ASSOCIATION OF IL-10 A-1082G GENE POLYMORPHISM WITH PRODUCTION AND SECRETION OF IL-10 IN WOMEN WITH FETAL GROWTH RESTRICTION
Objective. To clarify risk factors for fetal growth restriction (FGR), to determine the features of a genotype by the interleukin (IL)-10 A-1082G polymorphism and its effect on the production and secretion of IL-10 by peripheral blood monocytes in women with FGR.Malyshkina A.I., Boiko E.L., Sotnikova N.Yu., Panova I.A., Fetisova I.N., Voronin D.N., Mileeva P.L.
Subjects and methods. A total of 209 pregnant women were surveyed. The investigators assessed IL-10A-1082G gene polymorphism by a real-time polymerase chain reaction assay, the intracellular production of IL-10 by monocytes by means of flow cytofluorometry, and IL-10 levels in the 24-hour monocyte culture supernatants by enzyme immunoassay.
Results. The IL-10 -1082AA genotype was more common in women with FGR. Changes were found in the synthesis and production of IL-10 by monocytes in relation to the allelic variant of the IL-10 A-1082G polymorphism.
Conclusion. The most significant risk factors for FGD are tobacco smoking, extragenital pathology, and a compromised obstetric and gynecological history. The results of the study may suggest that the L-10 A-1082G polymorphism is an additional factor regulating the immune balance during pregnancy when FGR develops.
Keywords
Currently, fetal growth restriction (FGR) remains one of the world’s greatest public health challenges due to its relatively high incidence (5-24%) and a large proportion of this pregnancy complication in the structure of perinatal morbidity and mortality [1,2]. In the past decade, the hypothesis of fetal programming has been a subject of debate in the literature. This theory suggests that the conditions affecting the fetus during its developmental phase determine its disease risk during the later stages. [3]. Most researches consider the FGR a multifactorial disease resulting from the combined action of maternal and fetal genetic factors, and environmental factors [4,5]. Some of FGR risk factors are associated with impaired functioning of a pregnant mother’s immune system [6]. A study of the serum cytokine profile of pregnant women reported a statistically significant decrease in the content of IL-10 in monocytes in women with FGR compared with the control group [6]; however, these changes were mainly associated with exogenous factors.
Recent data confirm the relationship between genetic risk factors and the development of FGR, but the results of these studies in different populations are contradictory [7,8]. Therefore, more studies are needed to investigate the polymorphisms of genes involved in FGR development. Elucidation of the genetic mechanisms of FGR formation will offer new possibilities for substantiating the prevention and treatment of FGR at the stage of pregnancy planning. A similar approach has been implemented in the examination of women for the polymorphism of thrombophilia genes. [9]. Several more recent studies investigating IL-10 gene polymorphism reported a significant association between this cytokine and the risk of developing acute pancreatitis and breast cancer [10,11].
The polymorphic -1082 A allele of the IL-10 gene is low-functional and downregulates the production of IL-10 cytokine protein molecule [12]. This observation implies that in women with FGR carrying the IL-10 -1082A allele the production and secretion of IL-10 by peripheral blood monocytes may be decreased. There is currently no research available exploring the relationship between IL-10 production and its gene polymorphism in FGR.
This study aimed to identify risk factors for FGR, investigate characteristic features of the genotype of IL-10 A-1082G gene polymorphism and its effect on the production and secretion of IL-10 by peripheral blood monocytes in women with FGR.
Materials and methods
A total of 209 women at 26-39 weeks’ gestation were examined at the obstetric clinic of the V.N. Gorodkov Research Institute of Maternity and Childhood of Minzdrav of Russia (Director - Dr.Med.Sci., Professor Malyshkina A.I.). The study group comprised 108 women whose fetuses had FGR. The women were categorized into two subgroups based on FGR severity. Subgroup I included 54 women whose fetuses were two weeks smaller than expected for their gestational age according to ultrasound (grade I FGR ); subgroup II included 54 women whose fetuses were 3-4 weeks smaller than expected for their gestational age (grade II-III FGR). The control group consisted of 101 pregnant women who did not develop FGR. The study participants resided in Ivanovo, Vladimir, and Kostroma regions (Central Federal District) of Russia and were Russian by ethnicity. Exclusion criteria were as follows: severe pre-eclampsia, severe non-obstetric comorbidities, multiple pregnancies, and fetal malformations.
