The expression profile of placental microRNAs as regulators of oxidative stress in fetal growth restriction
Objective. To evaluate the profile of differentially expressed microRNAs associated with oxidative stress and placental vascular dysfunction in fetal growth restriction (FGR).Gusar V.A., Timofeeva A.V., Kan N.E., Chagovets V.V., Ganichkina M.B., Frankevich V.E.
Materials and methods. Real-time quantitative RT-PCR was used to evaluate the placental tissue expression of microRNAs: miR-16-5p, miR-26b-5p, miR-100-5p, miR-125b-5p, miR-146a-5p, miR-182-5p, miR-199a-5p, miR-221-3p, miR-451a, and miR-574-3p in pregnant women with early- and late-onset FGR and control groups in the corresponding period.
Results. Placental tissues from pregnant women with FGR were found to have significant changes in the expression levels of the microRNAs playing an important role in the regulation of oxidative stress: miR-125b-5p, miR-221-3p, miR-451a, and miR-574-3P in early-onset FGR and miR-451a and miR-574-3p in late-onset FGR. The DAVID database.v.6.8 could identify the potential targets of the above microRNAs that are determined as the key participants of a number of biological pathways: TGFβ-, TNFα-, VEGF-, HIF1α-, FOXO-, and MAPK-signaling pathways.
Conclusion. The evaluation of the expression of the tissue-specific microRNAs regulating the balanced work of pro- and antioxidant system genes allows further determination of their diagnostic potential in the umbilical cord plasma and blood from pregnant women with FGR.
Keywords
Fetal growth restriction (FGR) is the most common pregnancy complication, affecting up to 5–10% of all pregnancies. One of the main causes of intrauterine growth restriction is placental insufficiency. It is caused by metabolic and hemodynamic disorders in “mother-placenta-fetus” functional system, resulting in reduced intake of nutrients and oxygen and impaired fetal growth [1, 2]. FGR may be diagnosed if the fetus weight is less than the 10th percentile for the corresponding gestational age in combination with ultrasound signs of FGR and abnormal blood flow in umbilical arteries and middle cerebral artery [1]. Small-for-gestational-age fetuses have 4-fold higher risk for: 1) perinatal mortality; 2) respiratory distress syndrome and necrotizing enterocolitis; 3) adverse neurological outcomes due to impaired brain structures development and myelination [2, 3].
Little is known about the mechanisms of FGR development. This complication of pregnancy is polyetiological. The main risk factors of FGR development are: 1) fetal factor (genetic and chromosomal abnormalities); 2) placental (impaired implantation and placentation); 3) maternal factor (vascular diseases, hypoxia, nutritional deficiency, exposure to toxiс environmental and infectious agents) [1]. Oxidative stress (OS) plays one of the leading roles in pregnancy complications, including FGR. OS is characterized by an imbalance between reactive oxygen species (ROS) production and the ability of a biological system to neutralize the reactive intermediates [4]. OS can occur due to placental ischemia/reoxygenation caused by maternal and fetal vascular malperfusion. OS leads to cell and tissue damage by means of free radicals and peroxides production [4].
Reactive oxygen species affect the expression of placental growth factor (PLGF) and vascular endothelial growth factor (VEGF). An increased superoxide and nitric oxide generation and peroxynitrite production lead to vascular dysfunction [5].
