Prevention of respiratory disorders in late preterm neonates born to mothers with abnormally invasive placenta
Nikonets A.D., Balashova E.N., Ionov O.V., Kirtbaya A.R., Zubkov V.V., Shmakov R.G., Degtyarev D.N.
Objective: To evaluate the efficacy of antenatal prevention of neonatal respiratory distress syndrome (RDS) at 34/0–36/6 weeks of gestation at preterm delivery in patients with abnormally invasive placenta and to determine the optimal timing and frequency of maternal antenatal corticosteroid (AC) administration in pregnant women with abnormally invasive placenta.
Materials and methods: This study included 226 late preterm neonates born to mothers with abnormally invasive placenta. The patients were divided into Group 1 (study group, n=80), which comprised children whose mothers received a full course of RDS prevention within 7 days before delivery, and Group 2 (control group, n=146), which consisted of children born to mothers who received antenatal RDS prevention for > 7 days before delivery. Furthermore, the children were divided into four subgroups: subgroup 1A (n=42) included children whose mothers received a single course of RDS prevention no more than seven days before delivery; subgroup 1B (n=35) included children whose mothers received two courses of RDS prevention, one of which was no more than seven days before delivery. Subgroups 2A (n=97) and 2B (n=45) represented children whose mothers had received RDS prevention more than seven days before delivery once and twice, respectively. The analyzed parameters included gestational age (GA) of the newborns, birth weight and length, sex, Apgar score at 1 and 5 minutes, frequency and duration of neonatal respiratory therapy (non-invasive respiratory support, invasive mechanical ventilation (IMV), high-frequency oscillatory ventilation (HFOV), maximum required mean airway pressure, frequency and duration of supplemental oxygen delivery, frequency of surfactant therapy and neonatal hypoglycemia, length of stay in the NICU, and total length of infant hospitalization.
Results: Infants born to mothers with abnormally invasive placentas who were given RDS prevention seven days before birth were 1.6 times less likely to require intubation and invasive respiratory therapy (RR [95%CI] 0.62 [0.39; 0.96]), 1.8 times less likely to require HFOV (RR [95%CI] 0.57 [0.35;0.93]), and 1.7 times less likely to require supplemental oxygen (RR [95%CI] 0.59 [0.39;0.87]). The required oxygen concentration in this group was significantly lower, and there was a significantly shorter total duration of respiratory support and a shorter length of stay in the NICU. When comparing cases with single and double courses of RDS prevention, considering the time of administration in relation to labor, no significant benefits of increasing the frequency of courses were found.
Conclusion: Antenatal RDS prevention during the latest 7 days before delivery is effective in reducing the severity of respiratory disorders in late preterm infants of 34/0-36/6 gestational age born to mothers with abnormally invasive placenta. The course of RDS prevention in the earlier stages of pregnancy is not decisive, and in patients with abnormally invasive placenta, an additional course is required during the week before the planned delivery.
Authors’ contributions: Nikonets A.D., Balashova E.N., Ionov O.V., Kirtbaya A.R., Shmakov R.G. – conception and design of the study; Balashova E.N., Nikonets A.D., Kirtbaya A.R. – formation of a database, data collection and analysis; Balashova E.N., Nikonets A.D. – statistical analysis; Nikonets A.D., Balashova E.N. – drafting of the manuscript; Ionov O.V., Kirtbaya A.R., Zubkov V.V., Shmakov R.G., Degtyarev D.N. – general supervision, information collection, editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The study was conducted according to the state assignment of planned research work 25-A19 “Prediction, diagnosis and treatment of patients with placenta accreta” No. R&D AAAA-A19-119021490133-6.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors’ Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Nikonets A.D., Balashova E.N., Ionov O.V., Kirtbaya A.R., Zubkov V.V., Shmakov R.G., Degtyarev D.N. Prevention of respiratory disorders in late preterm neonates born to mothers with abnormally invasive placenta.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (1): 90-100 (in Russian)
https://dx.doi.org/10.18565/aig.2023.265
Keywords
There has been a recent increase in the incidence of abnormally invasive placenta; however, the impact of this pregnancy complication on neonatal outcomes and respiratory morbidity has not been reported in a large multicenter clinical trial. Existing literature data are primarily focused on comparisons of the gestational age of children, their weight-for-age ratio, and the need for neonatal intensive care.
Several clinical studies have shown that abnormally invasive placenta is associated with an increased incidence of respiratory distress syndrome (RDS), longer need for neonatal respiratory support, and the use of continuous positive airway pressure [1, 2]. Our previous analysis demonstrated the dependence of the severity of respiratory and cardiovascular disorders on the presence of an abnormally invasive placenta in the mother, leading to a more severe course in the early neonatal period and a higher incidence of RDS in children of similar gestational age (GA) born to mothers without an abnormally invasive placenta [3].
