The features of respiratory and cardiovascular disorders in preterm infants born to mothers with abnormally invasive placenta
Objective. To study the features of respiratory and cardiovascular disorders in preterm infants born to mothers with abnormally invasive placenta (AIP) in comparison with preterm infants of similar gestational age (GA) born to mothers without AIP.Balashova E.N., Ionov O.V., Kirtbaya A.R., Nikonets A.D., Mikheeva A.A., Vasilchenko O.N., Zubkov V.V., Shmakov R.G., Degtyarev D.N.
Materials and methods. This was a retrospective cohort study of 843 premature newborns, 59 of them were born to mothers with AIP. All newborns were divided into two groups: the study group included 59 preterm infants of GA 33–36/6 weeks born to mothers with AIP and the control group consisted of 784 preterm infants of corresponding GA born to mothers without AIP. Additionally, all newborns were divided into subgroups depending on GA: 33–34 weeks and 35–36/6 weeks. There was a comparative analysis of GA, birth weight and length, Apgar score at 1 and 5 minutes, the incidence of respiratory distress syndrome (RDS), congenital pneumonia, transient tachypnea, the need and duration of using invasive mechanical ventilation (IMV) and high-frequency oscillatory ventilation (HFOV), surfactant administration, vasopressor and inotrope therapy, length of stay in the NICU.
Results. Preterm infants of the study group had much greater body weight and length. The leading cause of respiratory disorders in the study group was RDS, which significantly exceeded the incidence of RDS in the control group (by 1.5 times). The need for IMV (RR [95% CI] 1.6 [1.05; 2.4]), HFOV (RR [95% CI] 1.79 [1.29; .47]), surfactant administration (RR [95% CI] 2.12 [1.19;3.78]), dopamine administration (RR [95% CI]
1.75 [1.32;2.32]), dobutamine administration (RR [95% CI] 1.96 [1.37;2.81]) were significantly higher in the study group. There was a 1.7-fold increase in the frequency of HFOV use and a 2.5-fold increase in the frequency of cardiotonic therapy use in the subgroup of newborns aged 33–34 weeks in the study group compared to the similar subgroup in the control group. In the subgroup of infants of GA 35–36/6 weeks who were born to mothers with AIP, there was a higher frequency of RDS (by 3.6 times), more frequent use of HFOV (RR [95% CI]
1.8 [1.2; 2.79]) and surfactant replacement therapy (RR [95% CI] 4.8 [1.35; 17.3]) compared to the control group.
Conclusion. Abnormally invasive placenta affects the incidence of respiratory and cardiovascular disorders in preterm infants. RDS was the main cause of respiratory disorders in preterm infants born to mothers with AIP. Cardiovascular disorders are an additional factor that accounts for the severity of the condition of infants born at 33–34 weeks of gestation.
Keywords
The excessive use of cesarean section in obstetric practice in recent decades has led to a significant increase in the frequency of placental attachment disorders in multigravidas [1, 2]. According to different authors, the frequency of abnormally invasive placenta (AIP) varies from 0.24% to 0.9% [3–6]. Depending on the degree of invasion, AIP can be divided into placenta accreta (chorionic villi pass through the decidual membrane and attach to the myometrium), placenta increta (chorionic villi invade the myometrium), and placenta percreta (the chorionic villi penetrate through the myometrium into the serous membrane and may invade the adjacent organs (bladder, intestines)). The optimal gestational age for delivery for women with AIP ranges from 34 to 36 weeks, and the management strategy depends mainly on the risk of preterm birth and a previous history of bleeding [7, 8]. The protocols presented by the foreign associations of obstetricians and gynecologists did not show a common opinion on the optimal timing of delivery; the difference between the recommended gestation at delivery is 2–3 weeks [8].
In the medical literature, there is not enough information about the health status of children born to mothers with AIP. As a rule, the information that is available contains the data on the prevalence of prematurity, the increased frequency of intrauterine growth retardation, and the need for intensive care in newborns [6, 9–11].
The present research is aimed at studying the frequency and characteristics of respiratory and cardiovascular disorders in the early neonatal period in preterm infants born to mothers with AIP, in comparison with preterm infants of similar gestational age (GA) born to mothers without AIP.
