Neonatal seizures in extremely preterm infants with very and extremely low birth weight: prevalence and transformation into structural epilepsy
Objective: To investigate the incidence of neonatal seizures and their transformation into structural epilepsy in children with extremely low birth weight (ELBW) and very low birth weight (VLBW).Suvorov I.A., Amirkhanova D.Yu., Degtyareva A.V., Degtyarev D.N., Albegova M.B., Kirtbaya A.R., Filippova E.A.
Materials and Methods: This retrospective analysis of the medical records included 297 patients born at 25–32 weeks' gestation with birth weight less than 1499 g, including 29 children with neonatal seizures. After discharge, patients with neonatal seizures were under outpatient supervision by a neurologist. Electroencephalography (EEG) was performed at the corrected age (CA) of 2, 6, 9, 12, 18, 24, and 36 months. Neurological outcome was assessed at CA of 36 months.
Results: Neonatal seizures were diagnosed in 29 patients (9.8%). Amplitude-integrated EEG (aEEG) and multichannel EEG in the neonatal period were performed in 246 patients. Among children with neonatal seizures, epileptiform activity on the aEEG and EEG tracings were observed in 16 (55%) and 20 patients (69%), respectively. Among patients with clinical manifestations of neonatal seizures (n=29), antiepileptic therapy was administered to 26 patients (89.7%). At CA of 6 months of treatment, six patients with neonatal seizures showed normalization of EEG data, which was an indication for discontinuation of antiepileptic therapy. According to EEG findings, four children retained epileptiform activity without convulsions; one child developed repeated epileptic seizures. These patients were diagnosed with structural epilepsy. Antiepileptic therapy was continued. All patients with neonatal structural epilepsy had status seizures.
Conclusion: Neonatal seizures were diagnosed in 9.8% of patients. Compared to aEEG, EEG is more sensitive in detecting epileptiform activity. Structural epilepsy was diagnosed in children with neonatal seizures, requiring anticonvulsant therapy for more than three years. The study findings show the importance of a comprehensive neurological examination and follow-up of patients with neonatal seizures.
Keywords
The World Health Organization estimated that approximately 15 million babies are born preterm annually worldwide, indicating a global preterm birth rate of about 10% [1]. Over the past decades, there has been a persistent trend towards an increase in the survival rates of extremely low birth weight (ELBW) and very low birth weight (VLBW) infants (85% and 100%, respectively), which is primarily associated with the improvement of all stages of neonatal care including improvement of skills of medical personnel in neonatal intensive care units [2–4].
Neonatal seizures are the most common neurological emergency in the neonatal period, and preterm infants are more likely to experience them than full-term babies [5, 6]. The neonatal seizure rate in preterm infants varies widely and, according to some authors, ranges from 5.7 to 14.7% [7].
Neonatal seizures are associated with adverse neurological outcomes in preterm infants [8, 9]. According to several studies, 1.7% of VLBW infants develop epilepsy during the first three years of life [5]. Similar results were obtained in other studies [6, 10, 11]. In EBMT infants, the risk of epilepsy before the age of 7 increases to 8.6% [12].
According to the literature, more than 65% of neonatal seizures can be missed due to their subclinical course. Continuous electroencephalographic (EEG) monitoring is the only way to detect neonatal seizures in children with suspected convulsive seizures. However, this method is not widely used in neonatal care in Russia and European countries [13, 14].
According to many authors, prematurity is one of the leading risk factors for the development of structural epilepsy, but this statement remains insufficiently studied and requires further investigation [15].
The present study aimed to investigate the incidence of neonatal seizures and their transformation into structural epilepsy in children with ELBW and VLBW.
Materials and methods
This retrospective analysis of the medical records of 297 patients born at 25–32 weeks' gestation with birth weight less than 1499 (450–1499) g, a body length of 31–41 cm, with first- and fifth-minute Apgar scores of 2–7 and 4–7 was conducted at the Institute of Neonatology and Pediatrics of V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
The exclusion criteria were birth weight ≥1500 g, gestational age over 32 weeks, congenital malformations of the brain, verified syndromic pathology, and the convulsive syndrome caused by metabolic disorders.