When analyzing the available modern clinical recommendations for the management of pregnant women with an abnormally invasive placenta, differences were noted regarding antenatal prevention of neonatal RDS. The International Society for Abnormally Invasive Placenta (IS-PAS) [4] and the American Society of Obstetricians and Gynecologists (ACOG) [5] recommend antenatal RDS prevention with GA according to local protocols for the management of women at risk of preterm labor, regardless of the presence of abnormally invasive placenta. The ACOG also recommends a single course of betamethasone for pregnant women at 34/0–36/6 gestational age at risk of preterm labor in the next 7 days and not having received previous courses [6]. In contrast, the Royal Society of Obstetricians and Gynecologists (RCOG) recommends a course of antenatal corticosteroids (AC) at 34/0–35/6 weeks of gestation for pregnant women with low-lying placenta and placenta previa, without specifying a strategy in the presence of an abnormally invasive placenta [7]. These recommendations can be applied to cases of abnormal placentation, because in the vast majority of cases, abnormal placentation is associated with placenta previa.
The FIGO recommendations do not address antenatal RDS prevention in pregnant women with abnormally invasive placenta. The Russian Society of Obstetricians and Gynecologists (RSOAG) recommends antenatal RDS prevention for women with abnormally invasive placenta until 36 weeks of gestation in cases of recurrent episodes of bloody discharge or uterine contractions, taking into account the high risk of emergency delivery and a repeat course of RDS prevention if more than 14 days have passed since the previous course of AC [8].
To date, the issue of antenatal RDS AC prevention in women with a gestational age of 34/0–36/6 weeks remains controversial [4, 9]. Previously, the use of antenatal RDS prevention in the late preterm birth cohort (34/0–36/6 weeks) in the mid-1990s was limited because of a lack of studies showing the efficacy of AC when administered after 34/0 weeks [10]. However, further studies revealed an increased incidence of neonatal morbidity and mortality in late preterm infants compared with preterm infants [11].
The first large study to show the efficacy of antenatal RDS prevention for late preterm delivery (34/0–36/6 weeks) was the Gyamfi-Bannerman С. et al. study published in 2016. This study demonstrated a reduction in the use of respiratory and surfactant therapies and supplemental oxygen in children whose mothers received antenatal prevention with betamethasone. In addition, the study group showed a significant reduction in the incidence of transient tachypnea of the newborn (TTN) and bronchopulmonary dysplasia. Among the negative effects, a higher incidence of neonatal hypoglycemia was noted [12].
This study aimed to evaluate the efficacy of antenatal prevention of neonatal RDS performed at 34/0–36/6 weeks of gestation during early delivery in patients with abnormally invasive placenta, and to determine the optimal timing and frequency of maternal AC administration in pregnant women with abnormally invasive placenta.
Materials and methods
This prospective comparative cohort study included 266 preterm infants born to mothers with an abnormally invasive placenta who were admitted to the A.G. Antonov Neonatal Intensive Care Unit named after prof. A.G. Antonov, Institute of Neonatology and Pediatrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, from January 2019 to December 2022. Informed consent for participation in the study was obtained from legal representatives of the newborns. The study was reviewed and approved by the Research Ethics Committee of Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Preterm infants with GA 34/0–36/6 weeks born to mothers with abnormally invasive placenta were included in the study. Children with congenital malformations, chromosomal anomalies, multiple pregnancies, and those who did not receive antenatal RDS prevention were excluded from the study (n=40). The analysis included 226 late preterm infants born to mothers with an abnormally invasive placenta, who received antenatal RDS prevention. Figure 1 shows the flowchart of patient selection.
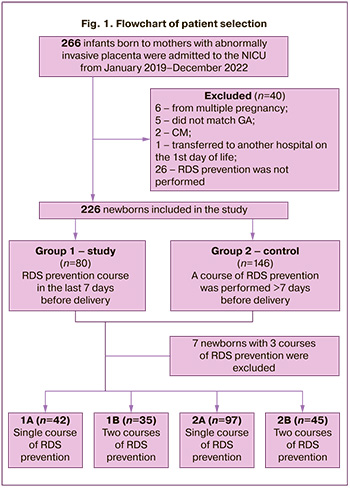
To evaluate the efficacy of AC antenatal RDS prevention in a cohort of late preterm infants born to mothers with abnormally invasive placenta, the children included in the study were divided into two groups. The study group (Group 1, n=80) included infants whose mothers had received a full course of RDS prevention in the last 7 days before delivery. The control group (Group 2, n=146) consisted of children born to mothers with an abnormally invasive placenta who received antenatal RDS prevention for >7 days before delivery.