Materials and Methods
A retrospective comparative cohort study included 941 preterm newborns who were admitted to the Neonatal Intensive Care Unit (NICU) between January 1, 2017 and December 2019 at the National Medical Research Centre for Obstetrics, Gynecology and Perinatology, Moscow, Russia.
The infants of GA 33–36/6 weeks were eligible for inclusion in the study. Newborns with congenital malformations, chromosomal abnormalities, hereditary metabolic diseases, and edematous form of hemolytic disease of a newborn (HDN) (n=98) were excluded from the study.
Among 843 preterm infants included in the study whose GA was 33–36/6 weeks, 59 children were born to mothers with AIP (the study group). The control group consisted of 784 preterm infants born to mothers without AIP (Figure 1).
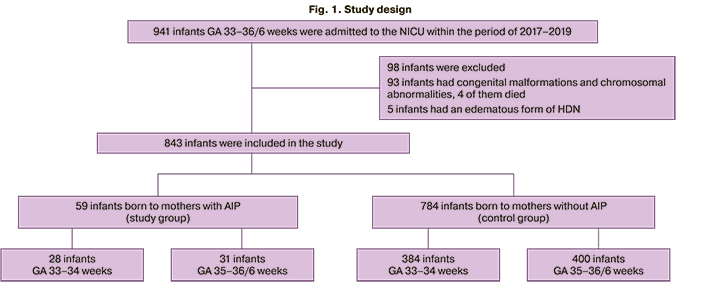
The infants underwent respiratory therapy with invasive and non-invasive methods, depending on the severity of respiratory disorders; in case of hemodynamic disorders, cardiotonic and vasopressor drugs were used. The follow–up period lasted from the moment of birth to the moment when the newborns were transferred to the Department of Neonatal Pathology and Preterm Infants for the second stage of nursing; the duration of this period ranged from a minimum of 2 days to a maximum of 33 days. The criteria for the transfer were spontaneous breathing without any respiratory disorders, stable hemodynamic parameters and no necessity for cardiotonic support, uptake of food in the amount of more than 50 ml/kg/day, adequate diuresis. On the 3rd day of life, the infants were made the final basic diagnosis for the diseases of the respiratory system (respiratory distress syndrome (RDS), congenital pneumonia, transient tachypnea of the newborn (TTN)).
The estimated variables included gestational age, birth weight and length indicators, sex, Apgar score at 1 and 5 minutes, type and incidence of respiratory disorders (RDS, congenital pneumonia, TTN), frequency and duration of invasive mechanical ventilation (IMV) and high-frequency oscillatory ventilation (HFOV), the use of surfactant replacement therapy, cardiotonic therapy (dopamine, dobutamine, adrenaline), as well as length of stay in the NICU.
Statistical analysis
Statistical processing of the data was performed using the IBM SPSS Statistics version 26.0. software package. When HFOV was used at a frequency of 19% in newborns in the control group and 43.5% in infants born to mothers with AIP, the required sample size according to the Lehr’s formula was 58 patients for the alpha risk of 5% and beta risk of 20% (power of the study is 80%). Before conducting a comparative analysis of quantitative data in the study and control groups, normal distribution of the data was checked (the Kolmogorov–Smirnov test, graphical data analysis). Due the absence of normal distribution of data, the methods of nonparametric statistics were used. The median (Me) and the 25%–75% interquartile range (IQR) were determined for the quantitative variables. The Mann–Whitney test was used to compare the quantitative variables between the two groups; in order to compare the binary variables, the Pearson’s 2 test was used, which was calculated using a 2×2 table; Fischer’s exact test was used for small samples. Relative risks (RR) with a 95% confidence interval (CI) were calculated to assess the impact of risk factors. The differences were considered statistically significant at the level of p<0.05.
Results
The comparative characteristics of the infants of the study and control groups are presented in Table 1.
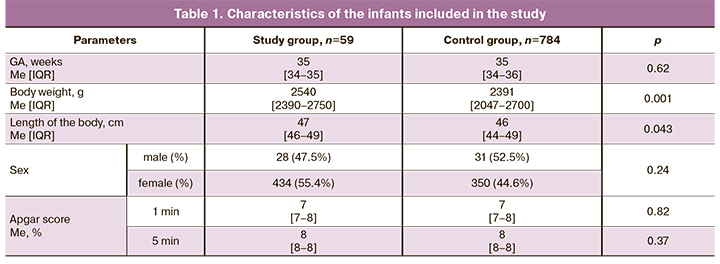
Despite the fact that both groups were evaluated for GA, the analysis of anthropometric data revealed that infants in the study group had significantly greater body weight and length. There were no differences in the ratio of boys and girls between the groups. The Apgar score did not differ between the groups, either.
