Congenital infection-associated genetic polymorphisms in children
Objective. To investigate the features of the genetic polymorphisms that determine the implementation and characteristics of the course of congenital infections in newborn infants, by taking into account their gestational age.Nikitina I.V., Donnikov A.E., Krogh-Jensen O.A., Lenyushkina A.A., Bystritsky A.A., Kryuchko D.S., Ionov O.V., Zubkov V.V., Degtyarev D.N.
Subjects and methods. The investigation enrolled 379 newborn infants with respiratory disorders of different origin, which required respiratory therapy. All the babies were born in the V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia, and treated at the Center’s Intensive Care Unit in January 2013 to December 2015. According to the infectious or non-infectious genesis of respiratory disorders, the infants enrolled in the investigation were divided into 2 study groups: 1) 161 babies with transient tachypnea of the newborn or respiratory distress syndrome; 2) 218 infants with intrauterine infection (congenital pneumonia or early neonatal sepsis). Based on their gestational age, the babies of the two study groups were divided into four subgroups: 1) 24–28 weeks; 2) 29–32 weeks; 3) 33–36 weeks; and 4) 37 or more weeks.
Results. The investigators revealed statistically significant differences between the genetic polymorphisms in the neonates born at different gestation ages who had an infectious or non-infectious cause of respiratory disorders, according to the distribution of the following genotypes and alleles: NOS3-786, NOS3-894, and IL1b at 29–32 weeks’ gestation, AGTR2, IL4R1902, IL8, GNB825, and HTR2A at 33–36 weeks, and IL8, ADD1, and ADRB3 at 37 or more weeks.
Conclusion. This investigation has shown that different genetic polymorphisms are associated with the development of infectious and inflammatory diseases in newborns at different gestational ages, which appears to reflect the process of maturation of the immune system. Not only the genes of innate immunity, but also the regulators of energy metabolism and vascular tone have been found to be involved in the development of infectious complications, which suggests that there is a complex mechanism for the development of neonatal septic conditions.
Keywords
The application of high technology in neonatology resulted in the increase of survival rate among premature newborns, especially among those with very low body weight (VLBW) and extremely low body weight (ELBW) at birth. The most widespread signs of various pathological processes in premature newborns at early neonatal period are respiratory disorders. Their existence may indicate both manifestation of early neonatal sepsis (ENS) and development of congenital pneumonia or severe respiratory distress syndrome (RDS). Early diagnosis of congenital infection in relation to such category of newborns still causes difficulties because of non-specific clinical symptoms in early premature newborns, fast generalization of infectious process and lack of highly-sensitive and specific biological markers [1]to investigate differences between early onset versus late-onset sepsis, and non-proven versus proven sepsis, and to examine differences in TTP by organism type using a retrospective observational study at the Neonatal Intensive Care Unit, Antwerp University Hospital, Belgium. The subjects were 1828 neonates with suspected sepsis who were treated with antimicrobials for at least 3 days. The TTP was recorded for all episodes of suspected sepsis in an approximately 6.5 year period. A total of 2916 blood cultures were collected, of which 437 (15%. Due to these reasons the study of genetic predisposition to the implementation of congenital infections in infants may provide better understanding of pathological processes, hasten diagnosis search, offer new ways of treatment and develop preventive measures.
The aim of the study was to investigate infection-associated genetic polymorphisms in newborns given their gestational age.
Materials and Methods
The research enrolled 379 infants with respiratory disorders of different origin, which required respiratory therapy. All the babies were born in the Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia, and treated at the Center’s Intensive Care Unit in January 2013 to December 2015. Exclusion criteria were congenital abnormalities and apparent intrauterine growth restriction (body weight at birth less than the 3rd percentile according to the standard anthropometric scale).
The parents of the newborns signed voluntary informed consent for their children to participate in the research. The research was approved by the Ethical Committee of the Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia.
 All newborns underwent clinical and laboratory examination in order to diagnose congenital infection. The examination included thoracic organs X-ray analysis, blood culture analysis, complete blood count with absolute leukocyte, platelet and neutrophil count as well as with neutrophil index count, protein level control in acute inflammation (С-reactive protein) on the first days of life. The results of the clinical and laboratory examination at 72 hours of age showed either the existence or the absence of genital infection in infants [2, 3]neonatal sepsis remains a frequent and devastating problem among hospitalized preterm neonates. Despite multiple attempts to address this unmet need, there have been minimal advances in clinical management, outcomes, and accuracy of diagnostic testing options over the last three decades. One strong contributor to a lack of medical progress is a variable case definition of disease. The inability to agree on a precise definition greatly reduces the likelihood of aligning findings from epidemiologists, clinicians, and researchers, which, in turn, severely hinders progress towards improving outcomes.\n\nRecent findings\nPediatric consensus definitions for sepsis are not accurate in term infants and are not appropriate for preterm infants. In contrast to the defined multi-stage criteria for other devastating diseases encountered in the NICU (e.g., bronchopulmonary dysplasia. Differential diagnosis of congenital pneumonia, sepsis, RDS or transient tachypnea of the newborn (TTN) was established according to the standard criteria [4, 5]. Depending on the genesis of the respiratory disorders infants were divided into two study groups. The first group comprised newborns with non-infectious respiratory disorders: TTN or RDS (n = 161), the second group comprised newborns with infectious respiratory disorders: congenital pneumonia or ENS (n = 218). The design of the study is shown in Fig. 1.