To compare the efficacy of repeated antenatal courses of AC in these patient groups, the children were further divided into 4 subgroups depending on the number and timing of the prophylactic courses. Subgroup 1A (n=42) included children whose mothers had received a single course of RDS prevention for less than 7 days before delivery. Subgroup 1B (n=35) included children whose mothers received 2 courses of RDS prevention, one of which was conducted not more than 7 days before delivery. Subgroups 2A (n=97) and 2B (n=45) were represented by children whose mothers received RDS prevention more than seven days before delivery, once and twice, respectively. Seven infants who received 3 courses of RDS prevention were excluded from the subgroups.
Characterization of the patient groups included GA, birth weight, body length, head circumference, sex, Apgar score at 1st and 5th minute, and timing of the last course of RDS prevention.
The primary endpoints of the study included incidence (RDS, congenital pneumonia (CP), and TTN) and severity criteria for respiratory distress, including the frequency and duration of respiratory therapy (non-invasive respiratory therapy, invasive mechanical ventilation (IMV) including high-frequency oscillatory ventilation (HFOV) used for severe respiratory distress and in case of ineffectiveness of conventional IMV), maximum required mean airway pressure, frequency, duration, and maximum concentration of supplemental oxygen, and frequency of surfactant replacement therapy.
Secondary endpoints were neonatal intensive care unit (NICU) length of stay, total neonatal hospitalization time, and incidence of hypoglycemia as a short-term adverse effect of AC in prenatal prevention.
Antenatal RDS prevention was performed according to the current clinical guidelines, namely, dexamethasone at a dose of 8 mg intramuscularly three times with an injection interval of 8 h (total dose of 24 mg) [13].
Statistical analysis
Statistical analysis was performed using the StatTech v. 3.0.9, and IBM SPSS, version 26.0. Sample size calculations were based on our previous study [3], in which the frequency of HFOV use in newborns in the control group was 23% and in children born to mothers with abnormally invasive placenta was 42%. With a type I error α=0.05, power of 80%, and control/study group ratio of 1.8, we estimated that 76 patients in the study group and 137 patients in the control group would be required. The sample size was calculated using G*Power V3.1. The distribution of continuous variables was tested for normality using the Shapiro-Wilk and Kolmogorov–Smirnov tests and graphical analysis of data. Given the absence of a normal distribution of the data, nonparametric statistical methods were used. Quantitative data are presented as the median (Me) and interquartile range (IQR) of 25–75 percentile. For clarity, the maximum oxygen demand was additionally presented as the arithmetic mean (M) with standard deviation (SD) and a 95% confidence interval (95% CI). Categorical variables are presented as counts and percentages. Comparisons of quantitative variables between two groups were analyzed using the nonparametric Mann–Whitney test, between three or more groups using the Kruskal–Wallis test, followed by pairwise comparison using the Mann–Whitney test with Bonferroni correction for multiple comparisons. The Pearson's χ2 test was used to compare categorical variables. The Yates correction was used when the expected frequencies were between 5 and 10. The relative risk (RR) with 95% confidence interval (CI) was calculated to assess the influence of risk factors. Differences between the groups were considered statistically significant at p<0.05.
Results
The characteristics of the neonates included in this study are presented in Table 1.
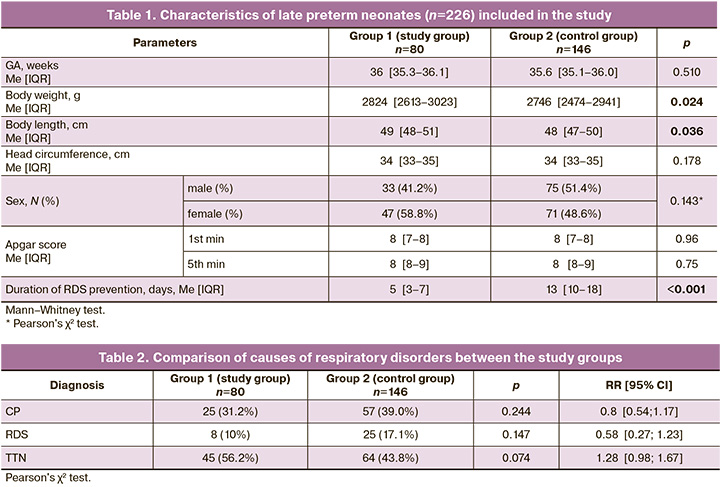
The groups were comparable in GA, birth weight, head circumference, and Apgar score at 1st and 5th minutes. Infants in the control group had significantly shorter body length at birth. The sex distribution was also comparable. The median time from the last course of RDS prevention to delivery was 5 days in the study group and 13 days in the control group (p<0.001).