MicroRNA (miRNA) are small non-coding RNA molecules which regulate various biological processes. miRNAs could shed new light on the pathogenesis of disorders associated with placental insufficiency. Particularly, some studies identified reduced miRNAs expression (miR-518b, miR-1323, miR-516b, miR-515-5p, miR-520h, miR-519d, and miR-526b) in patients with FGR [6, 7]. It was detected that members of the miR-200, miR-21, miR-23a/b family are involved in OS mediated vascular dysfunction [7, 8]. Increased miR-210 expression induces the production of reactive oxygen species. miR-210 plays a key role in mitochondrial metabolism and is considered to be hypoxia indicator [7, 9]. It is worth mentioning that preeclampsia has been the main subject of most studies focused on an investigation of miRNAs expression in OS associated pregnancy complications. It was discovered that miR-1, miR-16, miR-20a, miR-26b, miR-29b, miR-126-3р, miR-144-3р, miR-146b-5р, miR-155, miR-181, miR-182, miR-195, miR-204, miR-210, miR-335 and miR-451а are differentially expressed miRNAs associated with OS [7, 10, 11]. In this connection, we evaluated the expression of both miRNAs which regulate antioxidant enzymes action and reactive oxygen species production in early- and late-onset FGR.
Materials and Methods
Forming the groups of participants
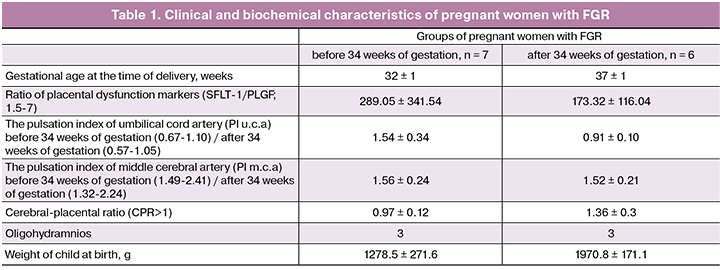
The study included 32 pregnant women who were under medical supervision at the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov, Ministry of Health of Russia. The patients were divided into four groups: 1) pregnant women with early-onset FGR who delivered before 34 weeks of gestation (n = 7); 2) pregnant women with late-onset FGR who delivered after 34 weeks of gestation (n = 6); 3) seven pregnant women without FGR with preterm labor who delivered before 34 weeks of gestation (30±2); 4) twelve pregnant women without FGR with normal pregnancy delivered after 34 weeks of gestation (37±1) (Table 1).
Fetal and placental Doppler velocimetry and identification of markers of placental dysfunction in pregnant women in the control groups (3 and 4) were not performed.
Exclusion criteria for participation in the study were: multiple pregnancy and pregnancy after IVF, severe somatic disorders in mothers, genetic pathology of the mother and/or fetus.
The diagnosis of FGR was determined on the basis of the estimated fetal weight below the 10th percentile for the corresponding gestational age. Fetal and placental Doppler velocimetry was used to detect the blood flow abnormalities in umbilical arteries, middle cerebral artery, uterine arteries. In addition to fetal weight, the abnormal pulsation index in the umbilical and middle cerebral arteries, amniotic fluid deficiency (oligohydramnion and anhydramnion) were taken into account. Women included in the study delivered their infants via cesarean section. Pathomorphological study was performed to detect ischemic lesions in placentas.
All studies were carried out using the informed consent signed by the patients and were approved by the Ethical Committee of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov, Moscow.
Total RNA/miRNA isolation from placental tissue
Tissue samples of the placenta were used as experimental samples. These samples were placed in liquid nitrogen and were stored at -75°C after washing in 0.9% sodium chloride solution. Then the tissue sample was homogenized in QIAzol Lysis Reagent and total RNA was isolated (miRNeasy MicroKit, QIAGEN, Germany), followed by enrichment with a low molecular weight miRNA fraction (RNeasy MinElute Cleanup Kit, QIAGEN, Germany). The quality control of the selected samples was performed on an Agilent 2100 bioanalyzer (Agilent Technologies) using the RNA 6000 Nano Kit, and the concentration was measured on a Qubit 3.0 fluorometer (Invitrogen). The obtained samples were stored at -75°C for the following analysis.
