Plasma cytokine profiles in preterm neonates with congenital pneumonia in the first week of life
Aim. To investigate plasma cytokine levels in preterm neonates with congenital pneumonia in the first week of life.Inviyaeva E.V., Nikitina I.V., Krechetova L.V., Krog-Jensen O.A., Vtorushina V.V., Lenyushkina A.A., Zubkov V.V., Degtyarev D.N.
Materials and methods. The study included 76 preterm neonates (gestational age (GA) 25–36 weeks, study group) and 13 healthy term infants (GA 37w, control group) born in our Center from January 2019 to November 2019. All preterm neonates developed clinical signs of systemic inflammatory response syndrome and respiratory failure during the first hours of life and were treated for congenital pneumonia in the neonatal intensive care unit (NICU). The children of the control group were born in satisfactory condition. Their early neonatal adaptation was uneventful. They were discharged home before the end of the first week of life. The study group was divided into two subgroups based on GA (25–31w and 32–36 w). Plasma cytokine profiles of the neonates were analyzed. Blood samples were taken on days 1, 3, and 7 of life in the study group and day 1 of life in the control group. Blood samples were collected into EDTA tubes. Plasma cytokine profiling included pro-inflammatory (IFNγ, TNFα, IL-1β, IL-2, IL-6, IL-12, IL-17), anti-inflammatory (IL-4, IL-5, IL-10, IL-13) chemokines (IL-8, MCP-1, MIP-1β), and growth factors (IL-7, G-CSF, GM-CSF).
Results. Very preterm neonates (GA 25–31w) with congenital pneumonia had higher concentrations of plasma pro-inflammatory mediators than preterm neonates of greater gestational age (GA 32–36w) and term infants
(GA 37w) on day 1 of life. The persistence of higher IL-6 and IL-8 concentrations in preterm neonates at GA 25–31w compared to preterm neonates at GA 32–36w up to day 7 of life indicates increased systemic inflammatory response from the moment of birth and incomplete relief of the inflammatory process in extremely immature children, despite comprehensive, intensive therapy.
Conclusion. Plasma cytokine profiling in preterm neonates is an effective laboratory tool for early diagnosis of congenital pneumonia and evaluating the effectiveness of therapy in the first week of life.
Keywords
The quality of nursing of preterm neonates is an essential indicator of social development and maternal and child health care. Preterm births account for 5 to 10%, not exceeding 1% for extremely preterm births (< 28 weeks) [1–3]. However, even in developed countries, preterm births, especially extremely preterm births, account for about half of perinatal losses [4].
It can be considered proven that a neonatal immune system differs significantly from its adult counterpart since intrauterine fetal development occurs with limited antigenic challenges. With a normally developing pregnancy, the relationship between maternal and fetal immune systems is balanced, which reflects the formation of maternal immune tolerance toward allogeneic fetal antigens. Among causes of prematurity, there may be an over-activation of the maternal immune system. It is believed that the maintenance of tolerance is provided by suppression of the Th1 type responses in early pregnancy and the prevalence of the Th2 type response in the middle of pregnancy. "Immunological provocation" from the fetus to the maternal organism can lead to preterm birth, and therefore, newborns' immune response is typically biased towards the anti-inflammatory Th2 response. However, suppression of Th1-type immune responses, in turn, causes a high susceptibility of the newborn to infectious complications, including the development of early neonatal sepsis and congenital pneumonia [3].
Congenital pneumonia is a multifactorial disease. Among the leading causes for the development of pneumonia in preterm infants, particular importance is given to the structural features of the respiratory system. They include lung tissue immaturity, leading to a ventilation-perfusion mismatch, inadequate alveoli expansion, which, in turn, is associated with the susceptibility to pathological processes [5, 6].
Immaturity of the immune system, which is more pronounced in infants born preterm, plays an important role in the development of pneumonia in newborns [5, 7]. The first level factor of protection against pathogens in newborns is innate immunity. Impaired innate immunity during the development of pneumonia in newborns, especially in preterm infants, is accompanied by an imbalance in cytokine production, leading to the development of systemic inflammatory response syndrome (SIRS) [6, 8].
Therefore, investigating characteristic features of plasma soluble immunoregulatory proteins (cytokines, chemokines, growth factors) in premature infants with congenital pneumonia seems to be especially relevant from the point of view of studying the variants of immunological response during the development of an infectious process, taking into account the morphofunctional maturity of premature infants and their gestational age (GW).
The present study aimed to investigate plasma cytokine levels in preterm neonates with congenital pneumonia in the first week of life.
