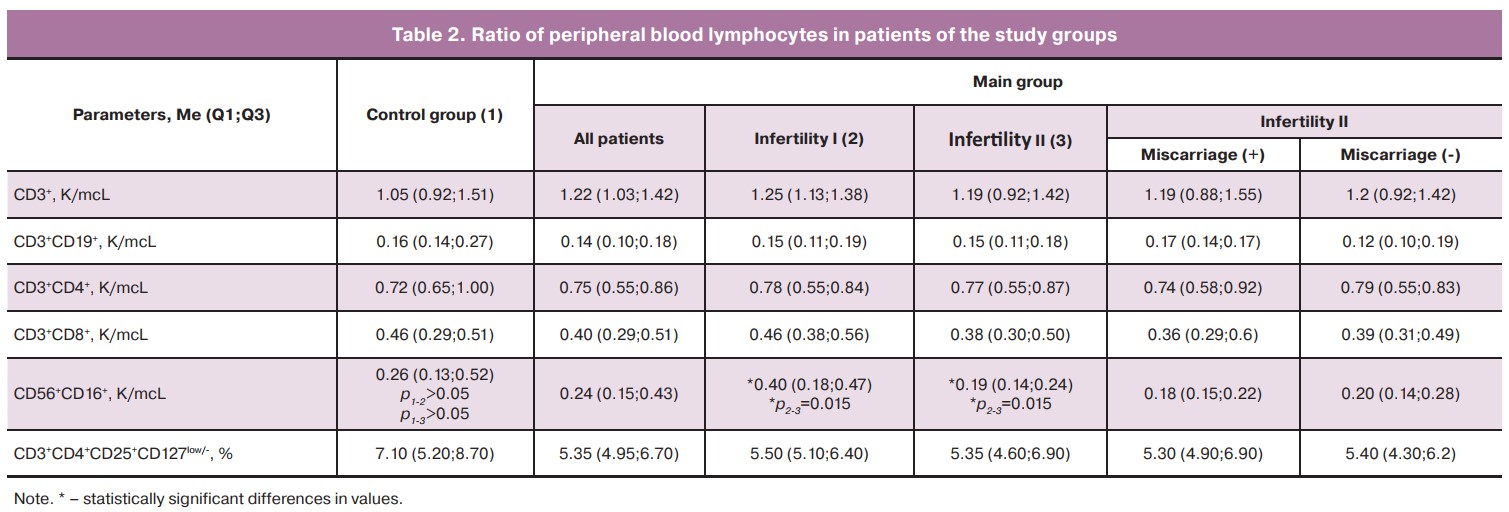
The multiple comparison of the data of women in the control group with patients with primary and secondary infertility of the main group (Fig. 1B) showed that the relative number of T-helpers (CD4%) was reduced in patients with primary infertility compared to women with secondary infertility (40.0 (38.1;44.2) and 45.5 (41.1;48.8), respectively, p<0.05), but it did not differ from the patients in the control group. The relative number of pbNK cells was higher in patients with primary infertility compared to the patients with secondary infertility (16.0 (12.7;20.1) and 11.7 (10.1;16.8)), but there was no statistical significance. However, the data on the absolute number of pbNK cells differed statistically and amounted to 0.40 (0.18;0.47) in patients with primary infertility and 0.19 (0.14; 0.24) in patients with secondary infertility, p<0.01. No other differences were found in the peripheral blood lymphocyte subpopulations, pbNK cells, and the number of Tregs (Table 2). We also revealed that the patients of the main group with primary infertility in comparison with the patients with secondary infertility and the control group had an increased number of pbNK cells with phenotype NKG2D+CD56dimCD16bright (37.0 (19.4;48.1), 17.1 (4.2;39.7) and 17.3 (5.3;39.9) respectively, p<0.01) и NKG2D+ CD56brightCD16dim (42.4 (23.0;64.7), 29.4 (11.7;40.7) and 27.5 (11.4;45.1) respectively, p<0.05), (Fig. 1B). The number of pbNK cells with phenotype NKG2D+CD56dimCD16dim did not differ between the compared groups.
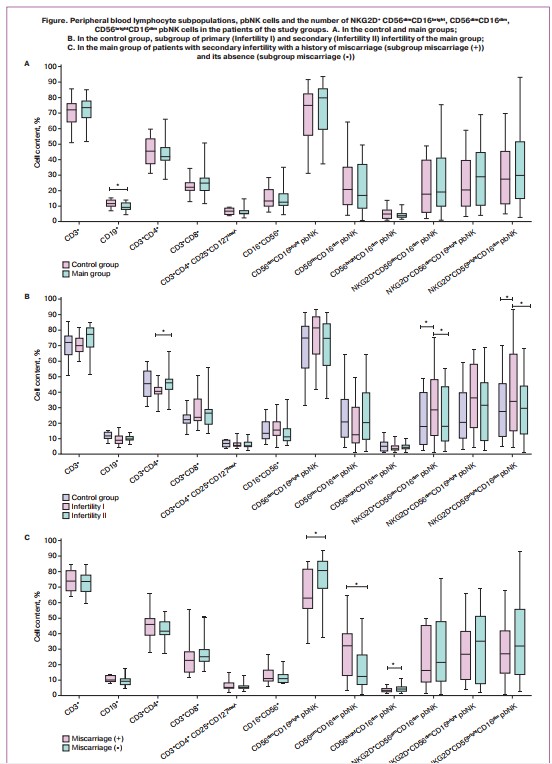
There were the following changes in the patient with a previous history of miscarriage compared to the patient without it: reduced number of CD56dimCD16bright (62.8 (57.2;80.0) and 82.9 (74.1;87.9), p<0.05) and CD56brightCD16dim (3.4(2.7;4.3) and 5.1(3.4;6.0), p<0.05) of pbNK cells, increased number of CD56dimCD16dim (33.4(16.4;39.6) and 10.1 (8.2;20.3), p<0.05) of pbNK cells (Fig. 1C).