Real-Time Quantitative Reverse Transcription Polymerase Chain Reaction (RT-PCR)
The reverse transcription reaction was performed using the miScript II RT Kit. Quantitative PCR was performed using StepOnePlus Real-Time PCR System to determine the level of miRNA expression in placental tissue (miScript SYBR Green PCR Kit). The following sense primers were used: hsa-miR-16-5p MIMAT0000069 (5ʹ-TAGCAGCACGTAAATATTGGCG), hsa-miR-26b-5p MIMAT0000083 (5ʹ- TTCAAGTAATTCAGGATAGGT), hsa-miR-100-5p MIMAT0000098 (5ʹ-AACCCGTAGATCCGAACTTGTG), hsa-miR-125b-5p MIMAT0000423 (5ʹ-TCCCTGAGACCCTAACTTGTGA), hsa-miR-146a-5p MIMAT0000449 (5ʹ-TGAGAACTGAATTCCATGGGTT), hsa-miR-182-5p MIMAT0000259 (5ʹ-TTTGGCAATGGTAGAACTCACACT), hsa-miR-199a-5p MIMAT0000231 (5ʹ-CCCAGTGTTCAGACTACCTGTTC), hsa-miR-221-3p MIMAT0000278 (5ʹ-AGCTACATTGTCTGCTGGGTTTC), hsa-miR-451a MIMAT0001631 (5ʹ-AAACCGTTACCATTACTGAGTT), hsa-miR-574-3p MIMAT0003239 (5ʹ-CACGCTCATGCACACACCCACA), SNORD68 (5’- ACATTCTCCGGAATCGCTGT). All stages of work were performed in accordance with the manufacturer recommendations (QIAGEN). Ct <34 was taken as the threshold level of expression. The miRNA expression level was determined by the 2-ΔΔCT method, using SNORD68 as a reference RNA.
Statistical analysis of the data
The statistical significance in miRNA expression levels differences in the groups was estimated with the Wilcoxon-Mann-Whitney method using scripts written in the R language [12].
Results and Discussion
One of the key mechanisms of FGR development is an impaired uteroplacental blood flow which can take place due to defective spiral arteries remodeling and prooxidant-antioxidant system unbalanced functioning. Thereby we selected miRNAs that play an important role in the pathogenesis of cardiovascular diseases according to the literature data [13–15]. We focused on the following miRNAs: miR-16-5p, miR-26b-5p, miR-100-5p, miR-125b-5p, miR-146a-5p, miR-182-5p, miR-199a-5p, miR-221-3p, miR-451a and miR-574-3p.
Differential expression analysis showed decreased miR-221-3p and miR-451a expression levels and increased miR-125b-5p and miR-574-3p expression levels in placental tissue of women with early-onset FGR compared to the control group (р < 0.03) (Fig. 1).
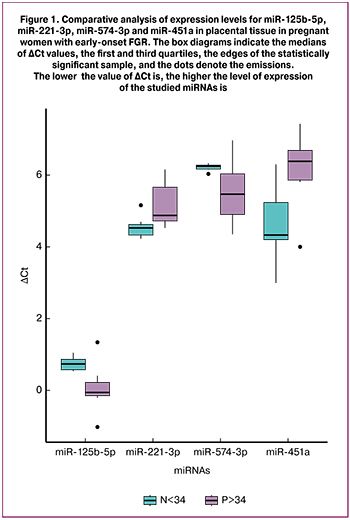
In the late-onset FGR group only miR-574-3p and miR-451a showed significant differences (Fig. 2).
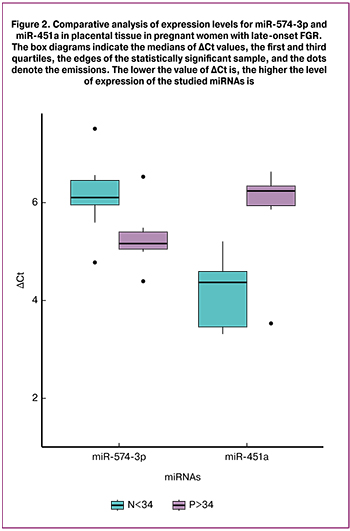
miR-574-3p and miR-451a expression levels in the late-onset FGR group changed in different directions, as well as in the early-onset FGR group: a significant increase in miR-574-3p expression level (p < 0.04) and decreased miR-451a expression level (p < 0.006) compared to the control group.