The incidences of TTN, RDS, and CP were compared to assess the effectiveness of antenatal RDS prevention. The causes of the respiratory disorders in the study groups are shown in Table 2.
There were no statistically significant differences in the incidence of CP, RDS, and TTN.
To compare the severity of respiratory disorders in the study groups, we analyzed the frequency of use of noninvasive respiratory therapy, IMV and HFOV, supplemental oxygen, surfactant replacement therapy, total duration of respiratory therapy, duration of HFOV and supplemental oxygen delivery, and, consequently, the length of stay of children in the NICU and hospital. The maximum required parameters of respiratory therapy, particularly the oxygen concentration and mean airway pressure, were also compared. The results are presented in Table 3.
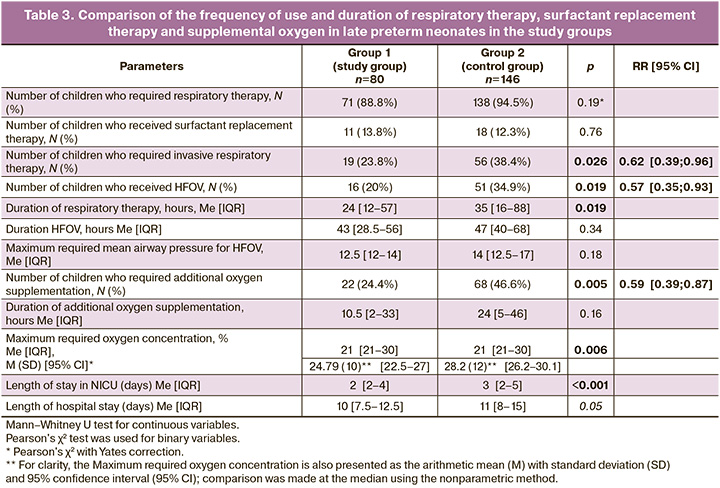
Children born to mothers with abnormally invasive placenta who received antenatal RDS prevention with AC seven days before birth were significantly less likely to undergo intubation and invasive respiratory therapy (IMV or HFOV) and less likely to require supplemental oxygen.
Although children in the study group were 1.6 times less likely to require invasive respiratory therapy and 1.8 times less likely to require conversion to HFOV, the duration of invasive respiratory support was not significantly different between the groups. However, the total duration of respiratory therapy was significantly longer in the control group. This, in turn, was reflected in a significantly longer length of stay in the NICU for the control group children, and the total duration of hospitalization in both groups was borderline of significance.
Children without RDS prevention required additional oxygen supplementation 1.7 times more often, and the required inspired oxygen concentration in this group was significantly higher.
There were no significant differences in the mean airway pressure during HFOV and duration of oxygen therapy between the groups.
The frequency of hypoglycemia did not differ between the groups: Group 1, 46/80 (57.5%); Group 2, 79/146 (54.1%) (p=0.624).
To determine the most effective antenatal RDS prevention regimen, the children were divided into subgroups according to the timing and frequency of antenatal AC administration. The characteristics of the subgroups are presented in Table 4.
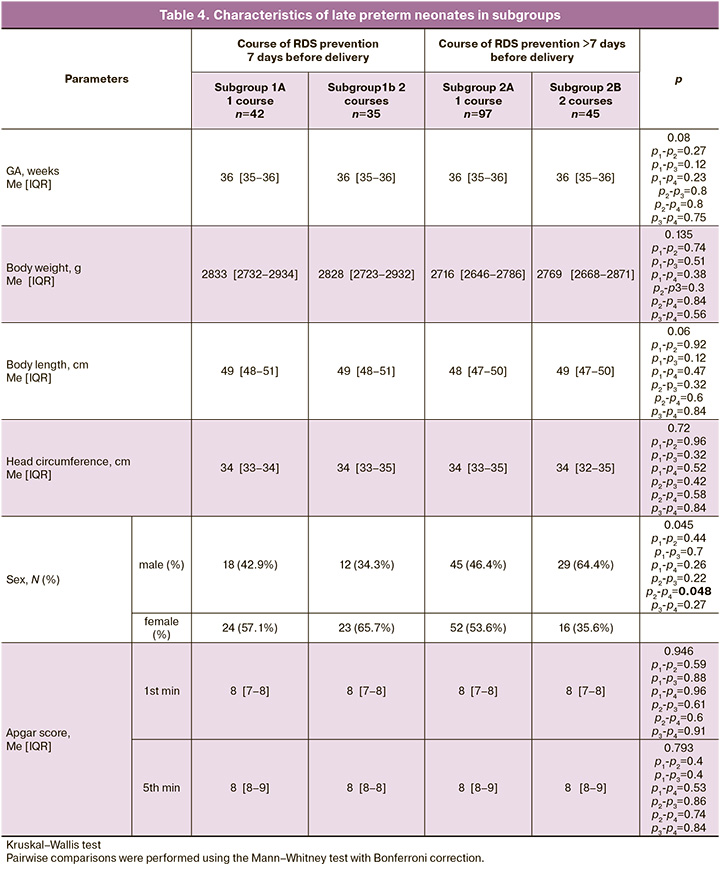
In subgroups 1A and 2B, the neonatal GA, anthropometric parameters, and Apgar scores did not differ between the groups.