Materials and methods
The study included 76 preterm neonates (GA 25–36 weeks, study group) and 13 healthy term infants (GA 37w, control group) born at the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia (from now on referred to as the Center) from January to November 2019. All preterm neonates developed clinical signs of systemic inflammatory response syndrome and respiratory failure during the first hours of life and were treated for congenital pneumonia in the neonatal intensive care unit (NICU). The children of the control group were born in satisfactory condition. Their early neonatal adaptation was uneventful, and they were discharged home before the end of the first week of life.
The exclusion criteria from the study were a syndromic form of hereditary pathology, congenital defects requiring urgent surgical correction, and hemolytic disease of the newborn.
Parents of newborns provided signed informed consent for their children to take part in the study. The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
All newborns in the study group after initial stabilization in the delivery room were admitted to the ICU, where in the first hours of life (before the start of drug therapy and enteral feeding) simultaneously with the baseline clinical and laboratory examination, according to the unified clinical protocol, peripheral venous blood was taken for immunological studies. In the control group, children from the first hours after birth were observed in the neonatal department of the obstetric hospital until they were discharged. Blood sampling in the control group was performed once on the first day of life, along with a standard clinical and laboratory examination without additional invasive manipulations.
Anthropometric data of preterm infants were assessed using the Fenton growth charts for boys and girls [9, 10].
A unified clinical protocol developed in the ICU of the Institute of Neonatology and Pediatrics of the V.I. Kulakov NMRC for OG&P included microbiological tests of the blood samples, chest X-ray, acute-phase proteins, a complete blood count including leukocytes, platelets, neutrophils, and calculation of the neutrophilic index (NI).
The final diagnosis was made on the third day of life based on detecting at least two clinical and one laboratory sign of infection. Focal lung infiltrates on the chest x-ray were regarded as absolute signs of congenital pneumonia. Increased bronchovascular markings were considered an indirect sign of congenital pneumonia and were the basis for this diagnosis only in combination with other clinical and laboratory markers of infection [11–13].
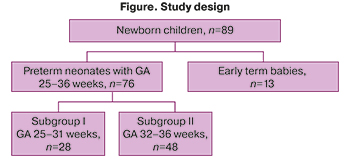 The study design is shown in the figure.
The study design is shown in the figure.
The study group's peripheral blood plasma cytokine profiles were analyzed on the first, third, and seventh days of life. Blood samples were collected in EDTA vacuum tubes.
Plasma cytokine profiling included proinflammatory (interferon (IFN)-γ, tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-2, IL-5, IL-6, IL-12, IL-17), anti-inflammatory (IL-4, IL-10, IL-13), chemokines (IL-8, monocyte chemoattractant protein (MCP)-1, macrophage inflammatory proteins (MIP) -1β and growth factors (IL- 7, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF). The analysis was performed on a flow-based laser Bio-Plex 200 system (Bio-Rad, USA) using the Bio-Plex Pro Human Cytokine 17-plex Assay commercial kit ( Bio-Rad, USA). According to the manufacturer's instructions of the Bio-Rad test system, for the preparation of EDTA plasma samples, blood samples were centrifuged at 4°C in two stages (at 1000g for 15 min and then at 10000g for 10 min) to remove platelets and sediments completely. Plasma samples were frozen and stored at -80°C until the analysis.
Statistical analysis
Statistical analysis was performed using the Microsoft Office Excel 2010 and MedCalc (version 16.8) software. Data distributions were tested for normality using the Shapiro–Wilk test. Quantitative variables showing normal distribution were expressed as means (M) and standard deviation (SD), and the Student's t-test was used to assess the significance of the differences. Non-normally distributed data were expressed as medians and interquartile ranges (IQRs). To determine the significance of differences between samples, the Mann–Whitney test was used to compare two groups or the Kruskal–Wallis test to compare three groups with subsequent post hoc analysis. Categorical variables were summarized using frequency counts and percentages. The Pearson χ2 test was used for categorical variables. Differences were considered significant at p<0.05.
Results and discussion
Table 1 summarizes the spectrum of diseases identified in the mothers of the examined neonates before and during this pregnancy.