Furthermore we detected that miR-125b-5p and miR-221-3p changed their expression only in early-onset FGR group. A larger cohort of pregnant women with FGR is required to check the specificity of these changes.
Hromadnikova et al. evaluated miRNA expression levels in placental tissue and umbilical cord blood in groups of pregnant women with arterial hypertension, preeclampsia and FGR [13, 14].
Decreased miR-125b-5p, miR-199a-5p, miR-221-3p and miR-574-3p expression levels were detected in placental tissue of patients with early-onset FGR [13]. The authors revealed decreased miR-199a-5p, miR-221-3p and miR-574-3p expression levels in the peripheral blood of pregnant women with early-onset severe preeclampsia. miR-199a-5p was associated with FGR regardless of gestational age and fetal condition (oligohydramnion/anhydramnion) [15]. In addition, a negative correlation between miR-221-3p expression level in the umbilical cord blood and maternal peripheral blood with FGR and the umbilical artery pulsation index was detected (r = −0.390, p = 0.017) [14].
In our study we did not detect statistically significant changes in miR-16-5p, miR-26b-5p, miR-100-5p, miR-146a-5p, miR-182-5p and miR-199a-5p expression levels in placental tissue of patients with FGR. Some other researchers revealed the differential expression of these miRNAs in arterial hypertension, preeclampsia and FGR [13–15]. It should be noted that miRNA expression levels discrepancy in our data and other researchers study results could apparently be explained by differences in: 1) methods used in collecting the material; 2) participants' gestational age in the compared groups; 3) somatic pathology in mothers.
Chen et al. revealed the opposite miR-221/222 regulatory influence on smooth muscle and endothelial cells proliferation and migration which was explained by multidirectional expression of miR-221/222 target genes (p27, p57, c-kit, eNOS, Ets-1) in the analyzed cell cultures [16]. It is also known that hypoxia induced miR-221/222 overexpression that indirectly led to a decrease in levels of endothelial nitric oxide synthase (eNOS) [8]. NOS is expressed in the placental syncytiotrophoblast, in vascular endothelial cells of the placenta and umbilical cord. Nitric oxide contributes to low vascular resistance in the placenta [17].
A decrease in miR-221-3p expression level in early-onset FGR could probably have an impact on placental blood flow and adequate oxygen supply of the fetus. Abnormal Doppler cerebro-placental ratio in pregnant women with early-onset FGR reflects placental insufficiency and fetal deterioration (Table 1).
In addition to the interaction with the above vascular system target genes, one of miR-221/222 targets is the proapoptotic protein PUMA, which initiates mitochondrial caspase-dependent apoptotic pathway. Zhang et al. showed miR-221/222-dependent inhibition of cell proliferation in transfected epithelial tumor cells by inducing apoptosis [18].