All newborns underwent clinical and laboratory examination in order to diagnose congenital infection. The examination included thoracic organs X-ray analysis, blood culture analysis, complete blood count with absolute leukocyte, platelet and neutrophil count as well as with neutrophil index count, protein level control in acute inflammation (С-reactive protein) on the first days of life. The results of the clinical and laboratory examination at 72 hours of age showed either the existence or the absence of genital infection in infants [2, 3]neonatal sepsis remains a frequent and devastating problem among hospitalized preterm neonates. Despite multiple attempts to address this unmet need, there have been minimal advances in clinical management, outcomes, and accuracy of diagnostic testing options over the last three decades. One strong contributor to a lack of medical progress is a variable case definition of disease. The inability to agree on a precise definition greatly reduces the likelihood of aligning findings from epidemiologists, clinicians, and researchers, which, in turn, severely hinders progress towards improving outcomes.\n\nRecent findings\nPediatric consensus definitions for sepsis are not accurate in term infants and are not appropriate for preterm infants. In contrast to the defined multi-stage criteria for other devastating diseases encountered in the NICU (e.g., bronchopulmonary dysplasia. Differential diagnosis of congenital pneumonia, sepsis, RDS or transient tachypnea of the newborn (TTN) was established according to the standard criteria [4, 5]. Depending on the genesis of the respiratory disorders infants were divided into two study groups. The first group comprised newborns with non-infectious respiratory disorders: TTN or RDS (n = 161), the second group comprised newborns with infectious respiratory disorders: congenital pneumonia or ENS (n = 218). The design of the study is shown in Fig. 1.
Anthropometric data of the premature infants were evaluated using Fenton Preterm Growth Chart, mature infants were assessed using weight-for-age charts of the World Health Organization.
Given the results of our previous studies, which pointed out the specifics of cytokine status and mRNA expression in newborns at different gestational age [6–8], in this study the newborns of the two main groups were divided into four subgroups (24–28 weeks, 29–32 weeks, 33–36 weeks, 37 or more weeks) (Fig. 1).
All the newborns included into the research underwent the blood collection into the test tubes with ethylene diamine tetraacetate acid (EDTA) on the first days of life. They were also exposed to the obligatory clinical and laboratory analysis on the admission to the Center’s Intensive Care Unit till the start of the specific therapy. Genotyping was conducted using polymorphic loci (the names of the functional groups are given according to the Gene Ontology Resource [9], see Table 1):

Genotyping was carried out by real-time PCR together with high-resolution melting curve (HRM) analysis using adjacent probes and diagnostic test-systems produced by Company DNA-Technology LLC, Russia.
DNA for genotyping was extracted from peripheral blood lymphocytes, collected with EDTA as anticoagulant using DNA extraction kit «PREP-GS-GENETICS» by Company DNA-Technology LLC, Russia. The method is based on the usage of strong chaotropic agents for cell lysis followed by the nucleic acid sorption on the solid support, sequential absorbent removal and the elution of DNA. The volume of the samples after the outflux comprised 100 mcl. DNA concentration, detected on DNA-minifluorimeter (Ноеfer, USA), was on average 50-100 mcl/ml. PCR-method and the measurement of melting temperature of oligonucleotide probes were conducted using Detecting thermocycler DT-964 (Company DNA-Technology LLC, Russia).
Statistical data processing was carried out with the help of SPSS Statistics version 23 software package (IBM, USA). Normality of distribution was assessed via the Kolmogorov-Smirnov test together with the Lilliefors and Shapiro-Wilk tests. Excess kurtosis and asymmetry were also assessed. Abnormal distribution was detected in all the subgroups. In order to find out statistical significance of differences among groups we used Mann–Whitney U-test. The results are shown in a median line (Ме) and interquartile range of the 25–75 percentile, minimum and maximum value (min-max). The differences were regarded as verifiable at the significance level of p < 0.05. To assess the correlational relationship, Spearman’s correlation coefficient was used. On the analysis of nominal variables Pearson’s chi-squared test together with Yates’ correction were brought into use, as well as Fisher’s exact test when there were few samples.
Results and Discussion
Clinical characteristics of the patients are shown in Table 2.

It should be noted that the major cause of respiratory disorders in the infants born before the 29th week of gestation were congenital pneumonia and ENS (one child in the subgroup of non-infectious respiratory disorders).
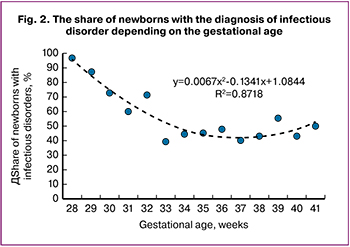 As demonstrated in Fig. 2, among the infants born before the 33rd week of gestation newborns with ENS and congenital pneumonia prevailed. In general, upon the gestational age increase, the non-infectious respiratory disorders rate also increased. From the 33rd week of pregnancy and further, the rate of non-infectious respiratory disorders in the newborns kept relatively permanent.
