Predictive value of testing for maternal plasma cell-free fetal DNA in fetal growth restriction
Aim. To investigate the relationship between changes in cell-free fetal DNA (cfDNA) level and placental morphology in fetal growth restriction (FGR) and determine its predictive value. Materials and methods. The study included 93 pregnant women, including 48 pregnant women with FGR (group I, 23 with early and 25 with late-onset FGR), and 45 women with uncomplicated pregnancy (group II, comparison group). The analysis included clinical and anamnestic data, the course of pregnancy and delivery, and the fetus and newborn health status. Maternal plasma cfDNA was measured by real-time polymerase chain reaction. Placentas were examined using the terminal deoxyuridine end-labeling (TUNEL) technique and immunofluorescence analysis. Results. The level of cfDNA in early-onset FGR [89.03 (44.52; 178.32) GE/ml] was statistically significantly lower than in the late FGR and the comparison group [244.14 (145.23; 422.47) and 211.05 (133.64; 567.81) GE/ml], (p <0.001). ROC analysis showed that testing for maternal plasma cfDNA at the threshold of 119.11 GE/ml had high sensitivity (73.1%) and specificity (79.3%) (AUC = 0.81, 95% CI 0.63–0.98) for detecting early FGR and predict the likelihood of newborn complications. The findings on placental architectonics included the predominance of fibrosis processes and the formation of extensive fibrinoid zones. This observation explains the decrease in cfDNA and impaired placental perfusion leading to FGR. It confirms the relationship between low cfDNA levels and the risk of having children with stunted growth. Also, there was strong positive correlations between the cfDNA level, body weight (r = 0.72; p <0.001) and length (r = 0.71; p <0.001) of newborns in cases of early-onset FGR. Conclusion. The study identified the diagnostic accuracy of cfDNA for differentiating early from late-onset FGR. The findings suggest that early and late-onset FGR have different underlying mechanisms. Testing for cfDNA allows for predicting the risk of having children with low body weight and height. The findings on the high diagnostic accuracy of cfDNA in detecting the early FGR suggest the prospects of using cfDNA as a predictive marker.Kan N.E., Tyutyunnik V.L., Khachatryan Z.V., Sadekova A.A., Krasnyi A.M.
Keywords
Currently, fetal growth restriction (FGR) remains one of the world's great-est public health challenges due to its relatively high incidence amounting to 5–18% [1–3]. Despite variable incidence among different populations, FGR is one of the leading causes of perinatal mortality and morbidity. At the same time, the lack of uniform diagnostic and prognostic criteria for FGR leads to insufficient effectiveness of its antenatal detection [4–6].
In recent years, cell-free fetal DNA (cfDNA), a novel promising biomarker, has been used in clinical practice for prenatal diagnosis and pregnancy-related complications. Cell-free fetal DNA is released into the maternal circulation due to apoptotic mechanisms occurring on trophoblastic cells [7–9]. Of particular importance is using this marker in FGR since the latter refers to placenta-associated pregnancy complications [10, 11]. The concept of the placental origin of cfDNA is generally accepted and has been confirmed in many studies [12–15]. The underlying mechanisms include apoptosis accompanying the normal development of the placenta, the release of cfDNA into maternal circu-lation due to cellular apoptosis and necrosis, and direct transmission of DNA through the placenta or amniotic membranes [16, 17]. Among the causes for the change in cfDNA levels is an increase in the nucleic acid release into the maternal bloodstream and decreased clearance. Existing literature provides contradictory data regarding the role of cfDNA in FGR.
Thus, D. Morano et al. [18] reported that statistically lower cfDNA values were observed only in patients with early-onset FGR, and cfDNA values corre-lated directly with placental volume and placental insufficiency. At the same time, the cause of changes in the cfDNA level in this complication of pregnancy remains debatable.
Thus, it is of interest to determine the prognostic and diagnostic value of cfDNA in FGR by studying placental morphology, which may help clarify the mechanisms of this pregnancy complication and identify a new non-invasive marker.