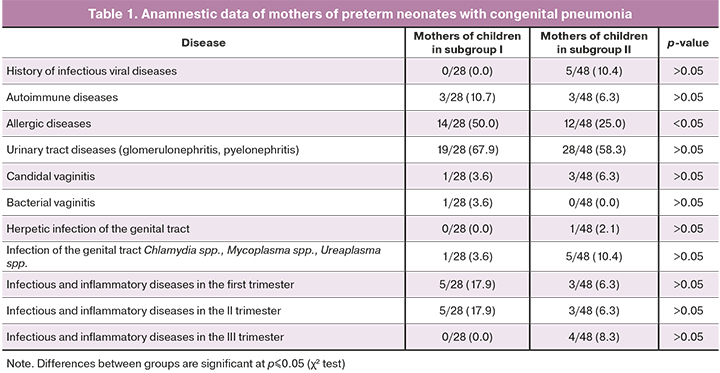
There were no statistically significant differences in past medical history and comorbidities between mothers of newborns of I and II subgroups except allergic diseases, which were significantly more common in mothers of newborns with GA 25–31 weeks (p<0.05).
The clinical characteristics of the newborns included in the study are presented in Table 2.
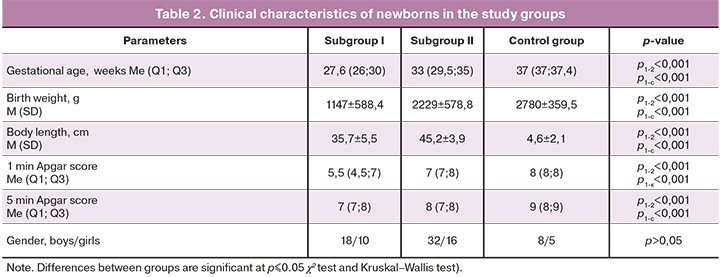
All newborns of the study group received two antibacterial drugs (penicillin + aminoglycoside) for seven days, followed by clinical and laboratory examination, including laboratory markers of the systemic inflammatory response.
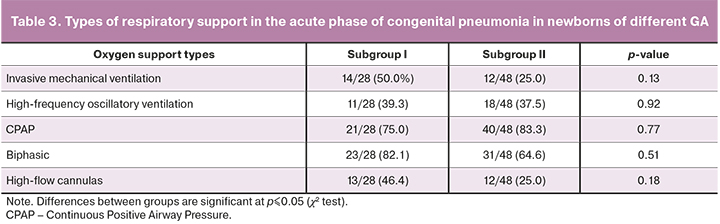
Respiratory therapy of newborns in both subgroups of the study group was differentiated according to the presence, severity, and dynamics of respiratory disorders. The frequency of its use in both groups was similar. The types of respiratory support in the acute phase of congenital pneumonia in newborns are presented in Table 3.
Disseminated intravascular coagulation (DIC) was observed only in subgroup I in 13 (46.4%) patients (p<0.001). Pulmonary hemorrhage were diagnosed in 7 newborns (25%) of this subgroup and in 2 (0.4%) subgroup 2 (p=0.019). Five (17.9%) and 5 (10.4%) neonates in subgroups I and II, respectively, had gastric (p>0.05). Skin hemorrhages were noted in 9 (32.1%) and 2 (0.4%) infants in subgroups I and II (p=0.005). Macro hematuria was observed in 3 (10.7%) very premature infants and non in subgroup II (p=0.03).
Intraventricular hemorrhages (IVH) of varying severity were diagnosed in 13 (46.4%) premature infants subgroup I and in 7 (14.6%) subgroup II, (p=0.02). Of these, 4 (14.3%) children in subgroup I and 5 (10.4%) in subgroup II had grade I IVH (p>0.05), 8 (28.6%) children in subgroup I and 1 (0.2%) in subgroup had grade II IVH (p=0.003). Grade III IVH was diagnosed in 1 (3.6%) and 1 (0.2%) newborns in subgroup I and II, respectively (p>0.05).
The subgroups were statistically significantly different in absolute leukocyte counts [9.9 (7.2; 14.5) and 12.6 (8.6; 18.1), p<0.001)] and absolute neutrophil counts [4.8 (2.5; 5.5) and 5.6 (3.7; 10.3), p<0.001)]. There were no significant differences in levels of other inflammation markers, for example, NI [0.04 (0.03; 0.11) and 0.03 (0.02; 0.06), p>0.05)] and C-reactive protein [0.7 (0.4; 1.2) and 0.7 (0.4; 1.3), p>0.05].
Therefore, compared with neonates in subgroup II, preterm neonates in subgroup I had changes in significantly more SIRS markers, manifestations of disseminated intravascular coagulation, and more often had hypoxic-hemorrhagic lesions of the central nervous system with the development of IVH of varying severity.
Analysis of cytokine levels in all studied groups on the first day of birth is presented in Table 4.