The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P (Minutes No. 5, 05/27/2021).
All children were in poor general condition at birth and were managed in the neonatal intensive care unit (NICU) and the neonatal and preterm intensive care unit (NPICU).
The main pathological conditions diagnosed in the neonatal period are presented in Table 1.
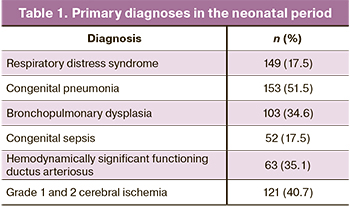
In the NICU and NPICU, all children underwent an assessment of the neurological status and neuro-sonography (NSG). Baseline evaluation also included amplitude-integrated electroencephalography (aEEG) (n=246), multichannel electroencephalography (EEG) (n=246), and brain magnetic resonance imaging (MRI) (n=167). The indications for EEG studies were suspicion of neonatal seizures, apnea of prematurity, severe birth asphyxia, acute cerebrovascular accident, and gestational age of 28 weeks or less. MRI was administered in patients with NSG-detected grade 2b and 3 intraventricular hemorrhages, acute cerebrovascular accident, suspicion of a congenital brain malformation, severe asphyxia at birth.
The neonatal neurological assessment included a standard neurological examination to assess cerebral, meningeal, focal symptoms, and unconditioned reflex activity.
Continuous aEEG tracing was performed using the Olympic CFM 6000, Natus Olympic Brainz Monitor with 5-channel recording with central and biparietal electrode placement; the duration of the tracing was ≥12 hours. Multichannel EEG tracing was performed for all patients using the Encephalan-EEGR-19/26 with a modified international scheme "10–20" with a reduced number of electrodes (Gibs F., Gibs E., 1950). The electrodes were placed in leads Fp1, Fp2, F3, F4, C3, C4, P3, P4, O1, O2, T5, T6, Fz, Cz. Registration was carried out in a monopolar lead with reference ear electrodes; in parallel, an ECG was recorded in standard lead 2. The quality of the electrode placement was controlled by impedancemetry; the resistance did not exceed 10 kΩ. The duration of the study was ≥1 hour. The EEG was assessed according to the following criteria: compliance of the bioelectrical brain activity adjusted for gestational age, the presence or absence of ictal and/or interictal epileptiform activity.
After discharge from the hospital, patients with neonatal seizures were followed in outpatient settings. A neurologist examined all children at the corrected age of 2, 4, 6, 9, 12, 18, 24, and 36 months. EEG in dynamics was performed at the corrected age of 2, 6, 9, 12, 18, 24, and 36 months. The age of 36 corrected months was chosen as the reference point for assessing the early neurological outcome. EEG data, the presence or absence of clinical seizures of the convulsive syndrome, and the need for antiepileptic therapy were analyzed.
Statistical analysis
The statistical analysis was performed using GraphPad Prism software (GraphPad Software, USA).
Results
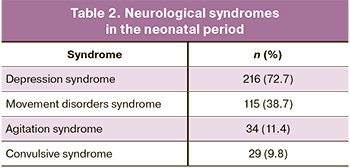
In the neonatal period, most of the patients were diagnosed with neurological disorders, the most frequently depression and motor disorder syndromes (Table 2).
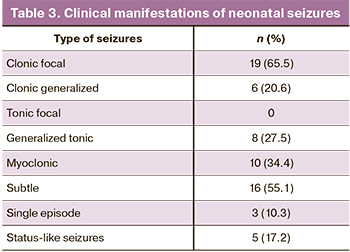
Among all children, 29 (9.8%) were diagnosed with neonatal seizures (the age of onset was 3–13 days of life). The diagnosis of neonatal seizures was based on clinical manifestations of epileptic seizures, regardless of EEG findings. The morphology of neonatal seizures is presented in Table 3. Most children had a combination of various types of seizures, more often focal clonic and subtle seizures.