The present study aimed to investigate the relationship between changes in cfDNA level and placental morphology in FGR and determine its predictive value.
Materials and methods
A total of 93 pregnant women met the eligibility criteria and were included in the study. Of them, 48 with FGR (23 with early and 25 with late-onset FGR) comprised group I, and 45 with uncomplicated pregnancy made up group II (comparison group).
The inclusion criteria for both groups were the age from 18 to 45 years, singleton pregnancy at 22–40 weeks, and signed an informed consent ap-proved by the ethical committee. The inclusion criteria for group 1 were FGR-complicated pregnancy. Exclusion criteria were severe extragenital pathology, multiple-gestation pregnancy after oocyte donation, fetal malformations, ma-ternal and fetal genetic diseases, acute phase or exacerbation of chronic infec-tious diseases in the mother, and large uterine fibroids.
All patients included in the study underwent a standard examination fol-lowing the order of the Ministry of Health of the Russian Federation dated No-vember 01, 2012, No. 572n "On approval of the Procedure for providing med-ical care in the profile of obstetrics and gynecology (except for the use of as-sisted reproductive technologies)." In addition, clinical and anamnestic data, characteristics of the course of pregnancies and delivery, the state of fetuses and newborns were studied.
Maternal plasma cfDNA was measured by real-time polymerase chain reac-tion. In addition, placentas were examined using the terminal deoxyuridine end-labeling (TUNEL) technique and immunofluorescence analysis.
Statistical analysis
Statistical analysis and plotting were performed using Statistica 10 and OriginPro 8.5 (USA) statistical software. The normality of the distribution was tested by the Shapiro–Wilk test. Equality of variance was assessed by Levene's test. Comparing numerical data with non-normal distribution was performed with nonparametric Mann–Whitney tests, and the Spearman Rank Test was used for the correlation analysis. Finally, categorical variables were compared using Fisher’s exact test. The diagnostic accuracy of variables was measured by using ROC analysis. ROC data are presented as an area under the curve with a 95% confidence interval (CI). The critical level of significance when the testing statistical hypothesis was considered at p <0.05.
Results and discussion
 All pregnant women included in the study were comparable in terms of main clinical and demographic characteristics. The mean age in groups 1 and 2 was 34 (29; 35) and 32 (29; 34) years, respectively. There were no statistically significant differences in the structure of somatic and gynecological diseases. Patients with FGR-complicated pregnancies were more likely to have a history of threatened miscarriage and oligohydramnios.
All pregnant women included in the study were comparable in terms of main clinical and demographic characteristics. The mean age in groups 1 and 2 was 34 (29; 35) and 32 (29; 34) years, respectively. There were no statistically significant differences in the structure of somatic and gynecological diseases. Patients with FGR-complicated pregnancies were more likely to have a history of threatened miscarriage and oligohydramnios.
Given the evidence that cfDNA levels increase with gestational age, groups were gestational age-matched. Thus, the medians of gestational ages in the study groups were comparable, making it possible to evaluate the results fur-ther.
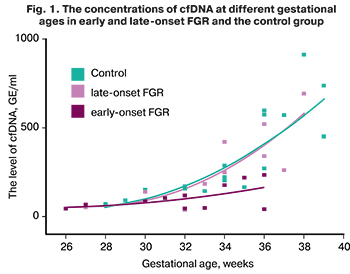
The concentrations of cfDNA at different gestational ages in early and late-onset FGR, and the control group, are shown in Figure 1.
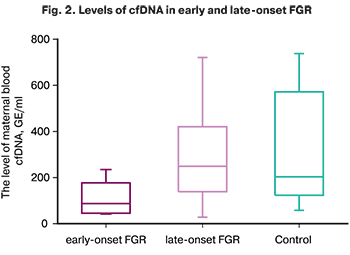 The level of cfDNA in early-onset FGR [89.03 (44.52; 178.32) GE/ml] was statistically significantly lower than in the late FGR and the comparison group [244.14 (145.23; 422.47) and 211.05 (133.64; 567.81) GE/ml], (p <0.001). At the same time, there were no statistically significant differences between the late FGR and the comparison group (p> 0.05). The study findings are shown in Figure 2.