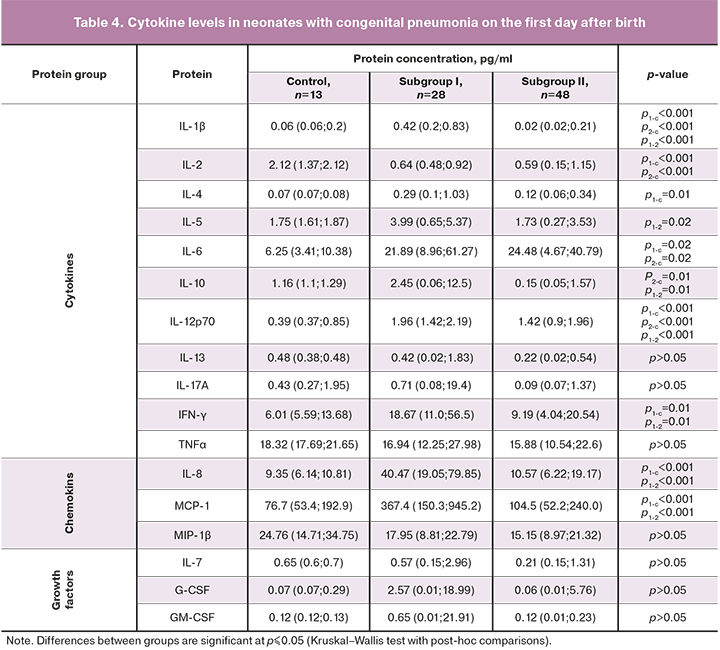
As can be seen from Table 4, plasma cytokine levels in subgroup I were significantly higher than in the control group. Significant differences were found in levels of the following cytokines: anti-inflammatory (IL-4, IL-10), pro-inflammatory (IFN-γ, IL-1β, IL-2, IL-5, IL-6, IL-12), and chemokines (IL-8, MCP-1). These findings correlate with the results of our previously published studies showing that umbilical cord plasma levels of cytokines IL-1β, IL-6, IFN-γ, IL-8, MCP-1 were significantly higher in preterm infants with congenital pneumonia than in children of similar GA with respiratory disorders of non-infectious origin [14].
IL-6 is known to be synthesized by activated macrophages and T cells and stimulates the immune response. According to many authors, the determination of its level plays an essential role in diagnosing early neonatal infections [14–20]. The feasibility of testing for serum IL-6 to monitor the development of the inflammatory response is also determined because it has a short half-life [21].
IL-8, a chemokine, is also a marker of neonatal sepsis and systemic inflammatory response. It is synthesized in the first phase of the immune response by macrophages, epithelial and endothelial cells containing Toll-like receptors. There is evidence that its increased levels are associated with an unfavorable outcome of pneumonia in adult patients. An excess of IL-8 correlates with increased inflammation in the lung tissue and the subsequent development of respiratory failure [21].
Concentrations of IL-1β, IL-6, IL-12 in preterm neonates with congenital pneumonia in subgroup II differed significantly from that in the control group; no differences were found in levels of other cytokines. These findings indicate a more significant imbalance in the immune system of extremely preterm infants in subgroup I, which can be associated with factors determined by GA as morphological and functional immaturity of the body as a whole, related to both the immune system and lung tissue. Besides, worthy of considerable attention is the large variability of growth factor levels on the first day after birth in preterm neonates of subgroup I, compared with the control group.
Cytokine concentrations in neonates with congenital pneumonia during the early neonatal period are presented in Table 5.
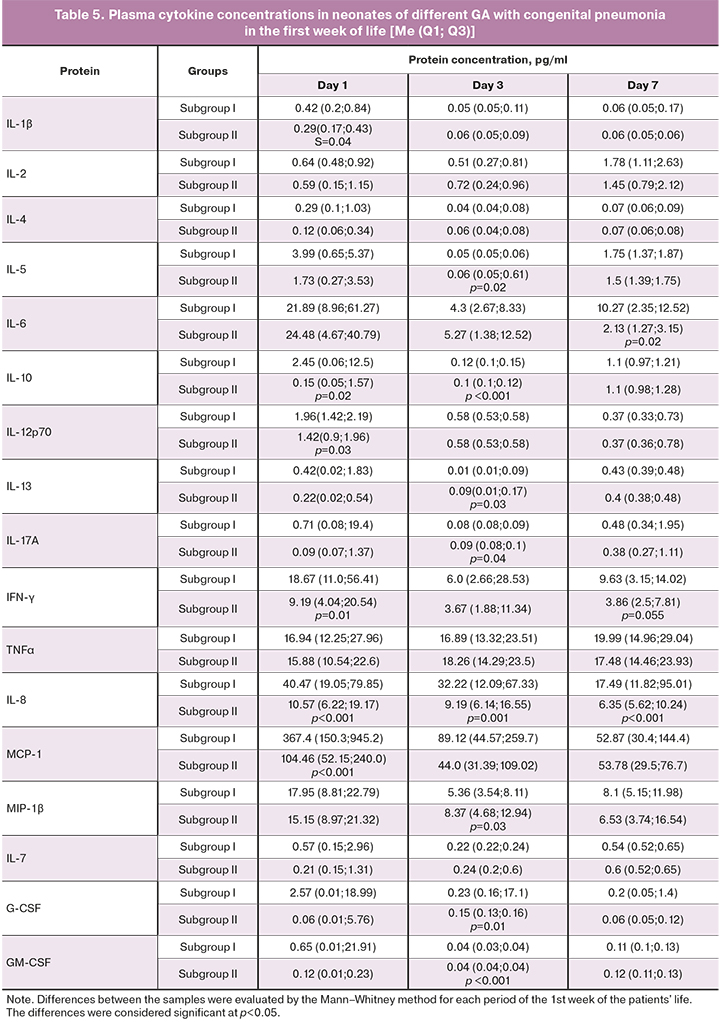
As indicated in table 5, on the first day after birth, the level of all cytokines in neonates of subgroup II was lower than in extremely preterm neonates in subgroup I. However, the differences were insignificant, except for proinflammatory MCP-1, IFN-γ, and IL-8, which had significantly lower levels.