The increased miR-125b expression level in early-onset FGR in our study plays an important role in pro- and antioxidant system regulation. Thus, miR-125b high expression level was detected in PC-12 neuronal line cells in oxygen-glucose deprivation/reoxygenation (OGD/R) in cerebral ischemia/reperfusion in vitro model. High expression level of miR-125b resulted in a decrease in protein kinase 2 level and NADHH oxidase (NOX2 and NOX4) activation. This led to free radicals level increase and neuron apoptosis [19]. The middle cerebral artery occlusion (MCAO) model in another study demonstrated the impact of miR-125b-5p overexpression on cystathionine-β-synthase (CBS) level decrease, the main protein which catalyzes H2S brain production. In contrast, an inhibition of miR-125b-5p expression in PC-12 cells line promoted H2S increased production and decreased levels of lactate dehydrogenase (LDH), malondialdehyde (MDA), superoxide and peroxide, and decreased number of apoptotic cells [20]. It is well known that in hypoxic cell damage conditions H2S protective effect correlates with a decrease in reactive oxygen species levels and an increase in antioxidants levels [21]. In turn, the main regulator of miR-125b-5p expression is the nuclear transcription factor NRF2 which is an important initiator of antioxidant protein genes (hemoxygenase 1, glutathione, glutathione transferase, thioredoxin-reductase 1) transcription [22]. So it can be concluded that an increase in miR-125b-5p expression level in placental tissue will shift the balance between the formation of antioxidant proteins and reactive oxygen species towards the latter. Some studies focused on oxidative stress indicators and total antioxidant status in placental tissue, amniotic fluid and blood plasma of pregnant women with FGR. Mert et al. revealed a significant increase in both blood plasma free radicals level and antioxidants in pregnant women with FGR and preeclampsia [23]. Currently, there are several investigations devoted to malonic dialdehyde (MDA) and catalase (CAT) evaluation in maternal and umbilical cord blood serum, myometrium and placenta of pregnant women with FGR and preeclampsia [24].
In our study we revealed a decrease in miR-451a expression level both in early-onset and late-onset FGR. miR-451a is involved in cellular protection against oxidative stress. In particular, in the research conducted by Wang et al., the investigators demonstrated that miR-451a had a cardioprotective effect in conditions of ischemia and oxidative stress [25]. The loss of miR-451a expression led to increased susceptibility of erythrocytes to oxidizing agents action due to a change in phosphoserine/threonine-binding protein 14-3-3zeta (KCIP-1) level, FoxO3 transcription factor inhibitor. FoxO3 is a positive regulator of antioxidant genes in erythrocytes. Erythropoietin is a well-known miR-451a expression modulator in the culture of SH-SY5Y neuron-like cells. It causes antioxidant, anti-apoptotic and neuroprotective effects by increasing the expression of some of the miR-451a target genes, including matrix metalloproteinase 9 (MMP9), cyclin-dependent kinase 2 (CDK2), erythropoietin receptor (EpoR), regulator of cell apoptosis (BCL2) [27].
As for miR-574-3p, we detected an increase in its expression in placental tissue of FGR patients. There is no data available in the literature concerning miR-574-3p participation in oxidative stress. However miR-574-3p expression levels are increased in cardiovascular diseases and in particular in myocardial infarction [28].
The analysis of databases (miRWalk, miRDB, miRTarbase, DAVID) allowed us to determine miR-125b-5p, miR-221-3p, miR-451a and miR-574-3p predictable targets associated with various pregnancy complications including FGR. They are important participants in regulation of various biological pathways presented in Figure 3.
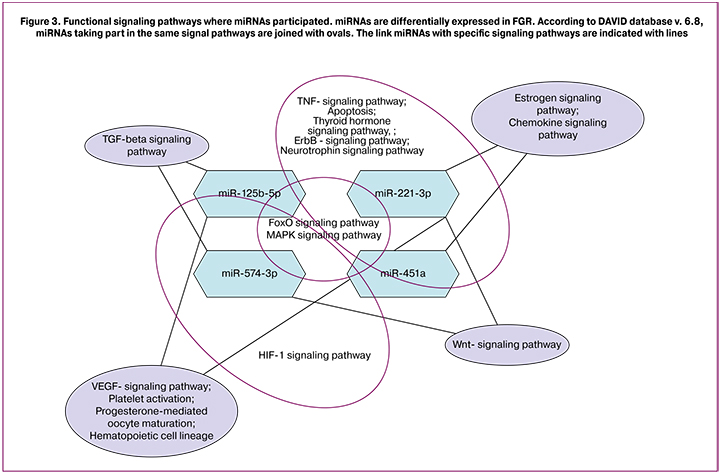
Thus, multi-level regulation of free radical oxidation and antioxidant protection is essential to ensure normal pregnancy development. miRNAs play a great role in this regulation process. miRNA expression level changes lead to a shift in pro- and antioxidant balance, causing placental tissues oxidative damage and impaired fetal growth and development respectively.