The level of cfDNA in early-onset FGR [89.03 (44.52; 178.32) GE/ml] was statistically significantly lower than in the late FGR and the comparison group [244.14 (145.23; 422.47) and 211.05 (133.64; 567.81) GE/ml], (p <0.001). At the same time, there were no statistically significant differences between the late FGR and the comparison group (p> 0.05). The study findings are shown in Figure 2.
Taking into account research evidence on the relationship between the level of cfDNA and placenta weight, as well as the results obtained indicating a sta-tistically significant decrease in the level of cfDNA only in the early form of FGR, the next stage of the study was to determine the relationship between the level of cfDNA and clinical criteria using the nonparametric Spearman rank correlation analysis. The p-values of <0.05 were considered to be statistically significant.
The placenta weight in the early FGR group was 248 (228; 307) g, which was statistically significantly lower than that in the late FGR group [335 g (305; 380, p <0.05)] and in the comparison group [432 g (340; 471, p <0.001)]. Also, there was strong positive correlations between the cfDNA lev-el, body weight (r=0.72; p <0.001) and length (r=0.71; p <0.001) of newborns in cases of early-onset FGR.
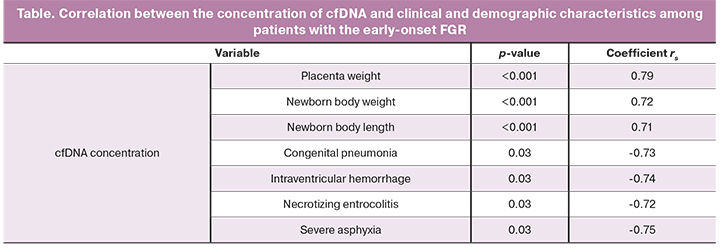
 Of particular interest was the analysis of perinatal outcomes. A strong neg-ative correlation was found between congenital pneumonia (r=-0.73; p=0.03), intraventricular hemorrhage (r=-0.74; p=0.03), necrotizing enterocolitis (r=-0.72; p=0.03), and severe asphyxia (r=-0.75; p=0.03).
Of particular interest was the analysis of perinatal outcomes. A strong neg-ative correlation was found between congenital pneumonia (r=-0.73; p=0.03), intraventricular hemorrhage (r=-0.74; p=0.03), necrotizing enterocolitis (r=-0.72; p=0.03), and severe asphyxia (r=-0.75; p=0.03).
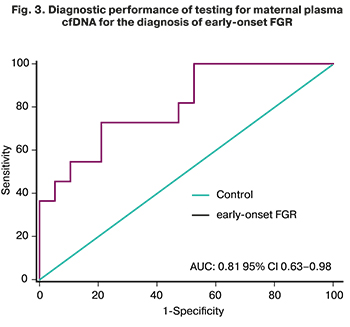
ROC analysis showed that testing for maternal plasma cfDNA at the threshold of 119.11 GE/ml had high sensitivity (73.1%) and specificity (79.3%) (AUC=0.81, 95% CI 0.63–0.98) for detecting early FGR and predict the likelihood of newborn complications such as congenital pneumonia, intra-ventricular hemorrhage, necrotizing enterocolitis and asphyxia severe in new-borns with an early form of FGR (Fig. 3).