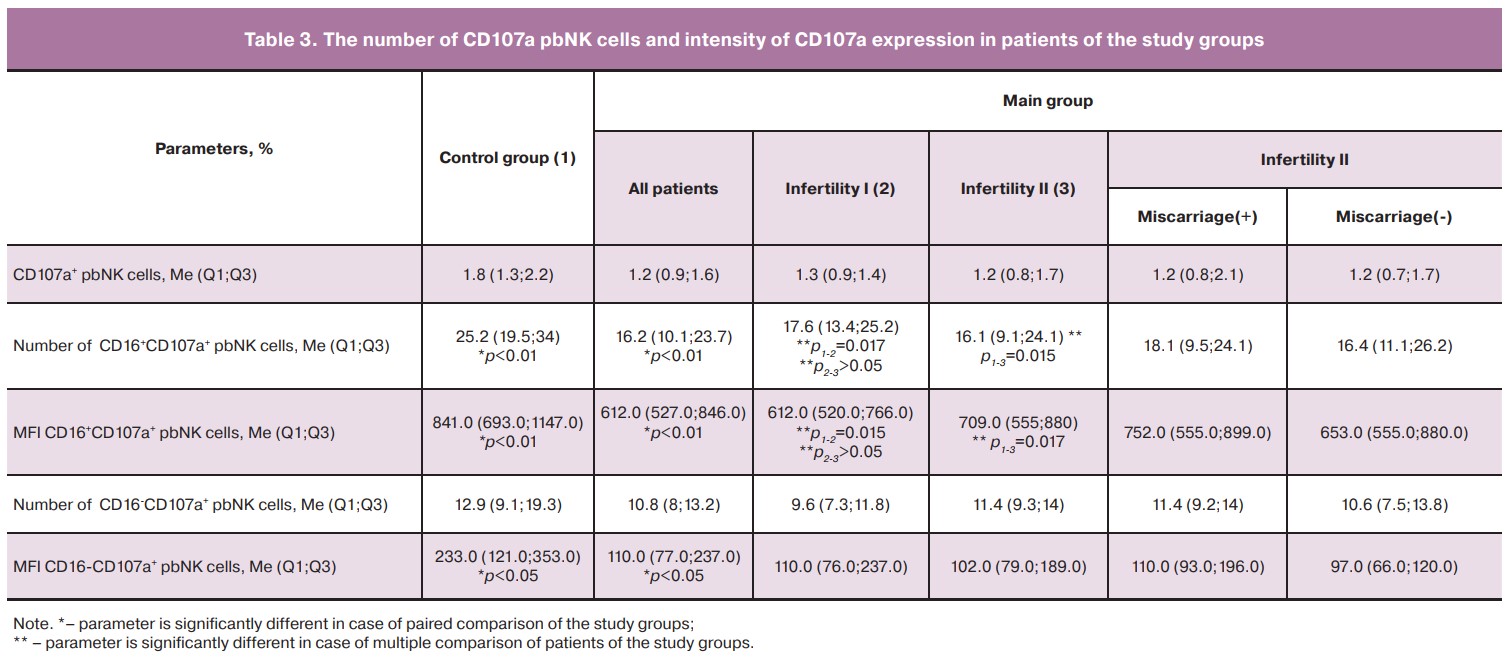
The assessment of CD 107a expression showed no difference in the total number of CD 107a+pbNK cells between the patients of the control and main groups. However, the assessment of the number of CD16+CD107a+pbNK cells, as well as the mean fluorescence intensity (MFI) of CD107a expression revealed that these parameters were lower in the patients of the main group, in both subgroups of primary and secondary infertility, compared to the patients of the control group (Table 3). MFI of CD16-CD 107a+ pbNK cells was also lower in the patients of the main group compared to ones in the control group (p<0.05).