The women underwent general clinical and laboratory investigations and ultrasound examination. The material for the study was peripheral venous blood. Polymorphism of the IL-10 A-1082G gene was estimated by real-time PCR using Lytech reagent kit (Moscow) by the manufacturer’s instructions. Intracellular production of IL-10 by peripheral blood monocytes was determined by flow cytofluorometry using monoclonal anti-IL-10 phycoerythrin-labeled antibodies (BD Bioscience, USA). Enriched monocyte culture was obtained using the standard method of double plastic adherence. The level of IL-10 in 24-hour supernatant cultures of peripheral blood monocytes was determined by ELISA using commercial ELISA-IL-10 kits (Cytokine LLC, Russia).
Statistical analysis was performed using Microsoft Office 2010 and Statistica for Windows 6.0. Continuous variables were expressed as arithmetic means and standard deviations (M (SD)) or as medians with the 25th and 75th percentiles (Me (Q1; Q3)). Categorical variables were compared by the χ2 test. Comparing numerical data between groups was performed with the Mann-Whitney test. Differences between the groups were considered statistically significant at p < 0.05. Relative risk (RR) and confidence intervals (CI) were calculated using OpenEpi.
Results and discussion
The clinical and anamnestic characteristics of the study participants are presented in Table 1. The mean age of the patients in the study and control groups was 28.46 (6.20and 29.27 (5.89) years, respectively (р > 0.05). There were no significant differences between the groups concerning the social status of the women (p > 0.05). Women with FGR were more likely to smoke (52.8%) than women in the control group (7.9%) (OR = 6.66, 95% CI: 3.35-13.26). Fifty percent (OR = 3.43, 95% CI: 2.35-5.00) of women with grade I FGR and 55.6% (OR = 3.85, 95% CI: 2, 60-5.70) with grade II-III FGR reported smoking (p < 0.001 in all cases). According to the literature, tobacco smoking leads to numerous changes in the pregnant woman›s body (increased blood pressure, platelet hyper-aggregation, plasma hypercoagulability and intravascular activation of blood coagulation) that may result in impaired placental circulation, chronic fetal hypoxia and FGR [13,14].
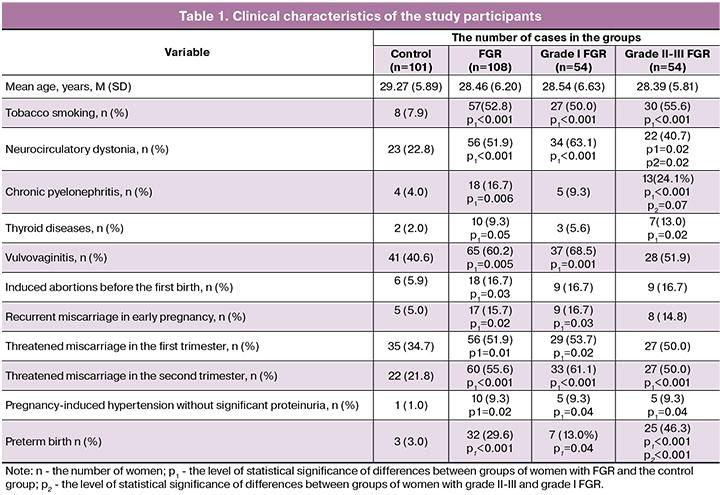
Among non-obstetric comorbidities, there was a higher incidence (51.9%) of neurocirculatory dystonia in women with FGR (OR = 1.77, 95% CI: 1.38-2.28); it was 63, 1% (OR = 3.08 95% CI: 1.96-4.85) and 40.7% (OR = 1.68, 95% CI: 1.11-2.55) among women with grade I and grade II-III FGR, respectively. Also, women with grade II-III FGR were more likely to have a history of chronic pyelonephritis (OR = 2.57, 95% CI: 1.78-3.72) and thyroid disease (OR = 2.42, 95% CI: 1.59-3.68). These observations are consistent with other studies that showed an important role of non-obstetric comorbidities in the development of FGR [15]. Women with grade I FGR were more likely to have a history of vulvovaginitis (OR = 2.15, 95% CI: 1.33-3.47) and recurrent miscarriage in early pregnancy (OR = 2.01, 95% CI: 1.27- 3.19). Women with FGR more often reported induced abortions before the first birth (OR = 1.54, 95% CI: 1.17-2.03).