As demonstrated in Fig. 2, among the infants born before the 33rd week of gestation newborns with ENS and congenital pneumonia prevailed. In general, upon the gestational age increase, the non-infectious respiratory disorders rate also increased. From the 33rd week of pregnancy and further, the rate of non-infectious respiratory disorders in the newborns kept relatively permanent.
The research revealed statistically significant differences between the distribution of allelic variants of some genes in the infants, born at different gestational age depending on the infectious and non-infectious cause of respiratory disorders (Fig. 3): NOS3-786, NOS3-894, and IL1b at 29-32 weeks of gestation; AGTR2, IL4R1902, IL8, GNB825, and HTR2A at 33-36 weeks, and IL8, ADD1, and ADRB3 at 37 or more weeks.
The data obtained enabled us to conclude that gene polymorphisms of the different functional groups are associated with the development of infectious diseases. In order to understand the possible role of the genes indicated above, we performed the analysis of the literature data.

NOS3 gene. Within this study we analyzed the distribution of the alleles and genotypes of the NOS3 gene two polymorphic loci (endothelial nitric oxide synthase, eNOS): 894 G>T in exon 7 and -786 T>C in promoter, associated with the changes in nitric oxide production (NO). No linkage disequilibrium for these loci found. NOS3 is constantly being expressed by the respiratory passages epithelium, its level is rising on physical exertion, stress and chronic hypoxia. NO is famous as a strong endogenous agent that takes part in angiogenesis, immunoregulation processes and the production of surfactants that cause the relaxation of the vascular smooth muscles. These processes increase the endothelium permeability and down-regulate platelets vascular adhesion. The downfall of the endogenous NO production level causes vasoconstriction which in its turn results in the blood pressure increase. NO is also involved in such processes as: neurons stimulation, neuron impulse transmission, gastrointestinal tract regulation and respiratory as well as urogenital system monitoring, in olfactory memory building, synaptic connection and in angiogenesis (formation of the new blood vessels).
The question on the role of NOS3 polymorphism in the genesis of respiratory disorders in the newborns is still barely touched upon in the Russian research studies. The only study conducted by O.V. Ionov et al. [10], highlighted the association of the C allele of the NOS3: -786 polymorphic loci with acute respiratory disorders and need for high frequency oscillation in newborns with congenital pneumonia (р = 0.028). NOS3, participating in oxidative stress reactions and NO synthesis, which works as a relaxant for endothelium, seems to influence endothelium permeability which, in its turn, causes ventilation-perfusion match, that results in the need for stringent respiratory therapy parameters.
The experiment showed that the -786 С allele of NOS3 gene promoter initiates the decrease of its activity. At the same time G replaced with T, resulting in Glu substituted with Asp in a 298 coding triplet inside mature protein, increases the NOS3 receptiveness to enzymatic degradation in vitro, which may lead to the lowering in NO production [11]. We were able to observe statistically significant rate increase in the alleles carriage, which are bound to the lowering of NO among children with infectious and inflammatory disorders born at 29–32 weeks of gestation (for -786 T > C 62.5% against 34.6%: OR = 3.15 (1.21–8.17), р= 0.02 and for 894 G>T 53.1% against 30.8%: OR = 2.55 (0.97–6.71), р = 0.05 according to autosomal and dominant pattern). At later gestational age no statistically significant differences in the alleles and NOS3 genotypes distribution were observed.
IL1b gene encodes the synthesis of the pro-inflammatory cytokine which is produced by activated macrophages and is considered to be a very important mediator for the inflammatory response. It is as well involved into cell processes including proliferation, differentiation and cell apoptosis. It is well-known that the Il1b level is growing on the development of RDS. During the research study of hsa-miR-92a-3p mRNA level expression (potentially regulating the transmission of IL-1β) in the infants born at 32 weeks and earlier and suffering from infectious respiratory disorders mRNA level increase in dark blood samples was detected (p=0.004) comparing to children suffering from TTN or RDS [8].
This study is also intended to analyze the distribution of the alleles of the two functionally significant polymorphisms: -31 T>C (rs1143627) and 3953 C>T (rs16944). –the 31T and 3953С alleles are associated with the higher production of IL1b, and T>C haplotype is characterized by maximum transcriptional activity.
While conducting research we managed to find statistically significant differences in genotypes and the alleles distribution of polymorphic loci: -31 T>C in premature children born at 29–32 weeks of gestation with congenital infection comparing to the group with TTN/RDS. The carriage of the С gene low-productivity allele was more frequently noted in the newborns with sepsis and congenital pneumonia, than in the newborns with RDS (68.8% versus 38.5% correspondingly, ОR = 3.52, 95% CI: 1.36–9.11, р = 0.008) according to the autosomal and dominant genetic inheritance pattern. Besides, though no statistically significant differences in the genotypes and alleles distribution of the second polymorphic locus were detected, C>T low-productivity haplotype frequency in patients with infectious complications was 2.8 times higher, than with RDS (4.8% versus 1.7%), but due to the rare occurrence of the haplotype these differences did not come to statistical significance.
In our previous research study, dedicated to the cytokine gene expression in premature children, born at 25–32 weeks of gestation, the buccal swab and dark blood sample taken on the first days showed tendency for the decrease in mRNA-gene expression of the congenital immunity of Il1b in premature children with a congenital infection [6]. These results may be obtained due to the congenital immune system deficiency, which may be manifested in the predisposition to infectious complications in extremely premature children.