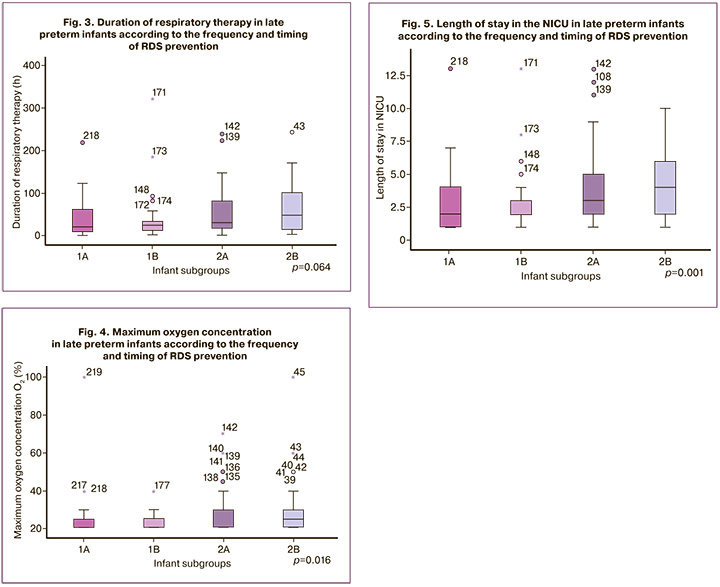
We compared the abovementioned parameters of respiratory therapy, duration of hospitalization in the NICU, and incidence of hypoglycemia in subgroups according to the timing and frequency of antenatal AC administration. The results are summarized in Figures 2–5.
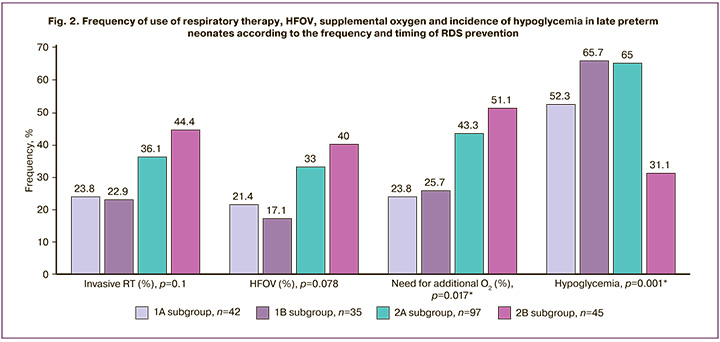
When comparing the frequency and duration of respiratory therapy and the frequency of need for supplemental oxygen in the subgroups, statistically significant differences were found in the frequency of supplemental oxygen use and maximum oxygen concentration required. Thus, the highest frequency of supplemental oxygen use – 23/45 (51.1%) and maximum oxygen concentration were observed in the subgroup of children whose mothers received two AC courses, the last of which was later than 7 days before delivery, compared with children whose mothers received an AC course in the last 7 days before delivery (p=0.017 and p=0.016, respectively). Moreover, in children who received an AC course > 7 days before birth, the frequency of supplemental oxygen use and maximum concentration did not differ significantly according to the frequency of AC courses (1 course, 42/97 (43.3%); 2 courses, 23/45 (51.1%)). There were no significant differences in the frequency of supplemental oxygen use and maximum oxygen concentration between subgroups 1A and 1B. With one course of RDS prevention administered 7 days before delivery, the frequency of supplemental oxygen use was 10/42 (23.8%); with two courses, one of which was administered 7 days before delivery, with a frequency of 9/35 (25.7%).
Similar results were obtained when comparing the duration of hospitalization in the NICU. The longest duration was observed in subgroup 2B (4 days on average), whereas the duration of NICU stay in the subgroups with RDS prevention in the last 7 days before delivery, regardless of the number of courses administered, averaged 2 days (p=0.001).
There were no significant differences in the frequency of HFOV use and invasive respiratory therapy, or in the duration of respiratory support in the subgroups.