Respiratory disorders were revealed in 100% of the infants of the study group and in 97% of the infants of the control group during the first hours after birth.
The main causes of respiratory disorders in infants of the study and control groups are presented in Table 2.
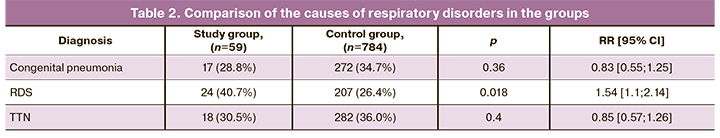
According to the data presented in Table 2, the leading cause of respiratory disorders in the newborns of the study group was RDS, which significantly exceeded its incidence in the control group. The probability of developing RDS in infants born to mothers with AIP was found to be 1.5 times higher (RR=1.54) than in infants of the control group. There was no statistically significant difference in the incidence of congenital pneumonia and TTN.
In order to compare the severity of respiratory disorders in the infants of the study and control groups, we conducted a comparative analysis of the number of newborns who required IMV and HFOV in the first days of life, as well as surfactant replacement therapy. To compare the incidence and severity of hemodynamic disorders, the frequency of use of vasopressors and cardiotonic drugs (dopamine, dobutamine and adrenaline) in the infants of the study and control groups was analyzed (Table 3).
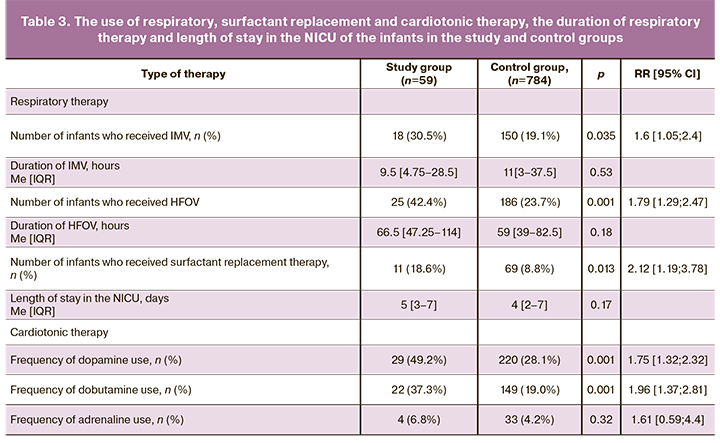
The infants born to mothers with AIP were found to require IMV and HFOV more frequently than infants of the same GA born to mothers without AIP. However, the duration of IMV did not differ significantly. Preterm infants born to mothers with AIP were 1.8 and 1.6 times more likely to receive HFOV and IMV, respectively, than infants of the similar GA born to mothers without AIP. The obtained data are indicative of a greater severity of respiratory failure in the infants of the study group, compared to ones of the control group.
Endotracheal surfactant preparations were administered more frequently to the infants of the study group: 11/59 (18.9%) cases versus 69/784 (8.8%) cases in the control group. The obtained data suggest that the increased need for replacement surfactant therapy reflects a more severe deficiency of endogenous surfactant in infants born to mothers with AIP. The use of surfactant in complex therapy in infants born to mothers with AIP was 2.1 times more likely than in newborns of the control group.
The comparative analysis of the need for cardiotonic and vasopressor therapy demonstrated dopamine and dobutamine were more frequently administered in the infants of the study group compared to the control group. However, the frequency of adrenaline use was low and did not differ between the groups. The relative risk of developing hemodynamic disorders, when newborns needed vasopressor and inotropic therapy, was almost two times higher in infants born to mothers with AIP, compared to newborns of the control group. Despite the greater severity of respiratory and cardiovascular disorders in preterm infants of the study group compared to the control group, these disorders were usually controlled within the first 3–7 days, after which the majority of these infants were transferred to the second stage of nursing during the first week of life. Length of stay in the NICU did not differ significantly between the groups.