On day 3, chemokine levels were similar except for IL-8, which was lower, and MIP-1β, which was significantly higher in newborns of subgroup II. It is known that MIP-1β form acute and chronic inflammatory responses in humans at sites of injury or infection. It plays a crucial role in inducing and modulating inflammatory responses, mainly by recruiting pro-inflammatory cells. They are essential for T cells chemotaxis from the bloodstream to the inflamed tissue and play an important role in regulating monocytes, dendritic cells, and NK cells transendothelial migration [20–22].
On day 7, in preterm neonates in subgroups II and I had similar levels of MIP-1β. At the same time, the levels of IL-6 and IL-8 significantly decreased, and there was a clear tendency towards a decrease in the level of IFN-γ.
The dynamics of the production of IL-6 and IL-8 in newborns' congenital pneumonia is essential for identifying the appropriate management strategy. Of note is the preservation of their high levels during the entire observation period in subgroup I. This finding confirms the assumptions that the degree of morphological and functional maturity of the lung tissue in this category of newborns may determine the effectiveness of the therapy. Indeed, the duration of treatment in the ICU for preterm neonates in subgroup I was 39 (23.5; 46.5) days, compared with 7 (4; 10) days in subgroup II (Mann–Whitney test, p<0.0001). Besides, 19 children in subgroup I (68%) with a confirmed diagnosis of congenital pneumonia included in the study required repeated courses of antibiotic therapy. In contrast, in subgroup II it was used only in 6 patients (12.5%), (2, p<0.001).
Based on the above, it can be assumed that differences in the cytokine profile (dynamics of IL-6, IL-8, and IFN-γ) may be necessary for predicting the effectiveness of treatment of extremely preterm infants with congenital pneumonia.
Immaturity of the immune system is more pronounced in infants born preterm than in normal-term neonates. Since the ontogeny of the immune system continues throughout gestation, it is natural that the immune system of very preterm infants is characterized by a quantitative and qualitative deficiency of factors of innate and adaptive immunity, compared with children born on time. Therefore, a more frequent generalization of the infectious process in preterm newborns is possible compared with children of older GA [23]. In addition, the low content of monocytes and neutrophils in preterm infants can lead to inadequate protection against pathogenic microorganisms compared with term babies [23]. Congenital pneumonia occurs due to fetal infection in the prenatal or intrapartum period [24–26]. It has been shown that activation of the immune system of an infected preterm newborn, in turn, aggravates the depletion of monocytes, which may determine the protracted course of congenital pneumonia in this cohort of patients [27].
High rates of allergic diseases in mothers suggest the influence of genetic or epigenetic factors on the rate of morphofunctional maturation of the immune system of preterm infants. On the other hand, women may have the risk of pathological immune reactions during implantation and placentation, which, in turn, can lead to the risk of pregnancy complications, placental insufficiency, early preeclampsia, and preterm birth. Extreme prematurity, low body weight, pronounced lung tissue immaturity, and imbalance of immune regulation are accompanied by a clinically longer course of the inflammatory process in the lung tissue, prolonged respiratory therapy, the need for repeat courses of antibiotic therapy, which entails a longer hospital stay.