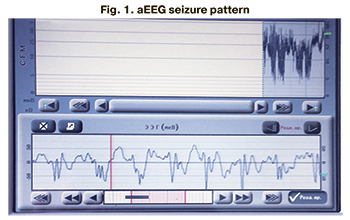
aEEG in the neonatal period was performed in 246 patients (82.8%), of which 230 (93.5%) had no ictal EEG abnormalities. Among all patients (n=29) with clinical seizure manifestations, the seizure aEEG pattern was registered in 16 patients (55%) (Fig. 1–3).
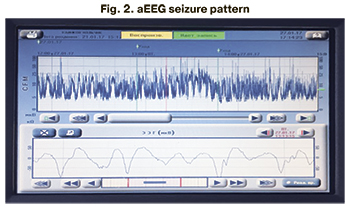
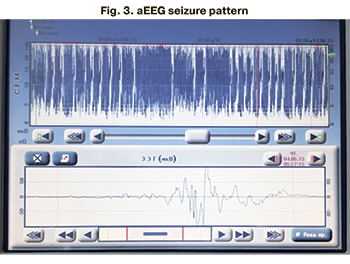
Multichannel EEG in the neonatal period was performed in 246 patients (82.8%). EEG epileptiform activity was registered in 20 patients (8.1%), while 226 patients (91.9%) had normal for the gestational age brain bioelectrical activity. Among all patients with neonatal seizures (n=29), epileptiform activity was recorded in 20 patients (68.9%); no EEG epileptiform activity was detected in 9 children (31.1%) (Table 4).

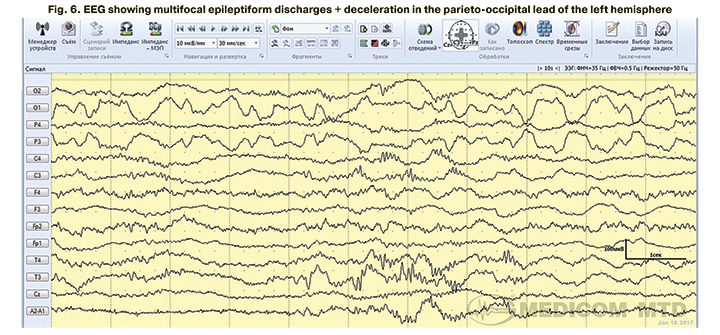
In 4 children with clinical manifestations of neonatal seizures confirmed by EEG, aEEG tracing showed no epileptiform activity. EEG epileptiform activity was represented mainly by focal (Fig. 4) and multifocal epileptiform patterns (Fig. 5, 6).
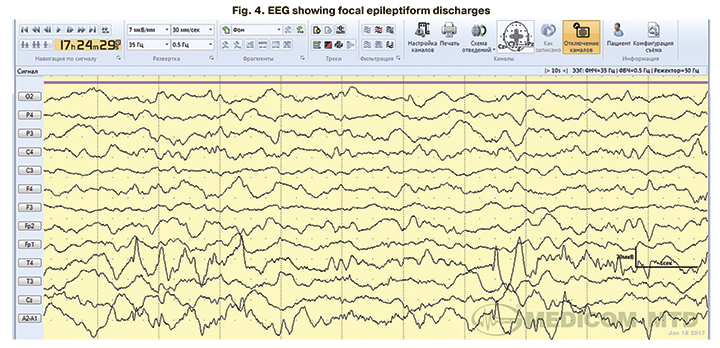
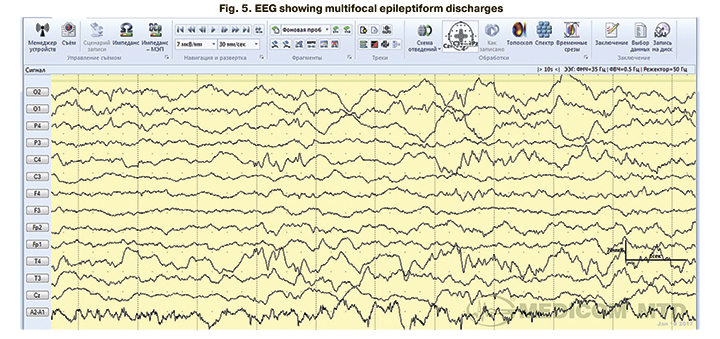
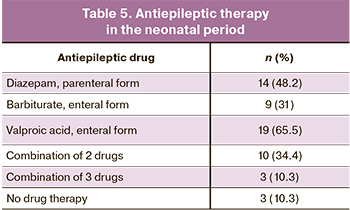
Among all children with clinical manifestations of neonatal seizures (n=29), 26 (89.7%) underwent antiepileptic therapy. The indications were repeated episodes of neonatal seizures and EEG epileptiform activity in children with a single convulsive episode. Antiepileptic treatment was not administered to three patients (10.3%) with single episodes of neonatal seizures not confirmed by pathological EEG activity (Table 5).