At the same time, according to the study carried out by Abu-Maziad A. et al. [12]case control study involving 535 preterm infants examining the roles of sequence polymorphisms in genes that mediate host immune responses to bacterial infection in newborn infants. A total of 49 single nucleotide polymorphisms (SNPs in the USA which included 535 premature children (23–36 weeks of gestation), the association of ENS with Il1b polymorphism was not defined. However, it should be noted that the researchers did not conduct the analysis of the gestational age of the patients. We did not find any statistically significant differences in the distribution of the alleles and genotypes of the IL1B gene after 32 weeks of gestation either.
AGTR2 gene encodes the receptors of the 2nd type of angiotensin II and, correspondingly, is considered to be the one of the key genes of renin-angiotensin system (RAS). The role of RAS pathway is widely studied in the processes of blood pressure regulation, kidney and cardiovascular activity. However, today it is acknowledged that RAS has several points of attack inside the organism, including pulmonary processes. Angiotensin 2, appearing RAS basic hormone, in vitro demonstrated itself as an inflammation activator, improving the synthesis of pro-inflammatory cytokines and chemokines through АТ1 и АТ2 receptors with the subsequent activation of NF-κB signaling pathway (nuclear factor kappa-light-chain-enhancer of activated B cells). NF-κB is a protein complex, regulating DNA transcription, cytokines production, and cell survival rate. NF-κB plays a pivotal role in the regulation of the immune response to the infection.
The main effects of angiotensin II are provoking apoptosis of pulmonary vascular endothelial cells and alveolar epithelial cells; playing an important role in the fibrosis response to acute lung injury, causing the transformation of -α growth factor expression in the lungs, promoting fibroblasts proliferation; inducing hypertrophic and hyperplastic growth of vascular smooth muscle cells (myointimal hyperplasia); regulating NO-synthetase expression [13, 14].
Therefore, RAS is likely to take an active part in the development of lung injury together with lung dysfunction. According to the latest research data, the absence of AGTR2 and its selective «silencing» improves the lung functioning results in an animal model. Thus, AGTR2 is considered to have a possible therapeutic effect in case of a diminished lung function in patients with cystic fibrosis, inflammation processes in lungs and bronchopulmonary dysplasia (BPD).
As a result we discovered that the T allele is associated with infectious complications in the newborns of 29–32 and 33–36 weeks of gestation. Total genotyping frequency of the Т allele in these age groups was 58% for sepsis versus 36% for RDS (р=0.00002). Apart from this the association observed may be described as both an autosomal and dominant (р=0.003), and as an autosomal and recessive (р=0.002) inheritance pattern. After 36 weeks of gestation the T allele frequency in the groups was not significantly different (54 and 58%, respectively).
Il4R gene encodes the receptor of the Il4 regulatory cytokine, which is the main regulator of Th1-Th2 bias and plays an important role in preventing excessive inflammatory process. The research also demonstrates a statistically significant association of the G allele carriage with the infectious respiratory disorders not only in premature infants at 33–36 weeks of gestation (ОR = 3.55, 95% CI: 1.59-7.93, р < 0.001). Protective role and polymorphism of the Il4R gene are described in several research studies, dedicated to the necrotizing enterocolitis (NEC) in the newborns [15]. In older children IL-4Rα is regarded as an important component of sensibilization and the induction of the sensitivity reaction. According to Li L. et al. [16], polymorphism of the IL4R gene is associated with bronchial allergy. The G allele carriage appeared to be statistically significant more often in the newborns at 33–36 weeks of gestation with the infectious respiratory disorders (53.2% versus 24.2%, respectively, р = 0,002, ОR = 3.55 (1.59–7.93) according to the autosomal and dominant inheritance pattern). The differences in two other groups were not statistically significant though there was the same tendency in the newborns at 29–32 weeks of gestation: the G allele frequency was 40.6% versus 30.8%.
IL8 gene encodes Interleukin-8 (IL-8) which is one of the main pro-inflammatory cytokines, synthetized by macrophages, epithelial and endothelial cells, containing Toll-like receptors. The increase of Il8 level results in the migration of the above-mentioned cells to the affected area where the phagocytosis stimulation takes place [17, 18]cord blood samples of neonates with septic bacterial infection were analyzed qualitatively and semiquantitatively by reverse transcriptase-polymerase chain reaction (RT-PCR. Such reactions as the increase in intercellular Са2+, exocytosis and respiratory burst necessary for phagocytosis are produced in the target cells. Also, Il8 is famous as an angiogenesis inductor.
The -251 А?Т polymorphism is situated in the promotor area, and the A allele is associated with the low level of IL-8. It is found that IL-8 causes chemotaxis and the activation of neutrophils in bacterial infections, which promotes further cytokines production.
According to the research of the congenital immune response in the newborns on the first days of life, the intense increase in IL8 level as a response to the infectious processes was observed only in the newborns at 25–31 weeks of gestation. At later gestational age the IL8 level did not change in inflammatory processes [7]. Genetically the predisposition associated with the lack of cytokine response appears in the decrease of the resistance to the infectious agents. In this research we discovered the association of the А/А low-productive genotype with inflammatory respiratory disorders in the premature infants born at 33-36 weeks of gestation (ОR=2.56, 95% CI: 1.09–6.02, р = 0.02). It is noteworthy that in the premature newborns this low-productive genotype is associated with non-inflammatory respiratory disorders (OR = 4.76 (1.18–19.15), р = 0.02), which may reflect the switching of the cytokine regulative mechanisms of the immune response before the birth delivery.