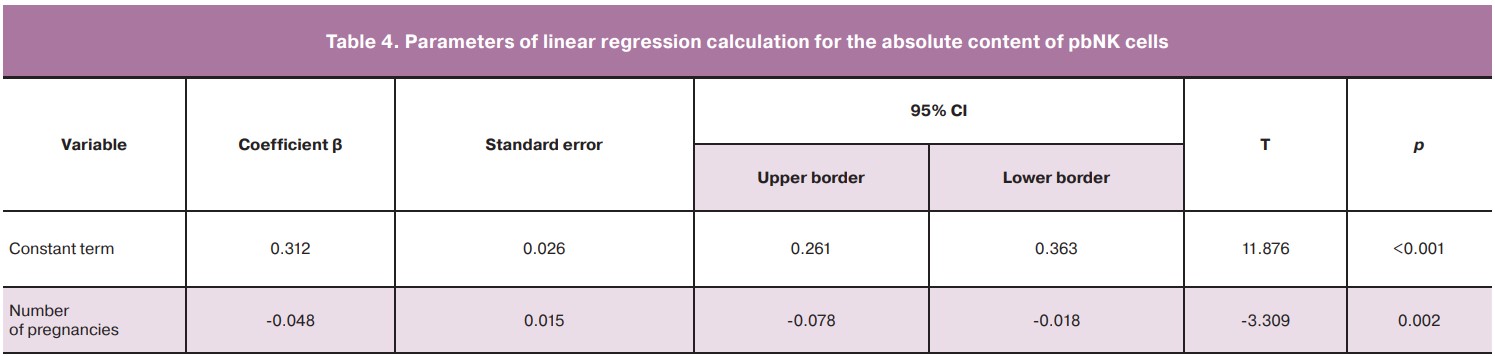
The patients of the main group had an inverse correlation of the number of pregnancies in the history with the absolute content of pbNK cells (rs=-0.55, p<0.01) and a direct correlation with MFI CD16+CD107a+pbNK cells (rs=0.41, p<0.01). In connection with the data obtained, a one-factor linear regression was calculated, where the absolute values of pbNK cells were used as a dependent variable, and the number of pregnancies in the history was used as a predictor (Table 4). On the basis of the calculation of linear regression coefficients, we obtained the equation of the predicted absolute content of pbNK cells: pbNK cells = 0.312-0.048×NP, where NP is the number of pregnancies in the history.
The standard error estimation of the model = 0.11 K/mcL, r2= 0.26 (F=9.39; p<0.01).
No outliers were detected, the residuals were distributed normally.
On the basis of the obtained equation, it can be assumed that if pregnancy does not occur, the absolute contents of pbNK cells are supposed to be more than 0.312 K/mcL.
Discussion
In the course of this study, the phenotypic characteristics of lymphoid cells were evaluated in patients with infertility and patients who had unsuccessful IVF attempts. We found that the relative number of B-lymphocytes was lower in the main group of patients compared to the patients in the control group. B-lymphocytes are an important component of the immune system; their functions include antigen presentation, antibody synthesis, cytokine secretion, and immunoregulation [22]. B-lymphocytes and their individual subpopulations (regulatory B-lymphocytes) contribute to the onset and development of pregnancy due to the induction of immunological tolerance to a semi-allogeneic fetus [23]. The decrease in the number of B-lymphocytes reflects the insufficient function of adaptive immunity in reproductive diseases. Thus, it was found that patients with repeated implantation failures in IVF protocols in comparison with healthy fertile women had a reduced number of IL-10 which produce peripheral blood B-lymphocytes [24]. The level of blood B-lymphocytes has been shown to have prognostic value in terms of pregnancy outcomes. Tu W. et al. (2020) assessed the contents of peripheral blood B-lymphocytes in a group of patients with repeated ART failures before and after the protocol and showed a decrease in their number in women whose pregnancy ended in miscarriage compared to live birth. The level of blood B-lymphocytes less than 15% before pregnancy increased the risk of miscarriage by 39% [25]. Therefore, the results of our study on a lower number of B-lymphocytes in patients with previous ineffective ART protocols are consistent with the findings of other studies.
We have obtained the data on changes in the immunological parameters of blood depending on the presence of previous clinical pregnancy. The patients of the main group with primary infertility showed a 2.1-fold increase in the absolute number of pbNK cells compared to the patients with secondary infertility. We also found an inverse relationship between the number of pregnancies in patients of the main group and the absolute content of pbNK cells. This relationship suggests a negative effect of an increase in the number of pbNK cells on the implantation and placentation processes. The threshold value of the absolute content of pbNK cells associated with the absence of pregnancy in the patient’s history was more than 0.312 K/mcL. There is insufficient data in the literature regarding the comparison of the number of pbNK cells in primary and secondary infertility. In most studies, patients with reproductive failures were compared with healthy fertile women. Thus, according to the results of meta-analysis, the number of pbNK cells in patients with infertility is significantly higher than in healthy women [11]. According to Triggianese P. et al. (2015), patients with primary infertility demonstrated an increased number of pbNK cells compared to the control group, but there were no significant differences with the group of patients who had habitual miscarriage [26]. Azargoon A. et al. (2019) showed an increase in the number of pbNK cells in patients with primary idiopathic infertility and habitual miscarriage compared to the patients of the control group [9].