To elucidate the mechanisms underlying the above findings, the next stage of the study was the immunofluorescence analysis of placentas in early and late FGR (Fig. 4). In the early-onset FGR, the findings showed abnormal pla-cental architectonics. There were intermediate immature villi and intermediate mature villi closely spaced with each other with the dominance of the stromal component and capillary hypoplasia. Excessive deposition of perivillous fi-brinoid led to the formation of afunctional zones and a decrease in the volume of the intervillous space. Chaotic sclerotic chorionic villi with an increased number of stromal cells and single microvessels were also found. These chang-es in the hypoplasia of the distal villi are associated with the incomplete gesta-tional transformation of the spiral arteries and are a manifestation of maternal malperfusion (Fig.4A). The study of placentas in the late FGR showed persis-tence of villous differentiation. In mature villi, compensatory-adaptive reac-tions were expressed. An increase in syncytiotrophoblast activity led to the formation of large syncytial knots located on the pedicle. It consisted of dense-ly located syncytiotrophoblast nuclei as to the rise in capillary volume. At the same time, small fibrinoid foci were observed, which did not lead to a decrease in the intervillous volume. A small number of intermediate immature, interme-diate mature, and chaotic sclerotic villi were noted (Fig. 4B). The placentas in the control group were characterized by a predominance of the terminal and mature intermediate villi and free from encrusting fibrinoid deposits. It was noted that the proportion of terminal villous endothelium exceeded the stromal component (Fig. 4B).
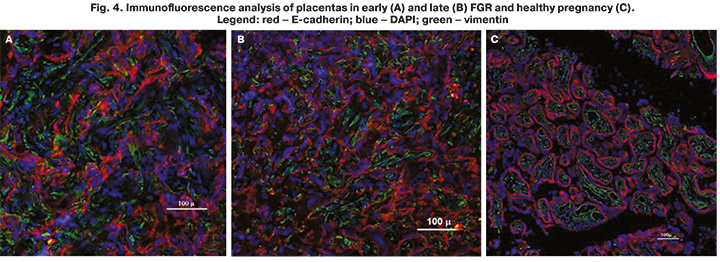
The study findings indicated a positive correlation between the level of cfDNA and the placenta weight. Thus, they served as one of the indirect con-firmations of the placental origin of cfDNA, which is consistent with other studies. Besides, the findings on placental architectonics showed the predomi-nance of fibrosis processes and the formation of extensive fibrinoid zones. This observation explains the decrease in cfDNA and the development of impaired placental perfusion, leading to FGR.
Currently, the assessment of the prognostic and diagnostic value of cfDNA, as one of the most studied non-invasive biomolecules, is of utmost priority in modern obstetrics, mainly due to the change in the level of this marker in vari-ous pregnancy complications before the onset of any symptoms.
Conclusion
The study identified the diagnostic accuracy of cfDNA for differentiating early from late-onset FGR. The findings suggest that early and late-onset FGR have different underlying mechanisms. Testing for cfDNA allows for predicting the risk of having children with low body weight and height. The findings on the high diagnostic accuracy of cfDNA in detecting the early FGR suggest the prospects of using cfDNA as a predictive marker.
References
- Железова М.Е., Зефирова Т.А., Канюков С.С. Задержка роста плода: современные подходы к диагностике и ведению беременности. Практическая медицина. 2019; 17(4): 8-14. [Zhelezova M.E., Zefirova T.A., Kanyukov S.S. Fetal growth restriction: modern approaches to the diagnosis and management of pregnancy. Practical medicine. 2019; 17(4): 8-14. (in Russian)].
- Ярыгина Т.А., Батаева Р.С. Задержка (замедление) роста плода: современные принципы диагностики, классификации и динамического наблюдения. Ультразвуковая и функциональная диагностика. 2019; 2: 33-44. [Yarygina T.A., Bataeva R.S. Fetal growth delay (deceleration): modern principles of diagnosis, classification and dynamic observation. Ultrasound and functional di-agnostics. 2019; 2: 33-44. (in Russian)].
- Gordijn S.J., Beune I.M., Thilaganathan B., Papageorghiou A., Bas-chat A.A., Baker P.N. et al. Consensus definition of fetal growth restriction: a Del-phi procedure. Ultrasound Obstet. Gynecol. 2016; 48(3): 333-9. https://dx.doi.org/10.1002/uog.15884.