HTR2A gene encodes the 2A serotonin receptor. Nowadays it is widely researched in neurology and psychiatry as a genetic marker, associated with the development of obsessive-compulsory disorders and schizophrenia [19]mRNA expression and methylation studies have reported inconsistent results. In this study, we examine HTR2A expression and methylation and the interaction with HTR2A polymorphisms to identify their biological significance in schizophrenia. Subjects included 25 schizophrenia and 25 control post-mortem brain samples. Genotype and mRNA data was generated by transcriptome sequencing. DNA methylation profiles were generated for CpG sites within promoter-exon I region. Expression, genotype and methylation data were examined for association with schizophrenia. HTR2A mRNA levels were reduced by 14% (p = 0.006. This gene was not examined earlier in the newborns. As part of the study the 102С/С genotype association with the congenital infection in the infants at 33-36 weeks of gestation subgroup was detected (ОR = 2.35 (1.07–5.16), р = 0.03). No such association was detected in other subgroups.
GNB3 gene encodes the guanine nucleotide-binding protein, which takes part in the lymphoblast and fibroblast differentiation, proliferative activity, intracellular transition of the α2-adrenergic receptor signaling [20]. The С for Т substitution at position 825 of exon 9 leads to the alternative splicing and synthesis of the protein shortened by 41 amino acids. The 825Т allele in the patients suffering from arterial hypertension is associated with the increase of the proliferative activity and vasoconstriction [21]. We did not find any studies on this gene polymorphism in the newborns. We defined the association of the Т/Т rare genotype with non-infectious respiratory disorders. Remarkably, the percent of the carriers of this genotype among the newborns with TTN/RDS was high in all the examined groups, but the most prominent association was observed at early gestation (ОR= 8.22 (0.81 – 83.04), р = 0.04 at gestational age of 29–33 weeks and ОR = 2.92 (0.92–9.28), р = 0.058 after 33 weeks of gestation).
ADD1 gene encodes alpha-adducin, a protein that is part of the cytoskeleton and is involved in the transition of the signal inside the cell. It can also interact with other membrane and skeleton proteins, which are responsible for the transition of the ions through the cell membrane. Adducins regulate actin dynamics that provoke the endothelial barrier function. Vascular endothelium, overlying the internal surface of the blood vessels, accurately controls the transmission of the dissolved matters, macromolecules, plasma proteins and inflammatory mediators and, therefore, provides the selective barrier between blood and surrounding tissue. In inflammatory conditions the intercellular detachment happens which becomes the cause of the vascular permeability increase. Adducins are the pivotal regulators of the actin polymerization process. They also control cell migration and the formation of the intercellular contacts. According to the latest research studies, adducin participates in the formation of the endothelial barrier cohesiveness and modulates Ca2+ that depends on the reorganization of the strengthening adhesion nodes between the cells, managing cell permeability. The 1378T (460Trp) allele in adult patients is associated with higher susceptibility to the sodium balance, which presumes predisposition to the arterial hypertension [22, 23]. In our study children with congenital pneumonia and ENS appeared to be the carriers of the 1378Т allele of ADD1 gene more often than full-term children with RDS and TTN (ОR = 3.08, 95% CI: 1.06-8.94, р = 0.036).
ADRB3 gene encodes Β3-adrenergic receptor (β3-AR, ADRB3), which is an important component of the sympathetic nervous system which regulates lipolysis and thermogenesis, angionesis and vasorelaxation processes. The increase of the local lipolysis occurs on the moment of receptor activation and, therefore, energy production which is the pathogenetic cause for many conditions. Endothelial β3-AR provoke vasorelaxation of the coronary arteries by having impact on the NO production. The 190 T>C (Trp64Arg) substitution leads to the decrease in the adenylylcyclase activity in response to the agonists activity, including endogenous catecholamine, adrenaline and noradrenaline [24]. The gene polymorphism is associated with the development of the essential arterial hypertension and vigorous physical stamina [25]. No research on the gene polymorphism in the newborns was carried out earlier. We associate the С allele with the development of the infectious complications in premature infants (ОR = 4.69 (1.29–17.07), р=0.01) according to the autosomal and dominant inheritance pattern. Statistically significant differences in the distribution of alleles and genotypes of the polymorphic locus were not defined in the newborns of the small gestational age.
Apart from this we carried out a preliminary analysis of the metabolic pathways including genes with the statistically significant differences defined between the distributions of allele variants. The analysis was conducted by means of Gene Ontology Resource [9]. As a result, the analysis showed that NOS3 and IL1b genes, where the differences were observed at 29-32 weeks of gestation, are simultaneously involved in the processes of the smooth muscles adaptation (GO:0014805, р= 6.1×10–8) as well as in the signaling pathway, mediated by lipopolysaccharides (GO:0031663, р = 2.9×10–6). For the rest two groups of genes, corresponding to the late gestational period, no statistically significant common biological processes were discovered. A small number of analyzed gene samples may be the possible reason for it. At the same time, this fact may become a reflection of a large number of factors and biological aspects influencing the predisposition of the newborns to the congenital infections.