In response to therapy, all patients with neonatal seizures showed improvement in clinical manifestations of seizure syndrome. According to the results of repeated EEGs in the neonatal period against the background of therapy, in 18 patients with neonatal seizures (62%), the normal bioelectric activity of the brain was registered. This allowed discontinuation of anticonvulsant therapy in the hospital. Eleven patients (38%) retained pathological activity in the form of focal/multifocal epileptiform activity, and antiepileptic therapy was continued.
After discharge from the hospital, children with neonatal seizures remained under outpatient medical supervision by a neurologist and underwent serial EEGs. Against the background of antiepileptic therapy in 6 patients with neonatal seizures, the brain bioelectrical activity normalized according to serial EEGs by six corrected months, which, together with the absence of clinical manifestations of seizures, was an indication to discontinue antiepileptic therapy. In 4 children, despite the ongoing therapy, EEG focal epileptiform activity persisted without clinical manifestations of the convulsive syndrome. One child developed repeated epileptic seizures at the corrected age of 9 months, accompanied by multifocal epileptiform EEG activity, which required correction of antiepileptic therapy. These five patients were diagnosed with structural epilepsy and received antiepileptic therapy under outpatient supervision by a neurologist and EEG control. All patients with diagnosed structural epilepsy in the neonatal period had status-like seizures.
At the age of 3, 24 patients (82.7%) out of 29 children with neonatal seizures had no clinical and EEG signs of seizure syndrome, and there was no need for antiepileptic therapy. In 5 patients (17.3%), according to EEG data, changes persisted in the form of a delay in cortical rhythm formation, epileptiform activity with a low index of representation, which was an indication for continuing the antiepileptic therapy.
Discussion
In our study of extremely preterm EBMT and VLBW infants, neonatal seizures were diagnosed in 9.8% of babies, consistent with the current literature, estimating the incidence of neonatal seizures in preterm infants as 5.7–14.7% [7]. It increases with decreasing gestational age and birth weight and is 3.4–12.7% among premature babies with birth weight <1500 g and 5.5–8.5% among preterm babies born at ≤30 week’s gestational age of [7].
In our study, the age of neonatal seizures onset was 3–13 days of life, which is consistent with the current literature. According to Pisani F., up to 90% of neonatal seizures in term infants occur on the first day of life. In contrast, extremely preterm infants born at a gestational age of ≤28 weeks are characterized by a relatively late onset of neonatal seizures, which is most likely is a reflection of the neurophysiological maturation of the brain of a preterm baby [16]. The characteristic features of neonatal seizures in extremely preterm infants are most likely associated with such physiological features of the brain as the incompleteness of synaptogenesis and the formation of the neuronal layer of the cortex, incompleteness, and insufficiency of myelination of cerebral structures, insufficient development of interhemispheric connections, incompletely formed limbic system and its connections with the stem structures [17, 18].