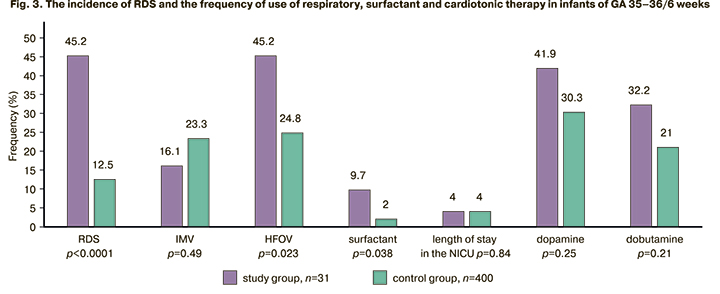
In order to clarify the dependence of the frequency and severity of respiratory disorders on GA, the infants of the study and control groups were divided into two subgroups: GA 33–34 weeks and 35–36/6 weeks. The subgroup of infants of GA 33–34 weeks included 28 newborns of the study group and 384 newborns of the control group; the subgroup of GA 35–36/6 weeks included 31 newborns of the study group and 400 newborns of the control group. The comparison of the data of these subgroups is presented in Figures 2 and 3.
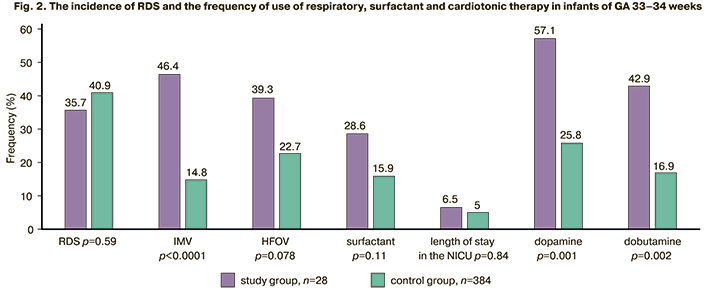
There was a significantly higher number of infants who needed IMV (conventional IMV (RR [95% CI] 3.1 [1.97; 4.98]), HFOV (RR [95% CI] 1.73 [1.05; 2.85]) and cardiotonic therapy among infants of GA 33–34 weeks born to mothers with AIP (dopamine RR [95% CI] 2.21 [1.54; 3.18]; dobutamine RR [95% CI] 2.53 [1.56; 4.1]) compared to a subgroup of infants of the same GA in the control group. Surfactant therapy was administered equally frequently in infants of GA 33–34 weeks, which is due to the identical frequency of RDS in this age group (RR [95% CI] 1.8 [0.96; 3.37]). The infants in the study subgroup of GA 33–34 weeks were found to have a significantly longer stay in the NICU (6.5 and 5 days, respectively), compared to the infants of the same GA born to mothers without AIP.
The infants born to mothers with AIP and whose GA was 35–36/6 weeks were more likely to receive HFOV (RR [95% CI] 1.8 [1.2; 2.79]) and surfactant replacement therapy (RR [95% CI] 4.8 [1.35; 17.3]) compared to the infants of the similar GA in the control subgroup. HFOV was 1.8 times more likely to be used in the study group, and surfactant replacement therapy was 4.8 times more likely to be administered in the study group than in the control group; this fact was due to a significantly higher frequency of RDS (RR [95% CI] 3.6 [2.3; 5.8]). However, there was no significant difference in the frequency of vasopressor and inotropic therapy use.
Length of stay in the NICU did not differ among the newborns of GA 35–36/6 weeks.