The results of our study showed changes in the functional activity of pbNK cells in patients of the main group, both with primary and secondary infertility, compared to the patients of the control group: the number of CD16+CD107a+ pbNK cells decreased by 1.36 and 1.58 times, respectively, and the intensity of CD107a expression was reduced by 1.37 and 1.2 times, respectively. A number of authors proposed a hypothesis regarding the migration of NK cells from peripheral blood to the endometrium, thereby the dysfunctional state of pbNK cells can determine the state of endometrial NK cells [27]. The obtained data may indicate excessive recruitment of functionally altered pbNK cells into the endometrium in patients with infertility and ART failures which may cause their insufficiency in the implantation and placentation processes. This conclusion is also confirmed by our correlation analysis of the relationship between the number of pregnancies and MFI severity of CD107a pbNK cells. These findings are consistent with the results of studies conducted by a number of scientists who demonstrated a low cytotoxic potential of NK cells in patients with reproductive losses [16, 28].
The patients of the main group with primary infertility showed an increase in the number of NKG2D+ CD56dimCD16bright and NKG2D+CD56brightCD16dim pbNK cells in comparison with the patients who had secondary infertility. The balance of signals of activating and inhibiting receptors on the surface of the NK cell is of key importance for its functioning. A number of studies have demonstrated a change in the receptor profile of NK cells during reproductive losses [17, 29, 30]. Transmembrane activating receptor NKG2D plays an important role in the development of various autoimmune diseases, viral and bacterial infections, complications and outcomes of transplantation. However, the data on its significance in the onset and course of pregnancy remain understudied [31].
When the NKG2D receptor binds to its ligand, the NK cell is activated and its cytotoxic activity increases [32]. In humans, the ligands of this receptor include MICA, MICB and UL1-6-binding proteins (ULBP1-6); their overexpression is characteristic of cells that are transformed, infected and exposed to other stress factors, so such cells become targets for NK cells. Improper receptor-ligand interaction can lead to impaired immune interaction [31]. One of the mechanisms for maintaining immunological tolerance to a semi-allogeneic fetus is the secretion of the soluble form of NKG2D ligands by syncytiotrophoblast cells [33, 34]. In addition, the expression of MICA and MICB molecules by human endometrial cells has been demonstrated [35]. The soluble forms of MICA, MICB and ULBP1 molecules in the umbilical cord blood of a full-term fetus were also shown to affect the decrease in the functional activity of the mother’s NK cells [36]. Besides, the functional status of NK cells is affected by polymorphisms of the CLCN1 gene encoding the NKG2D protein, which can lead to autoimmune disorders, pregnancy loss and transplant rejection [37]. The association of polymorphisms rs1049174 G/C and rs2617170 TT of the NKG2D gene with the risk of habitual miscarriage has been established [38, 39].
Thus, a change in binding NKG2D to ligands can both increase and decrease receptivity and this way can lead to a dysfunctional state of the NK cell and have an adverse effect on the implantation and placentation processes. There is a possibility of abnormal expression of soluble forms of NKG2D ligands by trophoblast cells during pregnancy, as well as a change in the expression level of the NKG2D receptor itself on NK cells due to binding to ligands in the endometrium at the preimplantation stages. The results of our study confirm the role of the NKG2D pbNK cell receptor in the reproductive function and require further study.
During the assessment of the subpopulation composition of pbNK cells, we identified such phenotypes as CD56dimCD16bright, CD56dimCD16dim, CD56brightCD16dim. It is believed that pbNK cells undergo successive stages of differentiation [40]. A number of authors distinguish a subpopulation of CD56dimCD16dim pbNK cells, which is comparable or even exceeds CD56brightCD16dim in terms of the number of cells [41]. CD56dimCD16dim cells exhibit a more mature phenotype compared to CD56brightCD16dim, and therefore they are assumed to be direct precursors of CD56dimCD16bright cells [41]. On the other hand, CD56dimCD16dim cells may also represent an intermediate stage between subsets of CD56dimCD16bright and CD56dimCD16-NK cells, since CD56dimCD16bright cells are able to acquire the CD56dimCD16-phenotype during degranulation [42]. At the same time, CD56dimCD16bright pbNK cells have a lower cytotoxic potential than CD56dimCD16dim cells, but it is comparatively greater than CD56brightCD16dim subpopulation.
The results of the study showed that the subpopulation composition of pbNK cells changed in the patients with secondary infertility and previous history of miscarriage. The number of cells of regulatory subpopulation CD56brightCD16dim and cytotoxic subpopulation CD56dimCD16bright decreased and at the same time the number of subpopulations CD56dimCD16dim pbNK cells increased. Not only a decrease in the number of cells of the regulatory subpopulation, but also a lack of active pbNK cells may be a link in the pathogenesis of miscarriage and lead to the termination of pregnancy in both IVF and natural cycle.