Due to neurophysiological immaturity, all newborns, especially preterm babies, are at risk of subclinical epileptic seizures and incorrect interpretation by the attending physician of clinical paroxysms due to the fragmentation and atypical seizure manifestation [19–21]. Equivalents of convulsive paroxysms in the neonatal period can be such motor phenomena as sucking, chewing, protruding tongue, eyeball movements, pedaling leg movements, rowing arms, diaphragm contraction, apnea, etc. In our observation, subclinical epileptic seizures were not registered in patients. However, 55.1% of children had paroxysms of subtle seizures, the epileptic genesis of which was confirmed by aEEG and EEG.
The morphology of seizures in our study patients was represented by the following types: clonic focal and generalized, tonic generalized, myoclonic, subtle; 17.2% of children had status-like seizures. Our findings are consistent with the Position paper by the ILAE Task Force on Neonatal Seizures about the type of neonatal seizures [22].
An EEG is recommended for all children with suspected neonatal seizures since, in addition to confirming the diagnosis, EEG results can indicate a possible etiology of paroxysms, evaluate the effectiveness of therapy, and be predictive of possible disease outcomes [23, 24]. Detecting EEG epileptiform activity in newborns is problematic because it is challenging to distinguish physiological transient acute waves from pathological types of activity [25]. Currently, aEEG is a routine method of neurophysiological diagnostics used at NICU, and it has high sensitivity and specificity in the diagnosis of paroxysmal conditions in newborns [26]. However, despite the widespread use of aEEG, in all cases of detection of neonatal seizures, a continuous EEG tracing is required to confirm the diagnosis. In our study, aEEG and EEG epileptiform activity were recorded in 6.5% and 8.1% of patients, respectively. This observation suggests higher sensitivity of the EEG in the diagnosis of pathological types of activity than aEEG.
We diagnosed structural epilepsy in 17.3% of children with neonatal seizures, which is consistent with the findings of Russian authors. They reported that the incidence of structural epilepsy in children with neonatal seizures in groups of different gestational ages is 18–25% [27]. All children with structural epilepsy in the neonatal period had had status-like seizures, which, in our opinion, reflects the damaging effect of prolonged and repeated epileptic seizures on the immature brain of a premature newborn.
Conclusion
Neonatal seizures were diagnosed in 9.8% of EBMT and VLBW babies at birth.
The morphology of seizures is represented by clonic focal, clonic generalized, tonic generalized, myoclonic, and subtle seizures.
Against the background of antiepileptic therapy, most children had a spontaneous remission of seizures in the neonatal period.
EEG is more sensitive in detecting epileptiform activity than aEEG.
Structural epilepsy was diagnosed in children with status-like neonatal seizures, which required anticonvulsant therapy for more than three years.
The study findings show the importance of a comprehensive neurological examination and follow-up of patients with neonatal seizures.
References
- Vogel J.P., Chawanpaiboon S., Moller A.-B., Watananirum K., Bonet M., Lumbiganon P. The global epidemiology of preterm birth. Best Pract. Res. Clin. Obstet. Gynaecol. 2018; 52: 3-12. https://dx.doi.org/10.1016/j.bpobgyn.2018.04.003.
- Patel R.M., Rysavy M.A., Bell E.F., Tyson J.E. Survival of infants born at periviable gestational ages. Clin. Perinatol. 2017; 44(2): 287-303. https://dx.doi.org/10.1016/j.clp.2017.01.009.
- Collaborative Study Group for Extremely Preterm and Extremely Low Birth Weight Infants. Survival and mortality rate of extremely preterm and extremely low birth weight infants admitted to neonatology departments. Zhonghua Er Ke Za Zhi. 2014; 52(10): 729-35.
- Glass H.C., Costarino A.T., Stayer S.A., Brett C.M., Cladis F., Davis P.J. Outcomes for extremely premature infants. Anesth. Analg. 2015; 120(6): 1337-51. https://dx.doi.org/10.1213/ANE.0000000000000705.