When comparing the frequency of hypoglycemia in the subgroups, significant differences were found. The highest incidence of neonatal hypoglycemia was observed in the group of children whose mothers were given RDS prevention twice, the last one in the period of 7 days before delivery – 23/35 (65.7%), and the lowest incidence of hypoglycemia was observed in the late double prevention – 14/45 (31.1%) (p=0.001).
Discussion
To date, the efficacy of antenatal RDS prevention in improving lung tissue maturation in late preterm infants born to mothers with abnormally invasive placenta has not been specifically studied [4].
A multicenter, randomized, placebo-controlled trial by Gyamfi-Bannerman С. et al. (2016) demonstrated a reduction in the use of continuous positive airway pressure (CPAP), high-flow cannula (HFC), supplemental oxygen, and surfactant therapy in infants whose mothers received antenatal betamethasone for late delivery [12]. These data partially agreed with the results of the present study. Specifically, we found a decrease in the frequency of supplemental oxygen use and a lower concentration of supplemental oxygen in children in the study group.
In addition, a study by Gyamfi-Bannerman С. et al. assessed the need for CPAP or HFC over 12 hours, which was significantly lower in children whose mothers received RDS prevention [12]. This indirectly correlates with the decrease in the total duration of respiratory therapy in the study group in our study. Children whose mothers did not receive timely RDS prevention stayed longer in the NICU than the children in the study group [12], which is comparable to our results.
We found no significant differences in the use of non-invasive respiratory therapy in general or in surfactant therapy. We also did not observe any significant differences in the incidence of TTN, RDS, and CP.
The observed discrepancies may be explained by the fact that not all women in the study group of Gyamfi-Bannerman С. et al. [12] completed the full course of prevention (only 60%). Additionally, those who had previously received RDS prevention were excluded from the study, whereas such women were included in our study.
In 2016, a systematic review and meta-analysis was conducted, including the above study [14].
According to the results of this meta-analysis, newborns whose mothers received antenatal AC at more than 34 weeks' gestation had a significantly lower risk of RDS, TTN, lower frequency of surfactant and IMV use, and a significantly shorter duration of supplemental oxygen delivery. These infants required a lower maximum inhaled oxygen concentration, shorter stay in the NICU, and higher Apgar scores than the control group [14].
Of the above indicators, our study identified significant differences in the frequency of IMV and oxygen supplementation as well as in the maximum required concentration of supplemental oxygen. The data on the decrease in the length of stay in the NICU of the children in the study group were also correlated.
A limitation to the use of antenatal RDS prevention in late pregnancy is the currently described short- and long-term side effects [15]. These include delayed fetal growth [16], neonatal hypoglycemia [12], and long-term outcomes such as poor school performance [17], increased vulnerability to stress-related physical and mental disorders [18], and metabolic disturbances in adulthood [19, 20].
In a retrospective cohort study, neonates born late preterm and preterm exposed antenatally to AC had significantly lower birth weight and length, and smaller head circumference. Moreover, the detected fetal growth restriction was exacerbated by repeated courses of AC [16].
In contrast, in our study, infants born to mothers with abnormally invasive placenta who received antenatal RDS AC prevention 7 days before birth had significantly higher birth weight and body length than the controls. The other anthropometric parameters were comparable between the groups, and no significant differences were found when these parameters were evaluated according to the multiplicity of the prevention courses administered.
A study by Gyamfi-Bannerman С. et al. showed a significant increase in the incidence of neonatal hypoglycemia in neonates whose mothers received antenatal AC courses [12]. In our study, the incidence of hypoglycemia in infants whose mothers received prevention in the last 7 days before delivery and in infants whose mothers received a single course of AC prevention more than 7 days before delivery did not differ significantly. Interestingly, the group of children whose mothers received two courses of RDS prevention later than 7 days before delivery had the lowest incidence of hypoglycemia. These findings cast doubt on the association between the incidence of hypoglycemia and the timing and frequency of AC administration.
Study limitations
The limitations of our study were the unequal number of children in the subgroups (the highest number (n=97) in the subgroup of children with single prevention more than 7 days before delivery and the lowest in the subgroup of children with two courses of prevention, the last of which was administered within 7 days before delivery (n=35). In addition, the proportion of maternal diabetes mellitus and gestational diabetes mellitus was not considered when analyzing the incidence of hypoglycemia.
Conclusion
The results of our study showed that antenatal AC for the prevention of RDS at 34/0–36/6 weeks in women with abnormally invasive placenta at least 7 days before delivery reduces the severity of respiratory distress, frequency and duration of invasive respiratory therapy, including HFOV, in late preterm infants, who have a more severe course of the early neonatal period and a higher incidence of RDS than in infants born to mothers without abnormally invasive placenta. The positive effects observed were independent of the number of RDS prevention courses, and the underlying factor was the duration of the prevention course. A course of RDS prevention performed earlier in pregnancy is not determinative, and an additional course in the week before the planned delivery in patients with an abnormally invasive placenta is required.