Discussion
In spite of the fact that abnormally invasive placenta increases the rate of complications, including hysterectomy [12], the literature data on perinatal neonatal outcomes of such pregnancies are not numerous. The main indicators that should be analyzed are the presence of prematurity, the increased frequency of birth of small children, the presence of asphyxia at birth, the need for hospitalization, and the length of stay in the NICU [3–5, 9–11, 13]. The studies of Moeini R. (2020), Gielchinsky Y. (2004), Mogos M. (2015) showed that there was a significant predominance of preterm infants and newborns with low weight and small length by gestational age in mothers with AIP; however, these data are not consistent with the results of our study [3, 4, 10]. According to our observations, infants born to mothers with AIP had significantly higher parameters of body weight and length (2540 g and 47 cm in the study group and 2391 g and 46 cm in the control group). A number of studies did not reveal a difference in the frequency of birth of infants with intrauterine growth retardation due to the absence of disorders of uteroplacental and fetoplacental blood flow. The authors of the studies suggested an increase in blood flow due to remodeling of the arteries in AIP [14–16]. In our study, the control group consisted of the newborns of the similar GA who were born and admitted to the NICU, including the infants with intrauterine disorders of uteroplacental and fetoplacental blood flow and infants with intrauterine growth retardation; therefore, there was a difference in the anthropometric parameters between the groups. The studies by Kassem G. (2013), Baldwin H. (2017), and Moeini R. (2020) [5, 10, 11] showed a lower Apgar score at 5 minutes in infants born to mothers with AIP; however, a cohort study conducted in Israel which included 310 patients born to women with AIP did not demonstrate a significant difference in these indicators [4]; the latter information is consistent with the results of our study. According to two studies [9, 10], infants born to mothers with AIP had to stay longer in the NICU; this fact is consistent with the data of our research. In the subgroup of infants of GA 33–34 weeks, length of stay in the NICU was significantly higher than in the control group of the similar GA, namely 6.5 days and 5.0 days, respectively. But there were no differences between the infants of GA 35–36/6 weeks in the study and control groups, and length of stay in the NICU in both subgroups was 4 days.
The obtained data demonstrate that infants born to mothers with AIP have more severe signs of respiratory and cardiovascular insufficiency than infants of a similar GA whose mothers did not have AIP. The newborns of the study group were 1.6 times more likely to require IMV, including HFOV (1.8 times); surfactant replacement therapy was used 2.1 times more often, and cardiotonic preparations were used almost 2 times more frequently to stabilize hemodynamic disorders. The most severe impairments in the period of early neonatal adaptation were observed in infants of GA 33-34 weeks.
In spite of the fact that the comparison of the subgroup of GA 33–34 weeks in the study group with newborns of the same GA in the control group did not reveal a difference in the frequency of use of surfactant replacement therapy, the infants of the study group were administered vasopressor and inotropic drugs more frequently. The need for dopamine and dobutamine in infants of the study group demonstrates that there are not only vascular tone disorders, but also there is an impairment in the contractile function of the myocardium. On the contrary, in preterm infants of GA 35–36/6 weeks in the study and control groups, there were no differences in the severity of cardiovascular disorders. At the same time, infants of GA 35–36/6 weeks born to mothers with AIP demonstrated a more frequent use of HFOV (1.8 times) and surfactant therapy (4.8 times), which was due to a high incidence of respiratory disorders caused by primary surfactant deficiency (3.6 times higher than in the control group).
The birth of infants at 33–34 weeks of gestation was not a planned delivery, but it was due to the onset of bleeding or fetal distress, that caused the delivery. In general, planned delivery was carried out within the period recommended by international associations. Thus, according to the protocols of the American College of Obstetricians and Gynecologists (ACOG, 2018), the Society of Maternal and Fetal Medicine (SMFM, 2019) and the Society of Obstetricians and Gynecologists of Canada (SOGC, 2019), the appropriate period of planned delivery in a stable patient is 34/0–35/7 weeks gestation [17, 18]. According to the recommendations of the Royal College of Obstetricians and Gynecologists of Great Britain (RCOG, 2019), planned delivery in the absence of bleeding should be performed at 35–36 weeks gestation, taking into account the optimal balance between the maturity of the fetus and the risk of preterm birth at this time [19]. The International Society for Abnormally Invasive Placenta (IS-AIP, 2019) recommends a wait-and-see approach until 36/0 weeks gestation in women without a previous history of preterm birth and bleeding; if the woman has a history of preterm birth, hemorrhage or premature rupture of the membranes, a planned delivery should be performed at 34/0 weeks gestation to avoid urgent complications [20]. The International Federation of Gynecology and Obstetrics (FIGO, 2018) does not recommend specific periods for delivery, but suggests that pregnant women should be managed depending on local resources, which include the ability of transfusiology and neonatal services to provide highly qualified emergency care. In case of insufficient resources, it is recommended to perform a delivery of a pregnant woman with AIP closer to 37 weeks gestation [21].
However, according to our study, delivery at late (35–36 weeks) gestation did not lead to a decrease in the frequency of use of HFOV and surfactant therapy in newborns; this fact confirms that the surfactant system is immature in infants and it results in the severity of the condition after birth in this group of newborns.