Multiple uterine curettages contribute to the development of chronic endometritis impairing endometrial vascularization, zygote’s attachment, and trophoblast invasion, leading to decidual tissue insufficiency and the development of FGR during subsequent pregnancies [16]. Among women with FGR, the most common pregnancy complications were threatened miscarriage in the first (OR = 1.40, 95% CI: 1.08-1.81) and the second trimester (OR = 1.94, 95 % CI: 1.50-2.51), and pregnancy-induced hypertension without significant proteinuria (OR = 1.84, 95% CI: 1.45-2.32). According to the literature, threatened miscarriage disrupts the interaction of invasive cytotrophoblast and maternal factors leading to the inadequate physiological transformation of the spiral artery walls, impairs placental blood supply resulting in placental insufficiency and FGR in the second trimester of pregnancy [6,17]. Patients with FGR were more likely to have a history of preterm delivery (OR = 2.09, 95% CI: 1.72-2.55), (Table 1).
According to the literature, an imbalance of cytokines may cause various pregnancy complications of [18–20]. Cytokines can exert both embryotoxic (IL-1 and IL-6) and embryo-protective (IL-4, IL-10) effects [21, 22]. The presence of polymorphic variants in the cytokine genes affecting their synthesis and functional activity may be a risk factor for a complicated course of pregnancy [6]. It would be a reasonable assumption that the interrelation of IL-10 synthesis with the allelic polymorphism of the IL-10 gene is one of the mechanisms for the development of FGR. This cytokine is one of the most important anti-inflammatory cytokines. IL-10 inhibits the release of Th1 cytokines, NF-κB signaling, expression of class II HLA molecules, the function of macrophages and dendritic cells [23]. IL-10 is known to be a regulatory cytokine that controls the immune response of the pregnant woman’s body and placenta formation [24]. Reduced level of IL-10 can disrupt the balance of immune processes during pregnancy, and losing control over the ongoing immunological processes, in turn, may lead to impaired differentiation and trophoblastic invasion. This may result in threatened miscarriage leading to the development of placental insufficiency and FGR [2, 6,17]. Analysis of A-1082G gene polymorphism showed that among all study participants 24.4%, 32.8%, and 42.8% women were homozygous for the IL-10 -1082G allele had a heterozygous genotype IL-10 -1082A/G and homozygous genotype IL-10 -1082A/A, respectively. Among women with FGR, 20 (19.1%) had the homozygous genotype for the -1082G allele. In 85 (80.9%) women in this group, the presence of a low-functional IL-10 -1082A allele in the genotype was detected. At the same time, 54 (51.4%) women were found to have a homozygous IL-10 -1082A/A genotype and 31 (29.5%) had a heterozygous IL-10 -1082A/G genotype.
On account of the subgroup with grade II-III FGR, homozygous genotype with the -1082A allele was more common among all women with FGR than in the control group (51.4% in the study group vs. 33.3% of the control group, p = 0.01 ; OR = 1.42, 95% CI: 1.09-1.84 and 58.5% in the subgroup with grade II-III FGR vs. 33.3% of the control, p = 0.005; OR = 1.92, 95% CI : 1.24, -2.98, respectively). No statistically significant differences in the rates of homozygous and heterozygous genotype with the -1082G allele were observed between women with FGR and the control group (p> 0.05) regardless of FGR severity. According to the literature, in the Caucasoid population, the prevalence of homozygotes with the IL-10 -1082G allele, heterozygous genotype IL-10 -1082G/A, and homozygous genotype IL-10 -1082A is 52.0-62.5%,13.0-49.9%, and 18.0-39.0%, respectively [25]. According to our findings, among the population of three regions of the Central Federal District of Russia (Ivanovo, Kostroma, Vladimir regions), homozygotes with the IL-10 -1082G allele were twice as rare as among the European population. Conversely, carriers of the heterozygous IL-10 -1082A/G genotype and the homozygous IL-10 genotype - 1082A/A were more common in Russian than in European populations [25]. Our analysis of rates of alleles and genotypes with IL-10 A-1082G polymorphism in pregnant women showed that the presence of a homozygous genotype with the -1082A allele increases the risk of developing FGR (p = 0.01; OR = 1.42, 95 % CI: 1.09-1.84) and grade II-III FGR (p = 0.005; OR = 1.92, 95% CI: 1.24-2.98).
At the same time, a combination of tobacco smoking and the presence of IL-10 -1082A/A or IL-10-1082G/A genotypes increased the risk of developing FGR during pregnancy. (OR = 3.19, 95% CI: 1.61-6.30) [patent for the invention No. 2664505 of 5.03.18].