Conclusion
The research study became one of the steps on the way to understand genetic predictors of the implementation of congenital infections in the newborns of various gestational age, including those suffering from VLBW and ELBW at birth. The involvement of the congenital immunity genes as well as the regulators of energy exchange and vascular tonus in the development of the infectious complications demonstrate the work of the complex mechanism of the infectious conditions development in the newborns. We showed that different genes are associated with the development of inflammatory and infectious diseases in the newborns at different gestational periods, (at 29–32 weeks of gestation – NOS3 and IL1b, at 33–36 weeks of gestation – AGTR2, IL4R, IL8, GNB3, HTR2A, at 37 weeks and more – IL8, ADD1, ADRB3), which seem to reflect the process of the maturation of the immune system. The development of the molecular and genetic technologies and further studies may facilitate the development of the new approaches in diagnosis and treatment of the congenital infection.
References
- Guerti K, Devos H, Ieven MM, Mahieu LM. Time to positivity of neonatal blood cultures: fast and furious? J Med Microbiol. 2011; 60(Pt 4):446–53. doi: 10.1099/jmm.0.020651-0
- Wynn J.L. Defining Neonatal Sepsis. Curr Opin Pediatr. 2016; 28(2):135–40. doi: 10.1097/MOP.0000000000000315
- Голубцова Ю.М., Дегтярев Д.Н. Современные подходы к профилактике, диагностике и лечению раннего неонатального сепсиса. Неонатология Новости Мнения Обучение. 2014; 2:15–25.[Golubtsova Yu.M., Degtyarev D.N. Modern approaches to preventing, diagnosing, and treating early-onset neonatal sepsis. Neonatologija Novosti Mnenija Obuchenie. 2014;2:15–25.(in Russ.)].
- Антонов А. Г., Байбарина Е.Н., Балашова Е.Н., Дегтярев Д.Н., Зубков В.В., Иванов Д.О., и др. Врожденная пневмония (клинические рекомендации). Неонатология: новости, мнения, обучение. 2017; 4:133-148. [Antonov A.G., Baybarina E.N., Balashova E.N., Degtyarev D.N., Zubkov V.V., Ivanov D.O., Ionov O.V., et al. Congenital pneumonia (clinical practice guidelines). Neonatologija Novosti Mnenija Obuchenie . 2017; 4:133-148.(in Russ.)]
- Володин Н.Н., Дегтярев Д.Н., Крючко Д.С. Клинические рекомендации. Неонатология. M.: ГЭОТАР-Медиа, 2019; 320 c.[Volodin N.N., Degtyarev D.N., Kryuchko D.S. Clinical recommendations. Neonatology. M.: GEOTAR-Media, 2019; 320 c.(in Russ.)]
- Никитина И.В., Непша О.С., Донников А.Е., Трофимов Д.Ю., Милая О.В., Дегтярева А.В., Ионов О.В., Зубков В.В., Дегтярев В.Н. Современные возможности молекулярно-генетических методов в диагностике раннего неонатального сепсиса у недоношенных новорожденных. Акушерство и гинекология. 2016; 12:106–13.[Nikitina I.V., Nepsha O.S., Donnikov A.E., Trofimov D.Yu., Milaya O.V., Degtyarevа A.V., Ionov O.V., Zubkov V.V., Degtyarev D.N. Modern possibilities of molecular genetic techniques in the diagnosis of early neonatal sepsis in preterm neonates. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2016; (12): 106-13. (in Russian)]http://dx.doi.org/10.18565/aig.2016.12.106-137.
- Никитина И.В., Жукова А.С., Ванько Л.В., Вторушина В.В., Матвеева Н.К., и др. Особенности цитокинового статуса у недоношенных новорожденных с заболеваниями легких инфекционного и неинфекционного генеза. Неонатология Новости Мнения Обучение. 2018; 6(4 (22)):16–23.[Nikitina I.V., Zhukova A.S., Vanko L.V., Vtorushina V.V., Matveeva N.K., et al. Cytokine status of preterm newborns with infectious and noninfectious diseases. Neonatologija Novosti Mnenija Obuchenie. 2018; 6(4 (22)):16–23.(in Russ.)].
- Тимофеева А.В., Никитина И.В., Гусар В.А., Чаговец В.В., Киртбая А.Р.,Ионов О.В. Циркулирующие мРНК как ранний индикатор инфекционно-воспалительных заболеваний у новорожденных. Неонатология. Новости. Мнения. Обучение. 2018; 6:34–8. [Timofeeva A.V., Nikitina I.V., Gusar V.A., Chagovets V.V., Kirtbaya Anna R., Ionov Oleg V., Degtyarev Dmitriy N., Kan N.E., Frankevich V.E. Circulating miRNA as an early indicator of infectious inflammatory diseases in the newborns. Neonatologija Novosti Mnenija Obuchenie. 2018; 6:34–8.(in Russ.)].
- The Gene Ontology Consortium. The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 2019; 47(D1): D330–8.doi: 10.1093/nar/gky1055.