- Стрижаков А.Н., Мирющенко М.И., Игнатко И.В., Попова Н.Г., Флорова В.С., Кузнецов А.С. Прогнозирование синдрома задержки роста плода у беременных высокого риска. Акушерство и гинекология. 2017; 7: 34-44. [Strizhakov A.N., Miryushchenko M.M., Ignatko I.V., Popova N.G., Florova V.S., Kuznetsov A.S. Prediction of fetal growth restriction in high-risk pregnant women. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2017; 7: 34-44. (in Russian)]. https://dx.doi.org/10.18565/aig.2017.7.34-44.
- Figueras F., Gratacos E. Update on the diagnosis and classification of fetal growth restriction and proposal of a stage-based management protocol. Fetal Diagn. Ther. 2014; 36(2): 86-98. https://dx.doi.org/10.1159/000357592.
- Rizzo G., Mappa I., Rizzo G., D'Antonio F. International gestational age-specific centiles for umbilical artery Doppler indices: a longitudinal prospec-tive cohort study of the INTERGROWTH-21st Project. Am. J. Obstet. Gynecol. 2020; 224(2): 248-9. https://dx.doi.org/10.1016/j.ajog.2020.08.110.
- Barker D.J. Adult consequences of fetal growth restriction. Clin. Ob-stet. Gynecol. 2006; 49(2): 270-83. https://dx.doi.org/10.1097/00003081-200606000-00009.
- Savchev S., Figueras F., Sanz-Cortes M., Cruz-Lemini M., Triunfo S., Botet F., Gratacos E. Evaluation of an optimal gestational age cut-off for the defi-nition of early-and late-onset fetal growth restriction. Fetal Diagn. Ther. 2014; 36(2): 99-105. https://dx.doi.org/10.1159/000355525.
- Красный А.М., Кан Н.Е., Тютюнник В.Л., Садекова А.А., Сарибекова А.Г., Кокоева Д.Н., Салпагарова З.Х., Меджидова М.К., Вторушина В.В., Кречетова Л.В. Прогнозирование преждевременных родов путем комбинированного определения цитокинов и внеклеточной ДНК. Акушерство и гинекология. 2019; 1: 86-91. [Krasnyi A.M., Kan N.E., Tyutyunnik V.L., Sadekova A.A., Saribekova A.G., Kokoeva D.N., Salpagarova Z.Kh., Medzhidova M.K., Vtorushina, V.V., Krechetova, L.V. Predicting preterm labor by combined testing of cytokines and cell-free DNA. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; 1: 86-91. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.1.86-91.
- Breveglieri G., D'Aversa E., Finotti A., Borgatti M. Non-invasive prenatal testing using fetal DNA. Mol. Diagn. Ther. 2019; 23(2): 291-9. https://dx.doi.org/10.1007/s40291-019-00385-2.
- Kwak D.W., Kim S.Y., Kim H.J., Lim J.H., Kim Y.H., Ryu H.M. Maternal total cell-free DNA in preeclampsia with and without intrauterine growth restriction. Sci. Rep. 2020; 10(1): 11848. https://dx.doi.org/10.1038/s41598-020-68842-1.
- Wertaschnigg D., Lucovnik M., Klieser E., Huber-Katamay J., Moertl M.G. Increased cell-free fetal DNA fraction in the first trimester: A sign of abnormally invasive placenta? Ultraschall Med. 2020; 41(5): 560-1. https://dx.doi.org/10.1055/a-0770-5209.
- Alberry M.S., Maddocks D.G., Hadi M.A., Metawi H., Hunt L.P., Ab-del-Fattah S.A. et al. Quantification of cell free fetal DNA in maternal plasma in normal pregnancies and in pregnancies with placental dysfunction. Am. J. Obstet. Gynecol. 2009; 200(1): 98.e1-6. https://dx.doi.org/10.1016/j.ajog.2008.07.063.