As is known, polymorphism-1082A in the promoter region of the IL-10 gene has a negative impact on the gestational process in the early pregnancy [26]. Given the association of this polymorphism with the risk of miscarriage, it can be assumed that impaired trophoblast invasion may cause the termination of pregnancy, but if pregnancy is preserved and prolonged, it can lead to the development of placental insufficiency and FGR [27].
Data on the production and secretion of IL-10 in the blood of women with FGR are presented in Table 2. Analysis of the production and secretion of IL-10 by peripheral blood monocytes depending on the female genotype with IL-10 A-1082G polymorphism showed that in women with grade I FGR, and genotype IL-10 -1082A/G or IL-10 -1082A/A the serum level of IL-10 was statistically significantly higher than in the control group (p = 0.02).
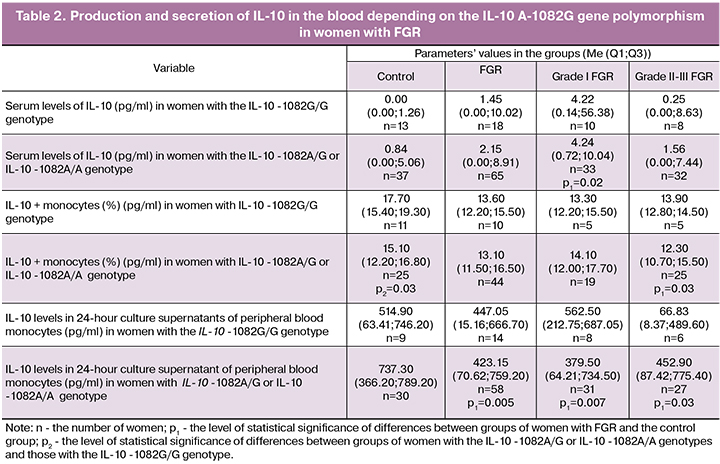
In the control group, the women with the genotype IL-10 -1082A/G or IL-10 -1082A/A had lower intracellular production of IL-10 by peripheral blood monocytes than women with the genotype IL-10 -1082G/G (p = 0.03). Only in women with grade II-III FGR and the genotype IL-10 -1082A/G or IL-10 -1082A/A, a decrease in intracellular production of IL-10 by peripheral blood monocytes was observed compared to the control group (p = 0.03).
The level of IL-10 in 24-hour supernatant cultures of peripheral blood monocytes was statistically significantly lower in women with genotypes IL-10 -1082A/G or IL-10 -1082A/A and grade I and grade II-III FGR than in the control group (p = 0.005, p = 0.007 and p = 0.03, respectively). There were no statistically significant differences in the levels of serum IL-10, the content of peripheral IL-10 + monocytes, and levels of IL-10 in 24-hour supernatant cultures of monocytes in women with FGR and the genotype IL-10 -1082G/G regardless of FGR severity (p> 0.05 in all cases).
Previous studies have shown that low IL-10 levels are associated with pregnancy complications, such as threatened miscarriage, pre-eclampsia, and FGR [18, 19, 20]. E. Nouvellon et al. In 2015 reported that the allelic variant of the IL-10 - 1082A gene is associated with low production of the IL-10 protein molecule [12]. This observation is consistent with our data on the decrease in intracellular production of IL-10 by peripheral monocytes among women in the control group, who had the IL-10 -1082A/G or IL-10 -1082A/A genotypes compared with women with the IL-10 -1082G/G genotype. However, in groups with FGR, we found no statistically significant differences in production and secretion of IL-10 depending on the genotype. It may imply that in women with FGR and a low-functional IL-10 -8282A allele, there are some additional mechanisms regulating IL-10 synthesis, possibly due to other cell populations that produce this cytokine intracellularly. A decrease in the IL-10 production in complicated pregnancies was observed in studies by Shi-Bin Cheng [28]. The direct association between low IL-10 levels and the development of FGR was shown by F.Y. Azizieh and R. Raghupathy [18]. On the contrary, A.V. Kudryashova et al (2009) observed an increase in the serum levels of IL-10 in a group of women with FGR [6], which is consistent with our findings in a subgroup of women with grade I FGR and the genotype IL-10 -1082A/G or IL-10 -1082A/A. The increase in the serum level of IL-10 may be attributed to its secretion by different cell populations and reflects the total cytokine content [6], which indirectly confirms the above assumption.