The obtained data on changes in pbNK cell parameters in patients who have never been pregnant in contrast to patients with the confirmed fact of clinical pregnancy require further study, especially when it comes to the population of endometrial NK cells. The changes can be explained by the phenomenon of fetomaternal microchimerism and the development of the concept which is called «immunological memory» of NK cells [43]. The «immunological memory» of NK cells refers to the presence of specific NK cells in a woman’s body induced by previous pregnancy; these cells are in a preactivated state and capable of active synthesis of cytokines and growth factors that facilitate the processes of implantation and placentation [44]. It is assumed that «memory» of NK cells is induced by any pregnancy that does not necessarily end in childbirth [45].
Conclusion
The patients with infertility and previous unsuccessful ART attempts show changes in the immunological blood parameters which may be involved in the impairment of the mechanisms of interaction with the embryo or fetus at various stages, both before and after implantation. The changes in the number and functional activity of NK cells in patients with ineffective ART protocols in primary and secondary infertility, as well as a previous history of miscarriage, may reflect immunological differences in the pathogenesis of these diseases. It is worth noting that the most significant changes in the quantitative and functional parameters of peripheral blood NK cells were detected in patients with primary infertility and previous unsuccessful ART attempts. Thus, it is reasonable to assess not only the number, but also the functional status of pbNK cells in patients with reproductive losses. It is necessary to conduct further studies including the assessment of the endometrial NK cells parameters and their correlation with changes in peripheral blood.
References
- Practice Committee of the American Society for Reproductive Medicine. Electronic address aao, Practice Committee of the American Society for Reproductive M. Evidence-based treatments for couples with unexplained infertility: a guideline. Fertil. Steril. 2020; 113(2): 305-22. https://dx.doi.org/10.1016/j.fertnstert.2019.10.014.
- Bashiri A., Borick J.L. Recurrent pregnancy loss: Definitions, epidemiology, and prognosis.In: Bashiri A., Harlev A., Agarval A., eds. Recurrent pregnancy loss. Springer; 2016: 3-18. https://dx.doi.org/10.1007/978-3-319-27452-2_1.
- Wendt K., Wilk E., Buyny S., Buer J., Schmidt R.E., Jacobs R. Gene and protein characteristics reflect functional diversity of CD56dim and CD56bright NK cells. J. Leukoc. Biol. 2006; 80(6): 1529-41. https://dx.doi.org/10.1189/jlb.0306191.
-
Euchner J., Sprissler J., Cathomen T., Fürst D., Schrezenmeier H., Debatin K.M. et al. Natural killer cells generated from human induced pluripotent stem cells mature to CD56(bright)CD16(+)NKp80(+/-) in-vitro and express KIR2DL2/DL3 and KIR3DL1. Front. Immunol. 2021; 12: 640672.
https://dx.doi.org/10.3389/fimmu.2021.640672.
- Castriconi R., Carrega P., Dondero A., Bellora F., Casu B., Regis S. et al. Molecular mechanisms directing migration and retention of natural killer cells in human tissues. Front. Immunol. 2018; 9: 2324. https://dx.doi.org/10.3389/fimmu.2018.02324.
- Michel T., Poli A., Cuapio A., Briquemont B., Iserentant G., Ollert M. et al. Human CD56bright NK cells: An Update. J. Immunol. 2016; 196(7): 2923-31. https://dx.doi.org/10.4049/jimmunol.1502570.
- Poli A., Michel T., Theresine M., Andres E., Hentges F., Zimmer J. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology. 2009; 126(4): 458-65. https://dx.doi.org/10.1111/j.1365-2567.2008.03027.x.
- Kolanska K., Suner L., Cohen J., Ben Kraiem Y., Placais L., Fain O. et al. Proportion of cytotoxic peripheral blood natural killer cells and T-cell large granular lymphocytes in recurrent miscarriage and repeated implantation failure: case-control study and meta-analysis. Arch. Immunol. Ther. Exp. (Warsz.). 2019; 67(4): 225-36. https://dx.doi.org/10.1007/s00005-019-00546-5.
- Azargoon A., Mirrasouli Y., Shokrollahi Barough M., Barati M., Kokhaei P. The state of peripheral blood natural killer cells and cytotoxicity in women with recurrent pregnancy loss and unexplained infertility. Int. J. Fertil. Steril. 2019; 13(1): 12-7. https://dx.doi.org/10.22074/ijfs.2019.5503.
- Toth B., Vomstein K., Togawa R., Böttcher B., Hudalla H., Strowitzki T. et al. The impact of previous live births on peripheral and uterine natural killer cells in patients with recurrent miscarriage. Reprod. Biol. Endocrinol. 2019; 17(1): 72. https://dx.doi.org/10.1186/s12958-019-0514-7.
- Seshadri S., Sunkara S.K. Natural killer cells in female infertility and recurrent miscarriage: a systematic review and meta-analysis. Hum. Reprod. Update. 2014; 20(3): 429-38. https://dx.doi.org/10.1093/humupd/dmt056.
- Zhang H., Huang C., Chen X., R, Böttcher B., Hudalla H., Strowitzki T. et al. The number and cytotoxicity and the expression of cytotoxicity-related molecules in peripheral natural killer (NK) cells do not predict the repeated implantation failure (RIF) for the in vitro fertilization patients. Genes Dis. 2020; 7(2): 283-9. https://dx.doi.org/10.1016/j.gendis.2019.03.005.