- Matsushita Y., Sakai Y., Torio M., Inoue H., Ochiai M., Yasuoka K. et al. Association of perinatal factors of epilepsy in very low birth weight infants, using a nationwide database in Japan. J. Perinatol. 2019; 39(11): 1472-9. https://dx.doi.org/10.1038/s41372-019-0494-7.
- Sun Y., Vestergaard M., Pedersen C.B., Christensen J., Basso O., Olsen J. Gestational age, birth weight, intrauterine growth, and the risk of epilepsy. Am. J. Epidemiol. 2008; 167(3): 262-70. https://dx.doi.org/10.1093/aje/kwm316.
- Pisani F., Facini C., Bianchi E., Giussani G., Piccolo B., Beghi E. Incidence of neonatal seizures, perinatal risk factors for epilepsy and mortality after neonatal seizures in the province of Parma, Italy. Epilepsia. 2018; 59(9): 1764-73. https://dx.doi.org/10.1111/epi.14537.
- Volpe J.J., Inder T.E., Darras B.T., de Vries L.S., du Plessis A.J., Neil J. et al. Volpe's neurology of the newborn, 6th ed. Elsevier; 2017. 1526p.
- Kharoshankaya L., Stevenson N.J., Livingstone V., Murray D.M., Murphy B.P., Ahearne C.E. et al. Seizure burden and neurodevelopmental outcome in neonates with hypoxic-ischemic encephalopathy. Dev. Med. Child Neurol. 2016; 58(12): 1242-8. https://dx.doi.org/10.1111/dmcn.13215.
- Jackson D.C., Lin J.J., Chambers K.L., Kessler-Jones A., Jones J.E., Hsu D.A. et al. Birth weight and cognition in children with epilepsy. Epilepsia. 2014; 55(6): 901-8. https://dx.doi.org/10.1111/epi.12622.
- Prasad A.N., Corbett B. Epilepsy, birth weight and academic school readiness in Canadian children: data from the national longitudinal study of children and youth. Epilepsy Res. 2017; 130: 101-6. https://dx.doi.org/10.1016/j.eplepsyres.2017.01.003.
- Falchi M., Palmas G., Pisano T., Meloni M., Gaspa G., Puddu M. et al. Incidence of epilepsy in extremely low-birthweight infants (<1,000 g): a population study of central and southern Sardinia. Epilepsia. 2009; 50(Suppl. 1): 37-40. https://dx.doi.org/10.1111/j.1528-1167.2008.01968.x.
- Nagarajan L., Palumbo L., Ghosh S. Classification of clinical semiology in epileptic seizures in neonates. Eur. J. Paediatr. Neurol. 2012; 16(2): 118-25. https://dx.doi.org/10.1016/j.ejpn.2011.11.005.
- Nunes M.L., Yozawitz E.G., Zuberi S., Mizrahi E.M., Cilio M.R., Moshé S.L. et al. Task Force on Neonatal Seizures, ILAE Commission on Classification and Terminology. Neonatal seizures: is there a relationship between ictal electroclinical features and etiology? A critical appraisal based on a systematic literature review. Epilepsia Ореn. 2019; 4(1): 10-29. https://dx.doi.org/10.1002/epi4.12298.
- Walsh S., Donnan J., Fortin Y., Sikora L., Morrissey A., Collins K. et al. A systematic review of the risks factors associated with the onset and natural progression of epilepsy. Neurotoxicology. 2017; 61: 64-77. https://dx.doi.org/10.1016/j.neuro.2016.03.011.
- Pisani F., Barilli A.L., Sisti L., Bevilacqua G., Seri S. Preterm infants with video‐ EEG confirmed seizures: outcome at 30 months of age. Brain Dev. 2008; 30(1): 20-30. https://dx.doi.org/10.1016/j.braindev.2007.05.003.