The influence of the AIP factor on the prevalence of hemodynamic disorders in preterm infants of GA 33–34 weeks and the frequency of RDS in late preterm newborns suggests that there may be a possible impairment in the synthesis of cortisol in the fetus in case of AIP. Further study is required to support this hypothesis.
Conclusion
Thus, abnormally invasive placenta increases the risk of respiratory and cardiovascular disorders in preterm newborns. The prevalence of respiratory or cardiovascular disorders in infants born to mothers with abnormally invasive placenta depends on the timing of delivery. Respiratory disorders in the early neonatal period in infants born to mothers with abnormally invasive placenta are due to the high frequency of RDS, which requires the use of IMV, especially HFOV, and surfactant replacement therapy; these disorders are mainly observed in newborns of GA 35–36/6 weeks. Cardiovascular disorders characterized by a decrease in vascular tone and contractile function of the myocardium are managed with vasopressor and inotropic therapy; this type of disorders is mainly observed in infants of GA 33-34 weeks who were born to mothers with abnormally invasive placenta. The delivery of women with abnormally invasive placenta at a later gestation of 35–36 weeks does not decrease the severity of the early neonatal period; therefore, it is necessary to transfer the timing of delivery to full-term if there are no other indications on the part of the mother or the fetus. This problem requires further study, including the development of methods for the prevention of RDS in late gestation.
References
- De Mucio B., Serruya S., Alemán A., Castellano G., Sosa C.G. A systematic review and meta-analysis of cesarean delivery and other uterine surgery as risk factors for placenta accreta. Int. J. Gynaecol. Obstet. 2019; 147(3): 281-91. https://dx.doi.org/10.1002/ijgo.12948.
- Шмаков Р.Г., Пирогова М.М., Васильченко О.Н., Чупрынин В.Д., Ежова Л.С. Хирургическая тактика при врастании плаценты с различной глубиной инвазии. Акушерство и гинекология. 2020; 1: 78-82. https://dx.doi.org/10.18565/aig.2020.1.78-82. [Shmakov R.G., Pirogova M.M., Vasilchenko O.N., Chuprynin V.D., Ezhova L.S. Surgery tactics for placenta increta with different depths of invasion. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2020; 1: 78-82 (in Russian)]. https://dx.doi.org/10.18565/aig.2020.1.78-82.
- Mogos M.F., Salemi J.L., Ashley M., Whiteman V.E., Salihu H.M. Recent trends in placenta accreta in the United States and its impact on maternal-fetal morbidity and healthcare-associated costs, 1998-2011. J. Matern. Fetal Neonatal Med. 2016; 29(7): 1077-82. https://dx.doi.org/10.3109/14767058.2015.1034103.
- Gielchinsky Y., Mankuta D., Rojansky N., Laufer N., Gielchinsky I., Ezra Y. Perinatal outcome of pregnancies complicated by placenta accreta. Obstet. Gynecol. 2004; 104(3): 527-30. https://dx.doi.org/10.1097/01.AOG.0000136084.92846.95
- Baldwin H.J., Patterson J.A., Nippita T.A., Torvaldsen S., Ibiebele I., Simpson J.M., Ford J.B. Maternal and neonatal outcomes following abnormally invasive placenta: a population-based record linkage study. Acta Obstet. Gynecol. Scand. 2017; 96(11): 1373-81. https://dx.doi.org/10.1111/aogs.13201.
- Zhang H., Dou R., Yang H., Zhao X., Chen D., Ding Y. et al. Maternal and neonatal outcomes of placenta increta and percreta from a multicenter study in China. J. Matern. Fetal Neonatal Med. 2019. 32(16): 2622-7. https://dx.doi.org/10.1080/14767058.2018.1442429.
- Gyamfi-Bannerman C. Society for Maternal-Fetal Medicine (SMFM) Consult Series #44: Management of bleeding in the late preterm period. Am. J. Obstet. Gynecol. 2018; 218(1): B2-8. https://dx.doi.org/10.1016/j.ajog.2017.10.019.
- Jauniaux E., Kingdom J.C., Silver R.M. A comparison of recent guidelines in the diagnosis and management of placenta accreta spectrum disorders. Best Pract. Res. Clin. Obstet. Gynaecol. 2021; 72: 102-16. https://dx.doi.org/10.1016/j.bpobgyn.2020.06.007.