- Wang Q., Li T.C., Wu Y.P., Cocksedge K.A., Fu Y.S., Kong Q.Y., Yao S.Z. Reappraisal of peripheral NK cells in women with recurrent miscarriage. Reprod. Biomed. Online. 2008; 17(6): 814-9. 10.1016/s1472-6483(10)60410-5.
- Ho Y.K., Chen H.H., Huang C.C., Lee C.I., Lin P.Y., Lee M.S., Lee T.H. Peripheral CD56(+)CD16(+) NK cell populations in the early follicular phase are associated with successful clinical outcomes of intravenous immunoglobulin treatment in women with repeated implantation failure. Front. Endocrinol. (Lausanne). 2019; 10: 937. https://dx.doi.org/10.3389/fendo.2019.00937.
- Ebina Y., Nishino Y., Deguchi M., Maesawa Y., Nakashima Y., Yamada H. Natural killer cell activity in women with recurrent miscarriage: Etiology and pregnancy outcome. J. Reprod. Immunol. 2017; 120: 42-7. https://dx.doi.org/10.1016/j.jri.2017.04.005.
- Fukui A., Funamizu A., Fukuhara R., Shibahara H. Expression of natural cytotoxicity receptors and cytokine production on endometrial natural killer cells in women with recurrent pregnancy loss or implantation failure, and the expression of natural cytotoxicity receptors on peripheral blood natural killer cells in pregnant women with a history of recurrent pregnancy loss. J. Obstet. Gynaecol. Res. 2017; 43(11): 1678-86. https://dx.doi.org/10.1111/jog.13448.
- Dons'koi B.V., Osypchuk D.V., Chernyshov V.P., Khazhylenko K.G. Expression of natural cytotoxicity receptor NKp46 on peripheral blood natural killer cells in women with a history of recurrent implantation failures. J. Obstet. Gynaecol. Res. 2021;47(3):1009-15. https://dx.doi.org/10.1111/jog.14631.
- Alter G., Malenfant J.M., Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods. 2004; 294(1-2): 15-22. https://dx.doi.org/10.1016/j.jim.2004.08.008.
- Ghiringhelli F., Menard C., Terme M., Flament C., Taieb J., Chaput N. et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J. Exp. Med. 2005; 202(8): 1075-85. https://dx.doi.org/10.1084/jem.20051511.
- Wang W.J., Hao C.F., Yi L., Yin G.J., Bao S.H., Qiu L.H., Lin Q.D. Increased prevalence of T helper 17 (Th17) cells in peripheral blood and decidua in unexplained recurrent spontaneous abortion patients. J. Reprod. Immunol. 2010; 84(2): 164-70. https://dx.doi.org/10.1016/j.jri.2009.12.003.
- Robertson S.A., Care A.S., Moldenhauer L.M. Regulatory T cells in embryo implantation and the immune response to pregnancy. J. Clin. Invest. 2018; 128(10): 4224-35. https://dx.doi.org/10.1172/JCI122182.
- LeBien T.W., Tedder T.F. B lymphocytes: how they develop and function. Blood. 2008; 112(5): 1570-80. https://dx.doi.org/10.1182/blood-2008-02-078071.
- Esteve-Solé A., Luo Y., Vlagea A., Deyà-Martínez Á., Yagüe J., Plaza-Martín A.M. et al. B regulatory cells: players in pregnancy and early life. Int. J. Mol. Sci. 2018; 19(7): 2099. https://dx.doi.org/10.3390/ijms19072099.
- Koushaeian L., Ghorbani F., Ahmadi M., Eghbal-Fard S., Zamani M., Danaii S. et al. The role of IL-10-producing B cells in repeated implantation failure patients with cellular immune abnormalities. Immunol. Lett. 2019; 214: 16-22. https://dx.doi.org/10.1016/j.imlet.2019.08.002.
- Tu W., Li Y., Ding Q., Wang L., Frempong S.T., Ruan J. et al. Association between peripheral CD19+ B cells and reproductive outcome in women with recurrent implantation failure. Clin. Lab. 2020; 66(1). https://dx.doi.org/10.7754/Lab.2019.190510.
- Triggianese P., Perricone C., Perricone R., De Carolis C. Prolactin and natural killer cells: evaluating the neuroendocrine-immune axis in women with primary infertility and recurrent spontaneous abortion. Am. J. Reprod. Immunol. 2015; 73(1): 56-65. https://dx.doi.org/10.1111/aji.12335.
- Strobel L., Vomstein K., Kyvelidou C., Hofer-Tollinger S., Feil K., Kuon R.J. et al. Different background: natural killer cell profiles in secondary versus primary recurrent pregnancy loss. J. Clin. Med. 2021; 10(2): 194. https://dx.doi.org/10.3390/jcm10020194.