- Пальчик А.Б., Федорова Л.А., Понятишин А.Е. Неврология недоношенных детей. М.: МЕДпресс-информ; 2014. 376c. [Palchik A.B., Fedorova L.A., Ponyatishin A.E. Neurology of premature babies. M.: MED press-inform; 2014. (in Russian)].
- Bolisetty S., Dhawan A., Abdel-Latif M., Bajuk B., Stack J., Lui K. New South Wales and Australian Capital Territory Neonatal Intensive Care Units’ Data Collection. Intraventricular hemorrhage and neurodevelopmental outcomes in extreme preterm infants. Pediatrics. 2014; 133(1): 55-62. https://dx.doi.org/10.1542/peds.2013-0372.
- Glass H.C., Shellhaas R.A., Wusthoff C.J., Chang T., Abend N.S., Chu C.J. et al. Contemporary profile of seizures in neonates: a prospective cohort study. J. Pediatr. 2016; 174: 98-103.e1. https://dx.doi.org/10.1016/j.jpeds.2016.03.035.
- Wietstock S.O., Bonifacio S.L., Sullivan J.E., Nash K.B., Glass H.C. Continuous video electroencephalographic (EEG) monitoring for electrographic seizure diagnosis in neonates: a single-center study. J. Child Neurol. 2016; 31(3): 328-32. https://dx.doi.org/10.1177/0883073815592224.
- Butler E., Mills N., Alix J.P., Hart A.R. Knowledge and attitudes of critical care providers towards neurophysiological monitoring, seizure diagnosis, and treatment. Dev. Med. Child Neurol. 2021; 63(8): 976-83. https://dx.doi.org/10.1111/dmcn.14907.
- Pressler R.M., Cilio M.R., Mizrahi E.M., Moshé S.L., Nunes M.L., Plouin P. et al. The ILAE classification of seizures and the epilepsies: modification for seizures in the neonate. Position paper by the ILAE Task Force on Neonatal Seizures. Epilepsia. 2021; 62(3): 615-28. https://dx.doi.org/10.1111/epi.16815.
- Beal J.C., Eisermann M., Misra S.N., Pearl P.L., Plouin P., Mizrahi E.M. et al. Seizures and epilepsy in preterm and term neonates, infants, children and adolescents. In: Schomer D.L., Lopes da Silva F.H., eds. Niedermeyer’s electroencephalography: basic principles, clinical applications and related fields. 7th ed. Oxford University Press; 2018. https://dx.doi.org/10.1093/med/9780190228484.003.0018.
- Koutroumanidis M., Arzimanoglou A., Caraballo R., Goyal S., Kaminska A., Laoprasert P. et al. The role of EEG in the diagnosis and classification of the epilepsy syndromes: a tool for clinical practice by the ILAE Neurophysiology Task Force (Part 2). Epileptic Disord. 2017; 19(4): 385-437. https://dx.doi.org/ 10.1684/epd.2017.0952.
- Worden L.T., Chinappen D.M., Stoyell S.M., Gold J., Paixao L., Krishnamoorthy K. et al. The probability of seizures during continuous EEG monitoring in high‐risk neonates. Epilepsia. 2019; 60(12): 2508-18. https://doi.org/10.1111/ epi.16387.
- Yuan X., Kang W., Song J., Guo J., Guo L., Zhang R. et al. Prognostic value of amplitude-integrated EEG in neonates with high risk of neurological sequelae. Ann. Clin. Transl. Neurol. 2020; 7(2): 210-8. https://dx.doi.org/10.1002/acn3.50989.
- Заваденко А.Н., Дегтярева М.Г., Медведев М.И., Рогаткин С.О., Гребенникова О.В. Динамическое клинико-нейрофизиологическое наблюдение детей различного гестационного возраста с неонатальными судорогами. Педиатрия. Журнал им. Г.Н. Сперанского. 2017; 96(1): 23-8. [Zavadenko A.N., Degtyareva M.G., Medvedev M.I., Rogatkin S.O., Grebennikova O.V. Dynamic clinical and neurophysiological observation of children of various gestational ages with neonatal seizures. Pediatrics. 2017; 96(1): 23-8. (in Russian)]. https://dx.doi.org/10.24110/0031-403X-2017-96-1-23-28.