References
- Spillane N.T., Zamudio S., Alvarez-Perez J., Andrews T., Nyirenda T., Alvarez M., Al-Khan A. Increased incidence of respiratory distress syndrome in neonates of mothers with abnormally invasive placentation. PLoS One. 2018;13(7): e0201266. https://dx.doi.org/10.1371/journal.pone.0201266.
- Munoz J.L., Kimura A.M., Julia J., Tunnell C., Hernandez B., Curbelo J. et al. Impact of placenta accreta spectrum (PAS) pathology on neonatal respiratory outcomes in cesarean hysterectomies. J. Matern. Fetal Neonatal Med. 2022;35(26):10692-7. https://dx.doi.org/10.1080/14767058.2022.2157716.
- Балашова Е.Н., Ионов О.В., Киртбая А.Р., Никонец А.Д., Михеева А.А., Васильченко О.Н., Зубков В.В., Шмаков Р.Г., Дегтярев Д.Н. Особенности дыхательных и сердечно-сосудистых нарушений у недоношенных детей, рожденных у матерей с врастанием плаценты. Акушерство и гинекология. 2021;5:85-93. [Balashova E.N., Ionov O.V., Kirtbaya A.R., Nikonets A.D., Mikheeva A.A., Vasilchenko O.N., Zubkov V.V., Shmakov R.G., Degtyarev D.N. The features of respiratory and cardiovascular disorders in preterm infants born to mothers with abnormally invasive placenta. Obstetrics and Gynecology. 2021;(5):85-93. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.5.85-93.
- Collins S.L., Alemdar B, van Beekhuizen H.J., Bertholdt C., Braun T., Calda P.et al. Evidence-based guidelines for the management of abnormally invasive placenta: recommendations from the International Society for Abnormally Invasive Placenta. Am. J. Obstet. Gynecol. 2019;220(6):511-26.https://dx.doi.org/10.1016/j.ajog.2019.02.054.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric Care Consensus No. 7: Placenta Accreta Spectrum. Obstet. Gynecol. 2018;132(6):e259-e275. https://dx.doi.org/10.1097/AOG.0000000000002983.
- Reddy U.M., Deshmukh U., Dude A., Harper L., Osmundson S.S. Society for Maternal-Fetal Medicine Consult Series #58: Use of antenatal corticosteroids for individuals at risk for late preterm delivery: Replaces SMFM Statement #4, Implementation of the use of antenatal corticosteroids in the late preterm birth period in women at risk for preterm delivery, August 2016. Am. J. Obstet. Gynecol. 2021;225(5):B36-B42. https://dx.doi.org/10.1016/j.ajog.2021.07.023.
- Jauniaux E., Alfirevic Z., Bhide A.G., Belfort M.A., Burton G.J., Collins S.L. et al.; Royal College of Obstetricians and Gynaecologists. Placenta Praevia and Placenta Accreta: Diagnosis and Management: Green-top Guideline No. 27a. BJOG. 2019;126(1):e1-e48. https://dx.doi.org/10.1111/1471-0528.15306.
- Министерство здравоохранения Российской Федерации. Клинические рекомендации «Патологическое прикрепление плаценты (предлежание и врастание плаценты)». 2023. [Ministry of Health of the Russian Federation. Clinical guidelines "Pathological attachment of the placenta (presentation and ingrowth of the placenta)." 2023. (in Russian)].
- Shanks A.L., Grasch J.L., Quinney S.K., Haas D.M. Controversies in antenatal corticosteroids. Semin. Fetal Neonatal Med. 2019;24(3):182-8.https://dx.doi.org/10.1016/j.siny.2019.05.002.
- Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. JAMA. 1995;273(5):413-8.https://dx.doi.org/10.1001/jama.1995.03520290065031.
- McIntire D.D., Leveno K.J. Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet. Gynecol. 2008;111(1):35-41. https://dx.doi.org/10.1097/01.AOG.0000297311.33046.73.
- Gyamfi-Bannerman C., Thom E.A., Blackwell S.C., Tita A.T., Reddy U.M., Saade G.R. et al.; NICHD Maternal-Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N. Engl. J. Med. 2016;374(14):1311-20. https://dx.doi.org/10.1056/NEJMoa1516783.
- Министерство здравоохранения Российской Федерации. Клинические рекомендации «Преждевременные роды». 2020. [The Ministry of Health of the Russian Federation. Clinical recommendations "Premature birth". 2020.(in Russian)].