Conclusion
On the first day of life, preterm neonates with congenital pneumonia and GA 25–31 weeks had higher plasma concentrations of proinflammatory mediators than preterm babies with GA 32–26 weeks and full-term babies (GA 37 weeks). The persistence of higher IL-6 and IL-8 concentrations in preterm neonates with GA 25–31 weeks compared with preterm neonates with GA 32–26 weeks up to day 7 of life indicates increased systemic inflammatory response from the moment of birth and incomplete relief of the inflammatory process in extremely immature children, despite comprehensive, intensive therapy.
References
- Chen Y., Li G., Ruan Y., Zou L., Wang X., Zhang W. An epidemiological survey on low birth weight infants in China and analysis of outcomes of full-term low birth weight infants. BMC Pregnancy Childbirth. 2013; 13: 242. https://dx.doi.org/10.1186/1471-2393-13-242.
- Menon R. Preterm birth: a global burden on maternal and child health. Pathog. Glob. Health. 2012; 106(3): 139-40. https://dx.doi.org/10.1179/204777312X13462106637729.
- Никитина И.В., Непша О.С., Донников А.Е., Трофимов Д.Ю., Милая О.В., Дегтярева А.В., Ионов О.В., Зубков В.В., Дегтярев Д.Н. Современные возможности молекулярно-генетических методов в диагностике раннего неонатального сепсиса у недоношенных новорожденных. Акушерство и гинекология. 2016; 12: 106-13. [Nikitina I.V., Nepsha O.S., Donnikov A.E., Trofimov D.Yu., Milaya O.V., Degtyarev A.V., Ionov O.V., Zubkov V.V., Degtyarev D.N. Modern possibilities of molecular genetic techniques in the diagnosis of early neonatal sepsis in preterm neonates. Obstetrics and Gynecology. 2016; 12: 106-13. (in Russian)]. https://dx.doi.org/10.18565/aig.2016.12.106-13.
- Каракушикова А.С., Рахимова К.В., Абдуллаева Г.М. Особенности иммунного статуса недоношенных детей с перинатальной патологией в раннем неонатальном периоде. Педиатрия. Журнал имени Г.Н. Сперанского. 2012; 91(1): 20-5. [Karakushikova A.S., Rakhimova K.V., Abdullayeva G.M. Features of the immune status of premature infants with perinatal pathology in the early neonatal period. Pediatrics. 2012; 91(1): 20-5.(in Russian)].
- Saini Y., Wilkinson K.J., Terrell K.A., Burns K.A., Livraghi-Butrico A., Doerschuk C.M. et al. Neonatal pulmonary macrophage depletion coupled to defective mucus clearance increases susceptibility to pneumonia and alters pulmonary immune responses. Am. J. Respir. Cell Mol. Biol. 2016; 54(2): 210-21. https://dx.doi.org/10.1165/rcmb.2014-0111OC.
- Dowling D.J., Levy O. Ontogeny of early life immunity. Trends Immunol. 2014; 35(7): 299-310. https://dx.doi.org/10.1016/j.it.2014.04.007.
- Jónsdóttir I. Maturation of mucosal immune responses and influence of maternal antibodies. J. Comp. Pathol. 2007; 137(Suppl. 1): S20-6. https://dx.doi.org/10.1016/j.jcpa.2007.04.007.
- Alia A., Kadi F.A., Yuniati T., Primadi A., Hidajat S., Sukadi A. Relationship between serum interleukin-6 levels and bronchopulmonary dysplasia in preterm infants at 28-34 weeks’ gestation with respiratory distress syndrome. Am. J. Clin. Med. Res. 2019; 7(1): 26-30. https://dx.doi.org/10.12691/ajcmr-7-1-5.
- Fenton T.R., Kim J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13: 59. https://dx.doi.org/10.1186/1471-2431-13-59.
- ВОЗ. Нормы для оценки роста детей. [Internet]. WHO; 2020 Dec 21. Available at: http://www.who.int/childgrowth/standards/ru/
- Ионов О.В., Никитина И.В., Зубков В.В., Митрохин С.Д., Крохина К.Н., Киртбая А.Р., Балашова Е.Н., Левадная А.В., Любасовская Л.А., Рюмина И.И., Дегтярев Д.Н., Крючко Д.С. Порядок обследования новорожденных с подозрением на инфекционную патологию и правила назначения антибактериальной терапии, принятые в отделении реанимации и интенсивной терапии новорожденных ФГБУ «Научный центр акушерства, гинекологии и перинатологии имени В.И Кулакова» Минздрава России. Неонатология: новости, мнения, обучение. 2014; 1: 95-106. [Ionov O.V., Nikitina I.V., Zubkov V.V. et al. The procedure for examination of newborns suspected of infectious pathology and rules of antibacterial therapy, adopted at department of resuscitation and intensive therapy of newborn at Kulakov Research Center for Obstetrics, Gynecology and Perinatology. Neonatology: News, Opinions, Training. 2014; 1: 95-106. (in Russian)].