We estimated the relative content of peripheral blood IL-10 + monocytes and spontaneous secretion of IL-10 by the cytokine content in 24-hour supernatant cultures of peripheral blood monocytes. A.V. Kudryashova et al. (2009) showed a decrease in the relative content of peripheral blood IL-10 + monocytes and level of IL-10 in 24-hour supernatant cultures of peripheral blood monocytes in women with FGR [6]. According to our data, a decrease in the level of peripheral IL-10 + monocytes was observed only in women with grade II-III FGR and IL-10 -1082A/G or IL-10 -1082A/A genotypes, while IL-10 levels in supernatants was low in all women with FGR and genotype IL-10 -1082A/G or IL-10 -1082A/A. No significant changes in the levels of IL-10 + monocytes and IL-10 in 24-hour supernatant cultures of peripheral blood monocytes were observed in women with the IL-10 -1082G/G genotype. The decrease in intracellular production and secretion of IL-10 by peripheral blood monocytes may be attributed to the presence of a low-functional allelic variant of the IL-10 - 1082A gene in women with FGR.
Conclusion
The most significant risk factors for FGR are tobacco smoking, non-obstetric comorbidities, and complicated obstetric and gynecologic history. The study results suggest that polymorphism of the IL-10A-1082G gene is an additional factor regulating the immune balance in pregnancy during the development of FGR.
References
- Ажибеков С.А., Путилова Н.В., Третьякова Т.Б., Пестряева Л.А. Роль генетически детерминированных особенностей энергетического обмена в формировании плацентарной недостаточности с исходом в синдром задержки роста плода. Акушерство и гинекология. 2016; 11: 11-5. [Azhibekov S.A., Putilova N.V., Tretyakova TB, Pestryaeva L.A. The role of genetically determined features of energy metabolism in the formation of placental insufficiency with outcome in fetal growth retardation syndrome. Obstetrics and gynecology. 2016; 11: 11-15. (in Russian)].
- Макаров И.О., Юдина Е.В., Боровкова Е.И. Задержка роста плода. Врачебная тактика. М.: МЕДпресс-информ; 2016. 56с. [Makarov I.O., Yudina E.V., Borovkova E.I. Fetal growth retardation. Medical tactics. M .: MEDpress-inform; 2016. 56 р. (in Russian)].
- Смирнова Н.Н., Куприенко Н.Б. Фетальное программирование патологии взрослых. Нефрология. 2012; 16(2): 111-7. [Smirnova N.N., Kuprienko N.B. Fetal programming of adult pathology. Nephrology. 2012; 16 (2): 111-117. (in Russian)].
- Стрижаков А.Н., Игнатко И.В., Карданова М.А. Критическое состояние плода: определение, диагностические критерии, акушерская тактика, перинатальные исходы. Вопросы гинекологии, акушерства и перинатологии. 2015; 14(4): 5-14. [Strizhakov A.N., Ignatko I.V., Kardanova M.A. Fetal critical condition: definition, diagnostic criteria, obstetric tactics, perinatal outcomes. Gynecology, obstetrics and perinatology issues. 2015; 14 (4): 5-14 (in Russian)].
- Фетисова И.Н., Посисеева Л.В., Поляков А.В. Наследственные факторы при различных формах нарушения функции супружеской пары. Иваново: Издательство “Иваново”; 2009. 240с.[Fetisova I.N., Posiseeva L.V., Polyakov A.V. Hereditary factors in various forms of dysfunction of a married couple. Ivanovo: Ivanovo; 2009. 240p. (in Russian)].
- Кудряшова А.В., Сотникова Н.Ю., Посисеева Л.В., Панова И.А., Веденеева М.В. Роль иммунной системы в формировании задержки внутриутробного развития плода. Иваново; 2009. 239с. [Kudryashova A.V., Sotnikova N.Yu., Posiseeva L.V., Panova I.A., Vedeneeva M.V. The role of the immune system in the formation of intrauterine growth retardation. Ivanovo; 2009. 239 p. (in Russian)].
- Tsai P.Y., Li S.H., Chen W.N., Tsai H.L., Su M.T. Differential miR-346 and miR-582-3p еxpression in аssociation with selected maternal and fetal complications. Int. J. Mol. Sci. 2017; 18(7). pii: E1570. https://dx.doi.org/10.3390/ijms18071570.
- Ye K.J., Dai J., Liu L.Y., Peng M.J. Network‑based gene function inference method to predict optimal gene functions associated with fetal growth restriction. Mol. Med. Rep. 2018 18(3): 3003-10. https://dx.doi.org/10.3892/mmr.2018.9232.