Conclusion
Nowadays circulating miRNAs have become a subject of scientific interest as non-invasive biomarkers of various obstetric syndromes. The early prediction of pregnancy complications using such biomarkers to monitor the health status both the mother and the fetus is of highest relevance. The main research perspectives include: 1) issue miRNAs diagnostic potential assessment in cord blood plasma and maternal blood plasma of patients with FGR; 2) search for correlations between miRNAs expression levels and concentrations of tissue oxidative stress and antioxidant response protein markers, as well as Doppler ultrasound parameters of fetal well-being.
References
- Devaskar S.U., Chu A. Intrauterine growth restriction: hungry for an answer. Physiology (Bethesda). 2016; 31(2): 131-46. https://dx.doi.org/10.1152/physiol.00033.2015.
- Audette M.C., Kingdom J.C. Screening for fetal growth restriction and placental insufficiency. Semin. Fetal Neonatal Med. 2018; 23(2): 119-25. https://dx.doi.org/10.1016/j.siny.2017.11.004.
- Miller S.L., Huppi P.S., Mallard C. The consequences of fetal growth restriction on brain structure and neurodevelopmental outcome. J. Physiol. 2016; 594(4): 807-23. https://dx.doi.org/.1113/JP271402.
- Schoots M.H., Gordijn S.J., Scherjon S.A., van Goor H., Hillebrands J.L. Oxidative stress in placental pathology. Placenta. 2018; 69: 153-61. https://dx.doi.org/10.1016/ j.placenta.2018.03.003.
- Myatt L., Cui X. Oxidative stress in the placenta. Histochem. Cell Biol. 2004; 122(4): 369-82. https://dx.doi.org/10.1007/s00418-004-0677-x.
- Higashijima A., Miura K., Mishima H., Kinoshita A., Jo O., Abe S. et al. Characterization of placenta-specific microRNAs in fetal growth restriction pregnancy. Prenat. Diagn. 2013; 33(3): 214-22.
- Rudov A., Balduini W., Carloni S., Perrone S., Buonocore G., Cristina M. Involvement of miRNAs in placental alterations mediated by oxidative stress. Oxid. Med. Cell. Longev. 2014; 2014: 103068. https://dx.doi.org/10.1155/2014/103068.
- Magenta A., Greco S., Gaetano C., Martelli F. Oxidative stress and microRNA in vascular diseases. Int. J. Mol. Sci. 2013; 14(9): 17319-46.
- Cicchillitti L., Di Stefano V., Isaia E., Crimaldi L., Fasanaro P., Ambrosino V. et al. Hypoxia-inducible factor 1-alpha induces miR-210 in normoxic differentiating myoblasts. J. Biol. Chem. 2012; 287(53):44761-71.
- Hu Y., Li P., Hao S., Liu L., Zhao J., Hou Y. Differential expression of microRNAs in the placentae of Chinese patients with severe pre-eclampsia. Clin. Chem. Lab. Med. 2009; 47(8): 923-9.
- Cross C.E., Tolba M.F., Rondelli C.M., Xu M., Abdel-Rahman S.Z. Oxidative stress alters miRNA and gene expression profiles in villous first trimester trophoblasts. Biomed. Res. Int. 2015; 2015: 257090. https://dx.doi.org/10.1155/2015/257090.
- Team R.C. R: A language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria, 2017. Available at: https://www.R-project.org/
- Hromadnikova I., Kotlabova K., Hympanova L., Krofta L. Cardiovascular and cerebrovascular disease associated microRNAs are dysregulated in placental tissues affected with gestational hypertension, preeclampsia and intrauterine growth restriction. PLoS One.| 2015; 10(9): e0138383. https://dx.doi.org/10.1371/journal.pone.0138383.