- Zhang Y., Huang C., Lian R., Xu J., Fu Y., Zeng Y., Tu W. The low cytotoxic activity of peripheral blood NK cells may relate to unexplained recurrent miscarriage. Am. J. Reprod. Immunol. 2021; 85(6): e13388. https://dx.doi.org/10.1111/aji.13388.
- Zhang Y., Zhao A., Wang X., Shi G., Jin H., Lin Q. Expressions of natural cytotoxicity receptors and NKG2D on decidual natural killer cells in patients having spontaneous abortions. Fertil. Steril. 2008; 90(5): 1931-7. https://dx.doi.org/10.1016/j.fertnstert.2007.08.009.
- Takeyama R., Fukui A., Mai C., Yamamoto M., Saeki S., Yamaya A., Shibahara H. Co-expression of NKp46 with activating or inhibitory receptors on, and cytokine production by, uterine endometrial NK cells in recurrent pregnancy loss. J. Reprod. Immunol. 2021; 145: 103324. https://dx.doi.org/10.1016/jri.2021.103324.
- Siemaszko J., Marzec-Przyszlak A., Bogunia-Kubik K. NKG2D natural killer cell receptor-A short description and potential clinical applications. Cells. 2021;10(6): 1420. https://dx.doi.org/10.3390/cells10061420.
- Schmiedel D., Mandelboim O. NKG2D ligands-critical targets for cancer immune escape and therapy. Front. Immunol. 2018; 9: 2040. https://dx.doi.org/10.3389/fimmu.2018.02040.
- Hedlund M., Stenqvist A.C., Nagaeva O., Kjellberg L., Wulff M, Baranov V., Mincheva-Nilsson L. Human placenta expresses and secretes NKG2D ligands via exosomes that down-modulate the cognate receptor expression: evidence for immunosuppressive function. J. Immunol. 2009;183(1): 340-51. https://dx.doi.org/10.4049/jimmunol.0803477.
- Mincheva-Nilsson L., Nagaeva O., Chen T., Stendahl U., Antsiferova J., Mogren I. et al. Placenta-derived soluble MHC class I chain-related molecules down-regulate NKG2D receptor on peripheral blood mononuclear cells during human pregnancy: a possible novel immune escape mechanism for fetal survival. J. Immunol. 2006; 176(6): 3585-92. https://dx.doi.org/10.4049/jimmunol.176.6.3585.
- Basu S., Pioli P.A., Conejo-Garcia J., Wira C.R., Sentman C.L. Estradiol regulates MICA expression in human endometrial cells. Clin. Immunol. 2008; 129(2): 325-32. https://dx.doi.org/10.1016/j.clim.2008.07.005.
- Cox S.T., Laza-Briviesca R., Pearson H., Soria B., Gibson D., Gomez S. et al. Umbilical cord blood plasma contains soluble NKG2D ligands that mediate loss of natural killer cell function and cytotoxicity. Eur. J. Immunol. 2015; 45(8): 2324-34. https://dx.doi.org/10.1002/eji.201444990.
- Zhao Y., Chen N., Yu Y., Zhou L., Niu C., Liu Y. et al. Prognostic value of MICA/B in cancers: a systematic review and meta-analysis. Oncotarget. 2017; 8(56): 96384-95. https://dx.doi.org/10.18632/oncotarget.21466.
- Hizem S., Mtiraoui N., Massaoudi S., Fortier C., Boukouaci W., Kahina A. et al. Polymorphisms in genes coding for the NK-cell receptor NKG2D and its ligand MICA in recurrent miscarriage. Am. J. Reprod. Immunol. 2014; 72(6): 577-85. https://dx.doi.org/10.1111/aji.12314.
- Abdian Asl A., Vaziri Nezamdoust F., Fesahat F., Astani A., Barati M., Raee P., Asadi-Saghandi A. Association between rs1049174 NKG2D gene polymorphism and idiopathic recurrent spontaneous abortion in Iranian women: A case-control study. J. Obstet. Gynaecol. 2021; 41(5): 774-8. https://dx.doi.org/10.1080/2020.1798906.
- Perera Molligoda Arachchige A.S. Human NK cells: from development to effector functions. Innate Immun. 2021; 27(3): 212-29. https://dx.doi.org/1177/17534259211001512.
- Dogra P., Rancan C., Ma W., Toth M., Senda T., Carpenter J. et al. Tissue determinants of human NK cell development, function, and residence. Cell. 2020; 180(4): 749-763.e13. https://dx.doi.org/10.1016/j.cell.2020.01.022.
- Amand M., Iserentant G., Poli A., Sleiman M., Fievez V., Sanchez I.P. et al. Human CD56(dim)CD16(dim) cells as an individualized natural killer cell subset. Front. Immunol. 2017; 8: 699. https://dx.doi.org/10.3389/fimmu.2017.00699.
- Gamliel M., Goldman-Wohl D., Isaacson B., Gur C., Stein N., Yamin R. et al. Trained memory of human uterine NK cells enhances their function in subsequent pregnancies. Immunity. 2018; 48(5): 951-962.e5. https://dx.doi.org/10.1016/j.immuni.2018.03.030.