Received 09.07.2021
Accepted 23.07.2021
About the Authors
Ivan A. Suvorov, Neurologist at the Department of Pediatric Counselling of the Institute of Neonatology and Pediatrics, V.I. Kulakov NMRC for OG&P, Ministry of Healthof Russia, +7(495)438-26-00, iwan.suv@mail.ru, https://orcid.org/0000-0002-1715-6381, 117997, Russia, Moscow, Ac. Oparina str., 4.
Dzhenneta Yu. Amirkhanova, Neurologist at the Department of Pediatric Counselling of the Institute of Neonatology and Pediatrics, V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia, +7(495)438-26-00, djenn83@mail.ru, https://orcid.org/0000-0002-5923-6646, 117997, Russia, Moscow, Ac. Oparina str., 4.
Anna V. Degtyareva, Dr. Med. Sci., Professor, Head of Pediatric Department, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, Professor of Neonatology Department of Institute for Children's Health, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University), +7(495)438-26-00, annadim@yahoo.com,
https://orcid.org/0000-0003-0822-751X, 117997, Russia, Moscow, Ac. Oparina str., 4.
Dmitriy N. Degtyarev, Dr. Med. Sci., Professor, Deputy Director for Research, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia; Head of the Department
of Neonatology, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University), +7(495)438-25-33, d_degtiarev@oparina4.ru,
https://orcid.org/0000-0001-8975-2425, 117997, Russia, Moscow, Ac. Oparina str., 4.
Marina B. Albegova, MD, Ph.D., Head of Consultative Pediatric Unit, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)438-26-00, albegova@yahoo.com, https://orcid.org/0000-0001-7833-7648, 117997, Russia, Moscow, Ac. Oparina str., 4.
Аnna R. Kirtbaya, Ph.D., Clinical Care Supervisor at the A.G. Antonov Neonatal Intensive Care Unit, Institute of Neonatology and Pediatrics, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, Associate Professor at the Department of Neonatology, Faculty of Pediatrics, I.M. Sechenov First MSMU, Ministry of Health of Russia
(Sechenov University), +7(495)438-22-77, a_kirtbaya@oparina4.ru, https://orcid.org/0000-0002-7628-8157, 117997, Russia, Moscow, Ac. Oparina str., 4.
Elena A. Filippova, Ph.D., Head of the Department of Ultrasound Diagnostics in Neonatology and Pediatrics, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)438-26-00, fla77@mail.ru, https://orcid.org/0000-0002-4964-1736, 117997, Russia, Moscow, Ac. Oparina str., 4.
Authors' contributions: Degtyarev D.N., Degtyareva A.V., Albegova M.B., Amirkhanova D.Yu., Suvorov I.A. – conception and design of the study, analysis and review of the results; Suvorov I.A., Amirkhanova D.Yu., Kirtbaya A.R., Filippova E.A. – data collection and analysis; Suvorov I.A., Amirkhanova D.Yu., Degtyareva A.V. – manuscript preparation; Degtyarev D.N., Degtyareva A.V. – manuscript editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The study was conducted within the framework of the state assignment: "Predictors and dynamics of neurological disorders in very preterm infants with extremely low and very low birth weight".
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study, including the depersonalized data of the study participants, the primary study protocol, and the data analysis plan, will be available on request from the corresponding author within a year of publication after approval from the principal investigator.
For citation: Suvorov I.A., Amirkhanova D.Yu., Degtyareva A.V., Degtyarev D.N., Albegova M.B., Kirtbaya A.R., Filippova E.A. Neonatal seizures in extremely preterm infants with very and extremely low birth weight: prevalence and transformation into structural epilepsy.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 10: 134-142 (in Russian)
https://dx.doi.org/10.18565/aig.2021.10.134-142