- Weiss S.L., Peters M.J., Alhazzani W., Agus M.S.D., Flori H.R., Inwald D.P. et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Pediatr. Crit. Care Med. 2020; 21(2): e52-106. https://dx.doi.org/10.1097/PCC.0000000000002198.
- Dellinger R.P., Levy M.M., Rhodes A., Annane D., Gerlach H., Opal S.M. et al.; Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 2013; 41(2): 580-637. https://dx.doi.org/10.1097/CCM.0b013e31827e83af.
- Жукова А.С., Никитина И.В., Ванько Л.В., Матвеева Н.К., Кречетова Л.В., Ионов О.В., Тимофеева Л.А., Дегтярев Д.Н. Содержание цитокинов в плазме крови недоношенных новорожденных детей в раннем неонатальном периоде. Иммунология. 2017; 3: 143-7. [Zhukova A.S., Nikitina I.V., Vanko L.V., Matveeva N.K., Krechetova L.V., Ionov O.V., Timofeeva L.A., Degtyarev D.N. The content of cytokines in the blood plasma of premature newborns in the early neonatal period. Immunology. 2017; 3: 143-7. (in Russian)].
- Mirzarahimi M., Barak M., Eslami A., Enteshari-Moghaddam A. The role of interleukin-6 in the early diagnosis of sepsis in premature infants. Pediatr. Rep. 2017; 9(3): 7305. https://dx.doi.org/10.4081/pr.2017.7305.
- Ye Q., Du L., Shao W.-X., Shang S. Utility of cytokines to predict neonatal sepsis. Pediatr. Res. 2017; 81(4): 616-21. https://dx.doi.org/10.1038/pr.2016.267.
- Hibbert J., Strunk T., Simmer K., Richmond P., Burgner D., Currie A. Plasma cytokine profiles in very preterm infants with late-onset sepsis. PloS One. 2020; 15(5): e0232933. https://dx.doi.org/10.1371/journal.pone.0232933.
- Prashant A., Vishwanath P., Kulkarni P., Sathya Narayana P., Gowdara V., Nataraj S.M., Nagaraj R. Comparative assessment of cytokines and other inflammatory markers for the early diagnosis of neonatal sepsis – a case control study. PLos One. 2013; 8(7): e68426. https://dx.doi.org/10.1371/journal.pone.0068426.
- Gilfillan M., Bhandari V. Neonatal sepsis biomarkers: where are we now? Res. Rep. Neonatol. 2019; 9: 9-20. https://dx.doi.org/10.2147/RRN.S163082.
- Никитина И.В., Жукова А.С., Ванько Л.В., Вторушина В.В., Матвеева Н.К., Кречетова Л.В., Крючко Д.С., Ионов О.В., Зубков В.В., Дегтярев Д.Н. Особенности цитокинового статуса у недоношенных новорожденных с заболеваниями легких инфекционного и неинфекционного генеза. Неонатология: новости, мнения, обучение. 2018; 6(4): 16-23. [Nikitina I.V., Zhukova A.S., Vanko L.V. et al. Cytokine status of preterm newborns with infectious and noninfectious diseases. Neonatology: News, Opinions, Training. 2018; 7(4): 16-23. (in Russian)]. https://dx.doi.org/10.24411/2308-2402-2018-14002.
- Satar M., Turhan E., Yapicioglu H., Narli N., Ozgunen F.T., Cetiner S. Cord blood cytokine levels in neonates born to mothers with prolonged premature rupture of membranes and its relationship with morbidity and mortality. Eur. Cytokine Netw. 2008; 19(1): 37-41. https://dx.doi.org/10.1684/ecn.2008.0118.
- Maurer M., von Stebut E. Macrophage inflammatory protein-1. Int. J. Biochem. Cell Biol. 2004; 36(10): 1882-6. https://dx.doi.org/10.1016/j.biocel.2003.10.019.
- Melville J.M., Moss T.J.M. The immune consequences of preterm birth. Front. Neurosci. 2013; 7: 79. https://dx.doi.org/10.3389/fnins.2013.00079.
- McAdams R.M., Juul S.E. The role of cytokines and inflammatory cells in perinatal brain injury. Neurol. Res. Int. 2012; 2012: 561494. https://dx.doi.org/10.1155/2012/561494.