- Hromadnikova I., Kotlabova K., Ivankova K., Vedmetskaya Yu., Krofta L. Profiling of cardiovascular and cerebrovascular disease associated microRNA expression in umbilical cord blood in gestational hypertension, preeclampsia and fetal growth restriction. Int. J. Cardiol. 2017; 249: 402-9. https://dx.doi.org/10.1016/j.ijcard.2017.07.045.
- Hromadnikova I., Kotlabova K., Hympanova L., Krofta L. Gestational hypertension, preeclampsia and intrauterine growth restriction induce dysregulation of cardiovascular and cerebrovascular disease associated microRNAs in maternal whole peripheral blood. Thromb. Res. 2016;137: 126-40.
- Liu X., Cheng Y., Yang J., Xu L., Zhang C. Cell-specific effects of miR-221/222 in vessels: molecular mechanism and therapeutic application. J. Mol. Cell. Cardiol. 2012; 52(1): 245-55. https://dx.doi.org/ 10.1016/j.yjmcc.2011.11.008.
- Sladek S.M., Magness R.R., Conrad K.P. Nitric oxide and pregnancy. Am. J. Physiol. 1997; 272(2, Pt 2): R441-63.
- Zhang C., Zhang J., Zhang A., Wang Y., Han L., You Y. et al. PUMA is a novel target of miR-221/222 in human epithelial cancers. Int. J. Oncol. 2010; 37(6): 1621-6.
- Liang Y., Xu J., Wang Y., Tang J.Y., Yang S.L., Xiang H.G. et al. Inhibition of MiRNA-125b decreases cerebral ischemia/reperfusion injury by targeting CK2α/NADPH oxidase signaling. Cell. Physiol. Biochem. 2018; 45(5): 1818-26. https://dx.doi.org/10.1159/000487873.
- Shen Y., Shen Z., Guo L., Zhang Q., Wang Z., Miao L. et al. MiR-125b-5p is involved in oxygen and glucose deprivation injury in PC-12 cells via CBS/H2S pathway. Nitric Oxide. 2018; 78(1): 11-21. https://dx.doi.org/10.1016/j.niox.2018.05.004.
- Yu Q., Lu Z., Tao L., Yang L., Guo Y., Yang Y. et al. ROS-dependent neuroprotective effects of NaHS in ischemia brain injury involves the PARP/AIF pathway. Cell. Physiol. Biochem. 2015; 36(4): 1539-51.
- Shah N.M., Zaitseva L., Bowles K.M., MacEwan D.J., Rushworth S.A. NRF2-driven miR-125B1 and miR-29B1 transcriptional regulation controls a novel anti-apoptotic miRNA regulatory network for AML survival. Cell Death Differ. 2015; 22(4): 654-64. https://dx.doi.org/ 10.1038/cdd.2014.152.
- Mert I., Oruc A.S., Yukse S., Cakar E.S., Buyukkagnıcı U., Karaer A., Danısman N. Role of oxidative stress in preeclampsia and intrauterine growth restriction J. Obstet. Gynaecol. Res. 2012; 38(4): 658-64. https://dx.doi.org/10.1111/j.1447-0756.2011.01771.x.
- Biberoglu E., Biberoglu K., Kirbas A., Daglar K., Genc M., Avci A., Danisman N. Circulating and myometrial markers of oxidative stress in pregnant women with fetal growth restriction. J. Obstet. Gynaecol. Res. 2015; 42(1): 29-35. https://dx.doi.org/10.1111/jog.12857.
- Wang X., Zhu H., Zhang X., Liu Y., Chen J., Medvedovic M. et al. Loss of the miR-144/451 cluster impairs ischaemic preconditioning-mediated cardioprotection by targeting Rac-1. Cardiovasc. Res. 2012; 94(2): 379-90.