- Huang X., Wang L., Zhao S., Liu H., Chen S., Wu L. et al. Pregnancy induces an immunological memory characterized by maternal immune alterations through specific genes methylation. Front. Immunol. 2021; 12: 686676. https://dx.doi.org/10.3389/fimmu.2021.686676.
- Goldman-Wohl D., Gamliel M., Mandelboim O., Yagel S. Learning from experience: cellular and molecular bases for improved outcome in subsequent pregnancies. Am. J. Obstet. Gynecol. 2019; 221(3): 183-93. https://dx.doi.org/10.1016/j.ajog.2019.02.037.
Received 10.06.2022
Accepted 07.09.2022
About the Authors
Valeriya A. Zagaynova, Junior Researcher, obstetrician-gynecologist at the Department of Assisted Reproductive Technologies, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, +7(812)328-98-33, zagaynovav.al.52@mail.ru, https://orcid.org/0000-0001-6971-7024, 199034, Russia, St. Petersburg, Mendeleevskaya line, 3.Igor Yu. Kogan, Corresponding Member of RAS, Dr. Med. Sci., Director of the D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology,
+7(812)328-98-33, http://orcid.org/0000-0002-7351-6900, 199034, Russia, St. Petersburg, Mendeleevskaya line, 3.
Sergey A. Selkov, Merited Scholar of the Russian Federation, Dr. Med. Sci., Professor, Head of the Department of Immunology and Intercellular Interactions, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, +7(812)328-98-50, https://orcid.org/0000-0003-1560-7529, 199034, Russia, St. Petersburg,
Mendeleevskaya line, 3.
Olesya N. Bespalova, Dr. Med. Sci., Deputy Director of the D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, +7(812)679-55-51,
https://orcid.org/0000-0002-6542-5953, 199034, Russia, St. Petersburg, Mendeleevskaya line, 3.
Inna O. Krikheli, PhD, Senior Researcher, obstetrician-gynecologist, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, +7(812)679-55-51,
https://orcid.org/0000-0002-5439-1727, 199034, Russia, St. Petersburg, Mendeleevskaya line, 3.
Valentina A. Mikhailova, PhD., Senior Researcher at the Laboratory of Intercellular Interactions, Department of Immunology and Intercellular Interactions, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, +7(812)328-98-50, https://orcid.org/0000-0003-1328-8157, 199034, Russia, St. Petersburg, Mendeleevskaya line, 3.
Alina A. Davydova, Junior Researcher at the Laboratory of Intercellular Interactions, Department of Immunology and Intercellular Interactions, D.O. Ott Research Institute
of Obstetrics, Gynecology and Reproductology, +7(812)328-98-50, https://orcid.org/0000-0001-5313-2910, 199034, Russia, St. Petersburg, Mendeleevskaya line, 3
Yulia P. Milyutina, PhD, Senior Researcher at Biochemistry Group, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, +7(812)679-55-51,
https://orcid.org/0000-0002-6542-5953, 199034, Russia, St. Petersburg, Mendeleevskaya line, 3.
Dmitry I. Sokolov, Dr. Bio. Sci., Head of the Laboratory of Intercellular Interactions, Department of Immunology and Intercellular Interactions, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology; Associate Professor at the Department of Immunology, Academician I.P. Pavlov First St. Petersburg State Medical University, +7(812)328-98-50, https://orcid.org/0000-0002-5749-2531, 199034, Russia, St. Petersburg, Mendeleevskaya line, 3.
Authors’ contributions: Kogan I.Yu., Bespalova O.N., Sokolov D.I., Zagaynova V.A. – developing the concept and design of the study; Zagaynova V.A., Krikheli I.O., Mikhailova V.A., Davydova A.A. – collecting and processing the material; Zagaynova V.A., Milyutina Yu.P. – statistical data processing; Zagaynova V.A. – writing the text; Kogan I.Yu., Sokolov D.I., Bespalova O.N., Selkov S.A. – editing the text.
Conflicts of interest: The authors declare that there are no conflicts of interest.
Funding: The study was supported by the Russian Foundation for Basic Research Grant № 20-315-90121, Evaluation of lymphocyte subpopulations, the number, subpopulation composition and expression of the CD 107a receptor by peripheral blood NK cells, and by the government program No. АААА-А20-120041390033-4, evaluation of NKG2D receptor expression.
Ethical Approval: The study was approved by the Ethical Review Board of D.O. Ott Research Institute of Obstetrics, Gynecology and Reproduction, St. Petersburg, Russia (Protocol No.100 – 19/12/2019).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Zagaynova V.A., Kogan I.Yu., Selkov S.A., Bespalova O.N., Krikheli I.O., Mikhailova V.A., Davydova A.A., Milyutina Yu.P., Sokolov D.I.
Peripheral blood NK-cells in women with unsuccessful attempts of assisted reproduction:
quantity, subpopulation composition and activation markers.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 9: 102-113 (in Russian)
https://dx.doi.org/10.18565/aig.2022.9.102-113