- Balayla J., Bondarenko H.D. Placenta accreta and the risk of adverse maternal and neonatal outcomes. J. Perinat. Med. 2013; 41(2): 141-9. https://dx.doi.org/10.1515/jpm-2012-0219.
- Moeini R., Dalili H., Kavyani Z., Shariat M., Charousaei H., Akhondzadeh A. et al. Maternal and neonatal outcomes of abnormal placentation: a case-control study. J. Matern. Fetal Neonatal Med. 2020 Apr 21: 1-7. https://dx.doi.org/10.1080/14767058.2019.1678128.
- Kassem G.A., Alzahrani A.K. Maternal and neonatal outcomes of placenta previa and placenta accreta: three years of experience with a two-consultant approach. Int. J. Womens Health. 2013; 5: 803-10. https://dx.doi.org/10.2147/IJWH.S53865.
- Шмаков Р.Г., Пирогова М.М., Васильченко О.Н., Чупрынин В.Д., Пырегов А.В., Ходжаева З.С., Клименченко Н.И., Федорова Т.А., Ежова Л.С., Быченко В.Г., Бойкова Ю.В. Органосохраняющие операции при аномальной инвазии плаценты (5-летний опыт Национального медицинского исследовательского центра акушерства, гинекологии и перинатологии имени академика В.И. Кулакова). Доктор.Ру. 2019; 11: 29-34. https://dx.doi.org/10.31550/1727-2378-2019-166-11-29-34. [Shmakov R.G., Pirogova M.M., Vasilchenko O.N., Chuprynin V.D., Piregov A.V., Khodzhaeva Z.S., Klimenchenko N.I., Fedorova T.A., Ezhova L.S., Bychenko V.G., Boykova Yu.V. Conservative surgery in abnormal placenta invasion (5-year experience of Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology). Doctor.Ru. 2019; 11(166): 29-34. (in Russian)]. https://dx.doi.org/10.31550/1727-2378-2019-166-11-29-34.
- Fitzpatrick K.E., Sellers S., Spark P., Kurinczuk J.J., Brocklehurst P., Knight M. The management and outcomes of placenta accreta, increta, and percreta in the UK: a population-based descriptive study. BJOG. 2014; 121(1): 62-70; discussion 70-1. https://dx.doi.org/10.1111/1471-0528.12405.
- Seet E.L., Kay H.H., Wu S., Terplan M. Placenta accreta: depth of invasion and neonatal outcomes. J. Matern. Fetal Neonatal Med. 2012; 25(10): 2042-5. https://dx.doi.org/10.3109/14767058.2012.678429.
- Lorie M.H., Anthony O.O., George A.M. Effect of placenta previa on fetal growth. Am. J. Obstet. Gynecol. 2010; 203(4): 330. e1-5. https://dx.doi.org/10.1016/j.ajog.2010.05.014.
- Jauniaux E., Dimitrova I., Kenyon N., Mhallem M., Kametas N.A., Zosmer N. et al. Impact of placenta previa with placenta accreta spectrum disorder on fetal growth. Ultrasound Obstet. Gynecol. 2019; 54(5): 643-9. https://dx.doi.org/10.1002/uog.20244.
- Society of Gynecologic Oncology; American College of Obstetricians and Gynecologists and the Society for Maternal–Fetal Medicine, Cahill A.G., Beigi R., Heine R.P., Silver R.M., Wax J.R. Placenta accreta spectrum. Am. J. Obstet. Gynecol. 2018; 219(6): B2-16. https://dx.doi.org/10.1016/j.ajog.2018.09.042.
- Hobson S.R., Kingdom J.C., Murji A., Windrim R.C., Carvalho J.C.A., Singh S.S. et al. No. 383-screening, diagnosis, and management of placenta accreta spectrum disorders. J. Obstet. Gynaecol. Can. 2019; 41(7): 1035-49. https://dx.doi.org/10.1016/j.jogc.2018.12.004.
- Jauniaux E., Alfirevic Z., Bhide A.G., Belfort M.A., Burton G.J., Collins S.L. et al.; Royal College of Obstetricians and Gynaecologists. Placenta praevia and placenta accreta: diagnosis and management: Green-top guideline no. 27a. BJOG. 2019; 126(1): e1-48. https://dx.doi.org/10.1111/1471-0528.15306.