- Yu D., dos Santos C.O., Zhao G., Jiang J., Amigo J.D., Khandros E. et al. MiR-451 protects against erythroid oxidant stress by repressing 14-3-3zeta. Genes Dev. 2010; 24(15): 1620-33.
- Alural B., Duran G.A., Tufekci K.U., Allmer J., Onkal Z., Tunali D. et al. EPO mediates neurotrophic, neuroprotective, anti-oxidant, and anti-apoptotic effects via downregulation of miR-451 and miR-885-5p in SH-SY5Y neuron-like cells. Front. Immunol. 2014; 4: 475. https://dx.doi.org/10.3389/fimmu.2014.00475.
- Boštjančič E., Zidar N., Glavač D. MicroRNAs and cardiac sarcoplasmic reticulum calcium ATPase-2 in human myocardial infarction: expression and bioinformatics analysis. BMC Genomics. 2012; 13(1): 552.
Received 21.06.2018
Accepted 22.06.2018
About the Authors
Gusar, Vladyslava A., PhD, senior researcher of Laboratory of Applied Transriptomics, Department of System Biology in Reproduction of the National Medical Research Center for Obstetrics, Gynecology, and Perinatology named after Academician V.I. Kulakov, Ministry of Health of Russian Federation.117997, Russia, Moscow, Oparina str. 4. Tel: +74955314444, add. 2197. E-mail: v_gusar@oparina4.ru. ORCHID ID https://orcid.org/0000-0003-3990-6224
Timofeeva, Angelika V., PhD, senior researcher of Laboratory of Applied Transriptomics, Department of System Biology in Reproduction of the National Medical Research Center for Obstetrics, Gynecology, and Perinatology named after Academician V.I. Kulakov, Ministry of Health of Russian Federation.
117997, Russia, Moscow, Oparina str. 4. Tel: +74955314444, add. 2197. E-mail: v_timofeeva@oparina4.ru. ORCHID ID https://orcid.org/0000-0003-2324-9653
Kan, Natalia E., MD, professor, head of Department of Obstetric Observatory, the National Medical Research Center for Obstetrics, Gynecology, and Perinatology named after Academician V.I. Kulakov, Ministry of Health of Russian Federation.
117997, Russia, Moscow, Oparina str. 4. Tel: +74955314444. E-mail: n_kan@oparina4.ru. ORCHID ID https://orcid.org/0000-0001-5087-5946
Chagovets, Vitaliy V., PhD, senior researcher of Laboratory of Proteomics and Metabolomics of Human Reproduction, Department of Systems Biology in Reproduction
of the National Medical Research Center for Obstetrics, Gynecology, and Perinatology named after Academician V.I. Kulakov, Ministry of Health of Russian Federation.
117997, Russia, Moscow, Oparina str. 4. Tel: +74955314444. E-mail: v_chagovets@oparina4.ru
Ganichkina, Maria B., graduate student, the National Medical Research Center for Obstetrics, Gynecology, and Perinatology named after Academician V.I. Kulakov,
Ministry of Health of Russian Federation.
117997, Russia, Moscow, Oparina str. 4. Tel: +74955314444. E-mail: m_ganichkina@oparina4.ru
Frankevich, Vladimir E., PhD, head of Department of Systems Biology in Reproduction, the National Medical Research Center for Obstetrics, Gynecology,
and Perinatology named after Academician V.I. Kulakov, Ministry of Health of Russian Federation.
117997, Russia, Moscow, Oparina str. 4. Tel: + 495 531-44-44. E-mail: v_frankevich@oparina4.ru. ORCHID ID https://orcid.org/0000-0002-9780-4579
For citations: Gusar V.A., Timofeeva A.V., Kan N.E., Chagovets V.V., Ganichkina M.B., Frankevich V.E. The expression profile of placental microRNAs as regulators of oxidative stress in fetal growth restriction. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; (1): 74-80. (in Russian)
http://dx.doi.org/10.18565/aig.2019.1.74-80