- Saccone G., Berghella V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. BMJ. 2016;355:i5044. https://dx.doi.org/10.1136/bmj.i5044.
- Haviv H.R., Said J., Mol B.W. The place of antenatal corticosteroids in late preterm and early term births. Semin. Fetal Neonatal Med. 2019;24(1):37-42. https://dx.doi.org/10.1016/j.siny.2018.10.001.
- Braun T., Sloboda D.M., Tutschek B., Harder T., Challis J.R., Dudenhausen J.W. et al. Fetal and neonatal outcomes after term and preterm delivery following betamethasone administration. Int. J. Gynaecol. Obstet. 2015;130(1):64-9. https://dx.doi.org/10.1016/j.ijgo.2015.01.013
- Stutchfield P.R., Whitaker R., Gliddon A.E., Hobson L., Kotecha S., Doull I.J. Behavioural, educational and respiratory outcomes of antenatal betamethasone for term caesarean section (ASTECS trial). Arch. Dis. Child. Fetal Neonatal Ed. 2013;98(3):F195-200. https://dx.doi.org/10.1136/archdischild-2012-303157.
- Alexander N., Rosenlöcher F., Stalder T., Linke J., Distler W., Morgner J., Kirschbaum C. Impact of antenatal synthetic glucocorticoid exposure on endocrine stress reactivity in term-born children. J. Clin. Endocrinol. Metab. 2012;97(10):3538-44. https://dx.doi: https://dx.doi.org/10.1210/jc.2012-1970
- Doyle L.W., Ford G.W., Davis N.M., Callanan C. Antenatal corticosteroid therapy and blood pressure at 14 years of age in preterm children. Clin. Sci. (Lond). 2000;98(2):137-42.
- Dalziel S.R., Walker N.K., Parag V., Mantell C., Rea H.H., Rodgers A., Harding J.E. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet. 2005;365(9474):1856-62. https://dx.doi.org/10.1016/S0140-6736(05)66617-2.
Received 14.11.2023
Accepted 24.11.2023
About the Authors
Anastasia D. Nikonets, MD, PhD Student at the Neonatal Intensive Care Unit named after Prof. A.G. Antonov, Institute of Neonatology and Pediatrics,Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-22-77,
nikon.na@yandex.ru, 117997, Russia, Moscow, Oparina str., 4, https://orcid.org/0000-0002-4717-1865
Ekaterina N. Balashova, MD, PhD, Leading Researcher at the Neonatal Intensive Care Unit named after Prof. A.G. Antonov, Institute of Neonatology and Pediatrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-2277,
e_balashova@oparina4.ru, 117997, Russia, Moscow, Oparina str., 4, https://orcid.org/0000-0002-3741-0770
Oleg V. Ionov, Dr. Med. Sci., Head of the Neonatal Intensive Care Unit named after Prof. A.G. Antonov, Institute of Neonatology and Pediatrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Oparina str., 4;
Professor of Neonatology Department at the Faculty of Pediatrics, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia, 119435, Russia, Moscow, B. Pirogovskaya str., 2-4, +7(495)438-22-77, o_ionov@oparina4.ru, https://orcid.org/0000-0002-4153-133X
Аnna R. Kirtbaya, Dr. Med. Sci., Clinical Care Supervisor at the Neonatal Intensive Care Unit named after Prof. A.G. Antonov, Institute of Neonatology and Pediatrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecologyand Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Oparina str., 4; Professor at the Neonatology Department at the Faculty of Pediatrics, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia, 119435, Russia, Moscow, B. Pirogovskaya str., 2-4, +7(495)438-2277, a_kirtbaya@oparina4.ru, https://orcid.org/0000-0002-7628-8157
Victor V. Zubkov, Dr. Med. Sci., Director of the Institute of Neonatology and Pediatrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Oparina str., 4; Professor of Neonatology Department, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia (Sechenov University), 119435, Russia, Moscow, B. Pirogovskaya str., 2-4, +7(495)438-22-66, v_zubkov@oparina4.ru,
https://orcid.org/0000-0001-8366-5208
Roman G. Shmakov, Dr. Med. Sci., Professor, Director of the Institute of Obstetrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics,
Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-72-00, r_shmakov@oparina4.ru, 117997, Russia, Moscow, Oparina str., 4,
https://orcid.org/0000-0002-2206-1002
Dmitriy N. Degtyarev, Dr. Med. Sci., Professor, Deputy Director, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Oparina str., 4; Head of Neonatology Department, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia (Sechenov University), 119435, Russia, Moscow, B. Pirogovskaya str., 2-4+7(495)438-23-88, d_degtiarev@oparina4.ru, https://orcid.org/0000-0002-2206-1002