- Collins S.L., Alemdar B., van Beekhuizen H.J., Bertholdt C., Braun T., Calda P. et al.; International Society for Abnormally Invasive Placenta (IS-AIP). Evidence-based guidelines for the management of abnormally invasive placenta: recommendations from the International Society for Abnormally Invasive Placenta. Am. J. Obstet. Gynecol. 2019; 220(6): 511-26. https://dx.doi.org/10.1016/j.ajog.2019.02.054.
- Allen L., Jauniaux E., Hobson S., Papillon-Smith J., Belfort M.A.; FIGO Placenta Accreta Diagnosis and Management Expert Consensus Panel. FIGO consensus guidelines on placenta accrete spectrum disorders: nonconservative surgical management. Int. J. Gynaecol. Obstet. 2018; 140(3): 281-90. https://dx.doi.org/10.1002/ijgo.12409.
Received 02.03.2021
Accepted 12.05.2021
About the Authors
Ekaterina N. Balashova, MD, PhD, leading researcher of the NICU named by professor Antonov A.G. of the Institute of neonatology and pediatrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. Tel.: +7(495)438-22-77. E-mail: e_balashova@oparina4.ru. ORCID: 0000-0002-3741-0770. 117997, Russia, Moscow, Oparina str., 4.Oleg V. Ionov, MD, PhD, Head of the NICU named by professor Antonov A.G. of the Institute of neonatology and pediatrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia; Associate Professor of Neonatology Department at the Faculty of Pediatrics,
I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia. Tel.: +7(495)438-22-77. E-mail: o_ionov@oparina4.ru. ORCID: 0000-0002-4153-133X.
117997, Russia, Moscow, Oparina str., 4.
Аnna R. Kirtbaya, MD, PhD, Chief of clinicians of the NICU named by professor Antonov A.G. of the Institute of neonatology and pediatrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia; Associate Professor of Neonatology Department at the Faculty of Pediatrics, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia. Tel.: +7(495)438-22-77.
E-mail: a_kirtbaya@oparina4.ru. ORCID: 0000-0002-7628-8157. 117997, Russia, Moscow, Oparina str., 4.
Anastasia D. Nikonets, resident of the NICU named by professor Antonov A.G. of the Institute of neonatology and pediatrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. Tel.: +7(495)438-22-77. E-mail: nikon.na@yandex.ru.
117997, Russia, Moscow, Oparina str., 4.
Alexandra A. Mikheeva, postgraduate student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology,
Ministry of Health of Russia. E-mail: shuratora@mail.ru. 117997, Russia, Moscow, Oparina str., 4.
Oksana N. Vasilchenko, MD, PhD, senior researcher of the Department of Innovative Technologies, Academician V.I. Kulakov National Medical Research Center f
or Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. Tel.: +7(495)438-30-47. E-mail: o_vasilchenko@oparina4.ru. ORCID: 0000-0001-9434-0011.
117997, Russia, Moscow, Oparina str., 4.
Victor V. Zubkov, MD, PhD, Director of the Institute of neonatology and pediatrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia; Professor of Neonatology Department, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia.
Tel.: +7(495)438-22-66. E-mail: v_zubkov@oparina4.ru. ORCID: 0000-0001-8366-5208. 117997, Russia, Moscow, Oparina str., 4.
Roman G. Shmakov, MD, PhD, Professor, Director of the Institute of obstetrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. Tel.: +7(495)438-72-00. E-mail: r_shmakov@oparina4.ru. ORCID: 0000-0002-2206-1002.
117997, Russia, Moscow, Oparina str., 4.
Dmitriy N. Degtyarev, MD, PhD, Professor, Deputy Director, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia; Professor of Neonatology Department, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia.
Tel.: +7(495)438-23-88. E-mail: d_degtiarev@oparina4.ru. ORCID: 0000-0001-8975-2425. 117997, Russia, Moscow, Oparina str., 4.
For citation: Balashova E.N., Ionov O.V., Kirtbaya A.R., Nikonets A.D., Mikheeva A.A., Vasilchenko O.N., Zubkov V.V., Shmakov R.G., Degtyarev D.N. The features of respiratory and cardiovascular disorders in preterm infants born to mothers with abnormally invasive placenta.
Akusherstvo i Ginekologiya / Obstetrics and gynecology. 2021; 5: 85-93 (in Russian)
https://dx.doi.org/10.18565/aig.2021.5.85-93