- Hooven T., Polin R.A. Pneumonia. Semin. Fetal Neonatal Med. 2017; 22(4): 206-13. https://dx.doi.org/10.1016/j.siny.2017.03.002.
- Kollmann T.R., Kampmann B., Mazmanian S.K., Marchant A., Levy O. Protecting the newborn and young infant from infectious diseases: lessons from immune ontogeny. Immunity. 2017; 46(3): 350-63. https://dx.doi.org/10.1016/j.immuni.2017.03.009.
- Сухих Г.Т., Трофимов Д.Ю., Бурменская О.В., Байрамова Г.Р., Непша О.С., Донников А.Е., Дуринян Э.Р., Бирюкова А.М. Профиль экспрессии мРНК генов цитокинов в вагинальных мазках женщин репродуктивного возраста при неспецифическом вагините и бактериальном вагинозе. Акушерство и гинекология. 2011; 7-2: 33-8. [Sukhikh G.T., Trofimov D.Yu., Burmenskaya O.V., Baimarova G.R., Nepsha O.S., Donnikov A.E., Durinyan E.R., Biryukova A.M. Cytokine mRNA gene expression profile in the vaginal smears of reproductive- age women with nonspecific vaginitis and bacterial vaginosis. Obstetrics and Gynecology. 2011; 7-2: 33-8. (in Russian)].
Received 29.03.2021
Accepted 06.04.2021
About the Authors
Eugenia V. Inviyaeva, Ph.D. (bio.sci.), Senior Researcher at the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.Tel.: +7(495)438-11-83. E-mail: e_inviyaeva@oparina4.ru. ORCID: 0000-0001-9878-3637. 117997, Russia, Moscow, Oparina str., 4.
Irina V. Nikitina, Ph.D., Leading Researcher at the Neonatal Intensive Care Unit №2, Institute of Neonatology and Pediatrics, Associate Professor at the Department
of Neonatology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Tel.: +7(495)438-22-77. E-mail: i_nikitina@oparina4.ru. ORCID: 0000-0002-1103-1908;
Reasearcher ID: AAH-3465-2019; SCOPUS Author ID: 57189233499. 117997, Russia, Moscow, Oparina str., 4.
Lubov V. Krechetova, Dr. Med. Sci., Head of the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Tel.: +7(495)438-11-83. E-mail: l_krechetova@oparina4.ru. ORCID: 0000-0001-5023-3476. 117997, Russia, Moscow, Oparina str., 4.
Olga A. Krogh-Jensen, Ph.D., Neonatologist at the Neonatal Intensive Care Unit №2 of the Institute of Neonatology and Pediatrics, V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia; Associate Professor at the Department of Neonatology, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University).
Tel.: +7(926)014-01-35. E-mail: o_krogh@oparina4.ru. ORCID: 0000-0002-5178-5659. 117997, Russia, Moscow, Oparina str., 4.
Valentina V. Vtorushina, Ph.D., Immunologist at the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Tel.: +7(495)438-11-83. E-mail: v_vtorushina@oparina4.ru. ORCID: 0000-0002-8406-3206. 117997, Russia, Moscow, Oparina str., 4.
Anna A. Lenyushkina, Ph.D., Head of the Neonatal Intensive Care Unit №2 of the Institute of Neonatology and Pediatrics, Associate Professor at the Department of Neonatology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Tel.: +7(495)531-44-44, ex. 2700, 2697. E-mail: a-lenushkina@yandex.ru.
ORCID: 0000-0001-8929-2991. 117997, Russia, Moscow, Oparina str., 4.
Viktor V. Zubkov, Dr. Med. Sci., Professor, Director of the Institute of Neonatology and Pediatrics, Head of the Department of Neonatology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia; Professor at the Department of Neonatology, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University).
E-mail: victor.zubkov@mail.ru. ORCID: 0000-0002-9697-9596. 117997, Russia, Moscow, Oparina str., 4.
Dmitriy N. Degtyarev, Dr. Med. Sci., Professor, Deputy Director for Research, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia; Head of the Department
of Neonatology, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University). Tel.: +7(495)438-25-33. E-mail: d_degtiarev@oparina4.ru.
ORCID: 0000-0001-8975-2425. 117997, Russia, Moscow, Oparina str., 4.
For citation: Inviyaeva E.V., Nikitina I.V., Krechetova L.V., Krog-Jensen O.A., Vtorushina V.V., Lenyushkina A.A., Zubkov V.V., Degtyarev D.N. Plasma cytokine profiles in preterm neonates with congenital pneumonia in the first week of life.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 8: 143-152 (in Russian)
https://dx.doi.org/10.18565/aig.2021.8.143-152