- Ионов О.В., Донников А.Е., Безлепкина М.Б., Никитина И.В., Байбарина Е.Н. Влияние полиморфизма генов NOS3, AGTR1, TLR9, DRD4 на тяжесть течения врожденной пневмонии у новорожденных детей. Акушерство и гинекология. 2019; 5: 102-111. [Ionov O.V., Donnikov A.E., Bezlepkina M.B., Nikitina I.V., Balashov E.N., Kirtbaya A.R., Kryuchko D.S., Baibarina E.N. Relationship between polymorphism in NOS3, AGTR1, TLR9, DRD4 genes and severity of congenital pneumonia in newborns. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; (5): 102-11. (in Russian)]. https://dx.doi.org/10.18565 /aig.2019.5.102-111.
- Tesauro M., Thompson W.C., Rogliani P., Qi L., Chaudhary P.P., Moss J. Intracellular processing of endothelial nitric oxide synthase isoforms associated with differences in severity of cardiopulmonary diseases: cleavage of proteins with aspartate vs. glutamate at position 298. Proc Natl Acad Sci U S A. 2000; 97(6): 2832–5. DOI: 10.1073/pnas.97.6.2832
- Abu-Maziad A., Schaa K.., Bell E.F., Dagle J.M., Cooper M., Marazita M.L., et al. Role of polymorphic variants as genetic modulators of infection in neonatal sepsis. Pediatr Res. 2010; 68(4): 323–9. doi: 10.1203/00006450-201011001-00632.
- Wagenaar G.T., Sengers R.M., Laghmani el H., Chen X., Lindeboom M.P., Roks A.J., Folkerts G., Walther F.J. Angiotensin II type 2 receptor ligand PD123319 attenuates hyperoxia-induced lung and heart injury at a low dose in newborn rats. Am J Physiol Lung Cell Mol Physiol. 2014; 307(3): L261-272. DOI: 10.1152/ajplung.00345.2013
- Jerng J.-S., Hsu Y.-C., Wu H.-D., Pan H.-Z., Wang H.-C., Shun C.-T., et al. Role of the renin-angiotensin system in ventilator-induced lung injury: an in vivo study in a rat model. Thorax. 2007; 62(6): 527–35. DOI: 10.1136/thx.2006.061945
- Henderson G., Craig S., Baier R.J., Helps N., Brocklehurst P., McGuire W. Cytokine gene polymorphisms in preterm infants with necrotising enterocolitis: genetic association study. Arch Dis Child Fetal Neonatal Ed. 2009; 94(2): F124–128. doi: 10.1136/adc.2007.119933
- Li L., Li Y., Zeng X.C., Li J., Du X.Y. Role of interleukin-4 genetic polymorphisms and environmental factors in the risk of asthma in children. Genet Mol Res GMR. 2016; 15(4): gmr15048873. http://dx.doi.org/10.4238/gmr15048873
- Berner R., Tüxen B., Clad A., Forster J., Brandis M. Elevated gene expression of interleukin-8 in cord blood is a sensitive marker for neonatal infection. Eur J Pediatr. 2000; 159(3): 205–10. doi: 10.1007/s004310050051
- Esposito S., Zampiero A., Pugni L., Tabano S., Pelucchi C., Ghirardi B., et al. Genetic polymorphisms and sepsis in premature neonates. PloS One. 2014; 9(7): e101248. doi: 10.1371/journal.pone.0101248
- Cheah S.-Y., Lawford B.R., Young R.Mc.D., Morris C.P., Voisey J. mRNA Expression and DNA Methylation Analysis of Serotonin Receptor 2A (HTR2A) in the Human Schizophrenic Brain. Genes [Internet]. 2017 Jan 4 [cited 2019 Apr 7];8(1). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5295009/
- Sheppard R., Hsich E., Damp J., Elkayam U., Kealey A., Ramani G., et al. GNB3 C825T Polymorphism and Myocardial Recovery in Peripartum Cardiomyopathy: Results of the Multicenter Investigations of Pregnancy-Associated Cardiomyopathy Study. Circ Heart Fail. 2016; 9(3): e002683. doi: 10.1161/CIRCHEARTFAILURE.115.002683.
- Wenzel R.R., Siffert W., Bruck H., Philipp T., Schäfers R.F. Enhanced vasoconstriction to endothelin-1, angiotensin II and noradrenaline in carriers of the GNB3 825T allele in the skin microcirculation. Pharmacogenetics. 2002; 12(6): 489–95. doi: 10.1097/00008571-200208000-00010
- Sousa A.C., Palma Dos Reis R., Pereira A., Borges S., Freitas A.I., Guerra G., Góis T., Rodrigues M., Henriques E., Freitas S., Ornelas I., Pereira D., Brehm A., Mendonça M.I. Relationship between ADD1 Gly460Trp gene polymorphism and essential hypertension in Madeira Island. Medicine. 2017; 96(42):e7861. doi: 10.1097/MD.0000000000007861
- Kugelmann D., Waschke J., Radeva M.Y. Adducin is involved in endothelial barrier stabilization. PloS One. 2015;10(5):e0126213. doi: 10.1371/journal.pone.0126213
- Piétri-Rouxel F., Manning B.St.J., Gros J., Strosberg A.D. The biochemical effect of the naturally occurring Trp64-->Arg mutation on human beta3-adrenoceptor activity. Eur J Biochem. 1997; 247(3):1174–9. DOI: 10.1111/j.1432-1033.1997.01174.x Li Y.-Y., Lu X.-Z., Wang H., Zhou Y.-H., Yang X.-X., Geng H.-Y., et al. ADRB3 Gene Trp64Arg Polymorphism and Essential Hypertension: A Meta-Analysis Including 9,555 Subjects. Front Genet. 2018; 9: 106. doi: 10.3389/fgene.2018.00106
Received 24.06.2019
Accepted 04.10.2019
About the Authors
Irina V. Nikitina, MD, Ph.D., Leading Researcher of the Neonatal Intensive Care Unit №2 of the Institute of neonatology and pediatrics National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I.Kulakov of Ministry of Healthcare of Russian Federation, Associate Professor of Neonatology Department National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I.Kulakov of Ministry of Healthcare of Russian Federation. Phone: +7 (495) 531 44 44, 2700, 2697;. е-mail: i_nikitina@oparina4.ruAddress: 117997, Russia, Moscow, Oparina street, 4.