- Illanes S., Parra M., Serra R., Pino K., Figueroa-Diesel H., Romero C. et al. Increased free fetal DNA levels in early pregnancy plasma of women who subsequently develop preeclampsia and intrauterine growth restriction. Prenat. Di-agn. 2009; 29(12): 1118-22. https://dx.doi.org/10.1002/pd.2372.
- Cebe F.S., Nur Tola E.N., Koşar P.A., Oral B. DNA methylation profiles of genes associated with angiogenesis in the samples of placenta in pregnancies complicated by intrauterine growth restriction. J. Matern. Fetal Neonatal Med. 2019 Oct 3: 1-9. https://dx.doi.org/10.1080/14767058.2019.1671344.
- Al Nakib M., Desbrière R., Bonello N., Bretelle F., Boubli L., Gabert J., Levy-Mozziconacci A. Total and fetal cell-free DNA analysis in maternal blood as markers of placental insufficiency in intrauterine growth restriction. Fetal Diagn. Ther. 2009; 26(1): 24-8. https://dx.doi.org/10.1159/000236355.
- Rafaeli-Yehudai T., Imterat M., Douvdevani A., Tirosh D., Benshalom-Tirosh N., Mastrolia S.A. et al. Maternal total cell-free DNA in preeclampsia and fetal growth restriction: Evidence of differences in maternal response to abnormal implantation. PLoS One. 2018; 13(7): e0200360. https://dx.doi.org/10.1371/journal.pone.0200360.
- Morano D., Rossi S., Lapucci C., Pittalis M.C., Farina A. Cell-free DNA (cfDNA) fetal fraction in early- and late-onset fetal growth restriction. Mol. Diagn. Ther. 2018; 22(5): 613-9. https://dx.doi.org/10.1007/s40291-018-0353-9.
Received 17.02.2021
Accepted 10.06.2021
About the Authors
Natalia E. Kan, Dr. Med. Sci., Professor, Deputy Director for Science, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russian Federation. Tel.: +7(926)220-86-55. E-mail: kan-med@mail.ru. Researcher ID: B-2370-2015, SPIN-код: 5378-8437,Authors ID: 624900, Scopus Author ID: 57008835600, ORCID ID: 0000-0001-5087-5946 117997, Russia, Moscow, Ac. Oparina str., 4.
Victor L. Tyutyunnik, Dr. Med. Sci., Professor, Leading Researcher at the Center for Clinical Research, Department of Research Administration, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russian Federation; Head Physician, Perinatal Center of
European Medical Center. Tel.: +7(903)969-50-41. E-mail: tioutiounnik@mail.ru. Researcher ID: B-2364-2015, SPIN-код: 1963-1359, Authors ID: 213217,
Scopus Author ID: 56190621500, ORCID ID: 0000-0002-5830-5099. 117997, Russia, Moscow, Ac. Oparina str., 4; 125040, Russia, Moscow, Pravda str. 15/1.
Zarine V. Khachatryan, Ph.D. student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health
of Russian Federation. Tel.: +7(909)656-24-56. E-mail: z.v.khachatryan@gmail.com. 117997, Russia, Moscow, Ac. Oparina str., 4.
Alsu A. Sadekova, Ph.D., Senior Researcher at the Cytology Laboratory, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russian Federation. Tel.: +7(495)438-22-72. E-mail: a_sadekova@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Aleksey M. Krasnyi, Ph.D. (bio. sci.), Head of the Cytology Laboratory, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russian Federation. Tel.: +7(495)438-22-72. E-mail: alexred@list.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
For citation: Kan N.E., Tyutyunnik V.L., Khachatryan Z.V., Sadekova A.A., Krasnyi A.M. Predictive value of testing for maternal plasma cell-free fetal DNA in fetal growth restriction.
Akusherstvo i Ginekologiya / Obstetrics and Gynecology. 2021; 6: 60-65 (in Russian)
http://dx.doi.org/10.18565/aig.2021.6.60-65