Andrew E. Donnikov, M.D., Ph.D., Head of Laboratory of molecular genetic methods, FSBI Research center for obstetrics gynecology and perinatology MOH Russia, Moscow, Russia. E-mail: a_donnikov@oparina4.ru ORCID: 0000-0003-3504-2406. Reasearcher ID: E-7178-2015. Scopus ID: 6505485697
117997, Moscow, st. Academician Oparin, 4.
Olga A. Krogh-Jensen, MD, PhD, Neonatologist, neonatal intensive care unit. Federal State Institution “Research Center for Obstetrics, Gynecology and Perinatology”
Ministry of Healthcare of Russian Federation. Associate professor of neonatal department, Pediatric Faculty, Federal State Autonomous Educational Institution of Higher Education I.M. Sechenov First Moscow State Medical University of the Ministry of Health of the Russian Federation (Sechenov University). е-mail: olgaborisevich@gmail.com
ORCID ID: 0000-0002-5178-5659
Address: 117997, Russia, Moscow, Oparina street, 4. Phone: +7 (495)531-44 44.
Anna A Lenushkina., MD, Ph.D., Head of the of the Neonatal Intensive Care Unit №2 of the Institute of neonatology and pediatrics National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I.Kulakov of Ministry of Healthcare of Russian Federation, Associate Professor of Neonatology Department. Address: 117997, Russia, Moscow, Oparina street, 4. Phone: +7 (495) 531 44 44, 2700, 2697; e-mail: a-lenushkina@yandex.ru
Andrey A. Bystritskiy, leading researcher. Laboratory of molecular genetic methods, FSBI Research center for obstetrics gynecology and perinatology MOH Russia, Moscow, Russia. E-mail: a_donnikov@oparina4.ru ORCID: 0000-0003-3504-2406. ReasearcherID: E-7178-2015. ScopusID: 6505485697
Moscow, Russia. 117997, Moscow, st. Academician Oparin, 4.
Darya S. Kryuchko, PhD, Professor of Institute of neonatology and pediatrics, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of Ministry of Healthcare of Russian Federation. Tel.: +7(926)011-72-32; e-mail: krdarya@gmail.com
Oleg V. Ionov, Ph.D., Head of the intensive care unit of the institute of neonatology and pediatrics FSBI Scientific Center for Obstetrics, Gynecology and Perinatology
of the Russian Health Ministry Address: 117997, Russia, Moscow, Oparina street, 4. Phone: +7(495) 438-22-77. E-mail: o_ionov@oparina4.ru
Viktor V. Zubkov, Director of Institute of neonatology and pediatrics, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of Ministry of Healthcare of Russian Federation, Professor of Neonatology Department National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I.Kulakov of Ministry of Healthcare of Russian Federation,
Professor of neonatal department, Pediatric Faculty at Federal State Autonomous Educational Institution of Higher Education I.M. Sechenov First Moscow State Medical University of the Ministry of Health of the Russian Federation (Sechenov University). E-mail: victor.zubkov@mail.ru ORCID: 0000-00020-9697-9596
Address: 117997, Russia, Moscow, Oparina street, 4.
Dmitriy N. Degtyarev, MD, PhD, Vice director of National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov
of Ministry of Healthcare of Russian Federation, Professor of Neonatology Department National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I.Kulakov of Ministry of Healthcare of Russian Federation, Professor, Head of neonatal department at Pediatric Faculty, Federal State Autonomous Educational Institution of Higher Education I.M. Sechenov First Moscow State Medical University of the Ministry of Health of the Russian Federation
(Sechenov University). Phone: +74954382533. E-mail: d_degtiarev@oparina4.ru ORCID: 0000-0001-8975-2425. Address: 117997, Russia, Moscow, Oparina street, 4.
For citations: Nikitina I.V., Donnikov A.E., Krogh-Jensen O.A., Lenyushkina A.A., Bystritsky A.A., Kryuchko D.S., Ionov O.V., Zubkov V.V., Degtyarev D.N., Congenital infection-associated genetic polymorphisms in children.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2019; 11: 175-85. (In Russian).
https://dx.doi.org/10.18565/aig.2019.11.175-185