- Трифонова Е.А., Габидулина Т.В., Агаркова Т.А., Сереброва В.Н., Бутко Ю.К., Ворожищева А.Ю., Юрьев С.Ю., Девятьярова Л.А., Минайчева Л.И., Степанов В.А. Анализ роли наследственной тромбофилии в развитии осложненного течения беременности. Фундаментальные исследования. 2012; 10-2: 337-44. [ Trifonova E.A., Gabidulina T.V., Agarkova T.A., Serebrova V.N., Butko Yu.K., Vorozhishcheva A.Yu., et al. Analysis of the role of hereditary thrombophilia in the development of a complicated course of pregnancy. Basic research 2012; 10: 337-344.(in Russian)]
- Zhou H., Liu A., Zhou B., Zhao C., Jin G. Interleukin-10 gene rs1800896 polymorphism increases risk of acute pancreatitis. Medicine (Baltimore). 2017; 96(48): e9006. https://dx.doi.org/10.1097/MD.0000000000009006.
- Maruthi G., Vinish Ramachander VR., Prasanna Latha komaravalli, Sureka TK., Parveen Jahan. Association of Il-10 gene polymorphisms (Rs 1800896, Rs1800872) in breast cancer patients. Int. J. Med. Sci. Clin. Invent. 2017; 4(6): 3050-61. 10.18535/ijmsci/v4i6.19.
- Cochery Nouvellon E., Nguyen P., Attaoua R., Cornillet Lefebvre P., Mercier E., Vitry F. et al. Interleukin 10 gene promoter polymorphisms in women with pregnancy loss: preferential association with embryonic wastage. Inflamm. Res. 2015; 64(12): 963-69.
- Артемьева Ж.Г., Поздеева М.С. Влияние условий перинатального периода на развитие ребенка. Молодой ученый. 2017; 15: 546-8. Доступно по: https://moluch.ru/archive/149/42053/. [Artemyeva Zh.G., Pozdeeva M.S. The influence of the perinatal period on the development of the child. Young scientist. 2017; 15: 546-548. Available at: https://moluch.ru/archive/149/42053/.(in Russian)].
- Shea A.K., Steiner M. Cigarette smoking during pregnancy. Nicotine Tob. Res. 2008; 10(2): 267-78.
- Волошина И.М. Взаимосвязь характера течения беременности и наличия в анамнезе соматической и стоматологической патологии. Здравоохранение Российской Федерации. 2014; 58(1): 43-7. [Voloshina I.M. The relationship between the nature of the course of pregnancy and the presence of a history of somatic and dental pathology. Healthcare of the Russian Federation. 2014; 58(1): 43-47. (in Russian)].
- Радзинский В.Е., Смалько П.Я. Биохимия плацентарной недостаточности. М.: РУДН; 2001. 275с. [Radzinsky V.E., Smalko P.Ya. Biochemistry of placental insufficiency. - M.: RUDN; 2001. 275p. (in Russian)]
- Газиева И.А., Чистякова Г.Н., Ремизова И.И. Предикторная значимость показателей функционального состояния эндотелия и регуляция ангиогенеза в I триместре беременности в развитии плацентарной недостаточности и ранних репродуктивных потерь. Вопросы гинекологии, акушерства и перинатологии. 2015; 14(2): 14-23. [Gazieva I.A., Chistyakova G.N., Remizova I.I. Predictor significance of endothelium functional status indicators and angiogenesis regulation in the first trimester of pregnancy in the development of placental insufficiency and early reproductive losses. Gynecology, obstetrics and perinatology issues. 2015; 14 (2): 14-23. (in Russian)].
- Azizieh F.Y., Raghupathy R. IL-10 and pregnancy complications. Clin. Exp. Obstet. Gynecol. 2017; 44(2): 252-58.
- Cornelius D.C., Cottrell J., Amaral L.M., LaMarca B. Inflammatory mediators: a causal link to hypertension during preeclampsia. Br. J. Pharmacol. 2018; 176(12): 1914-21. https://dx.doi.org/10.1111/bph.14466.
- Al-Azemi M., Raghupathy R., Azizieh F. Pro-inflammatory and anti-inflammatory cytokine profiles in fetal growth restriction. Clin. Exp. Obstet. Gynecol. 2017; 44(1): 98-103.
- Kwak-Kim J., Bao S., Lee S.K., Kim J.W., Gilman-Sachs A. Immunological modes of pregnancy loss: inflammation, immune effectors, and stress. Am. J. Reprod. Immunol. 2014; 72(2): 129-40. https://dx.doi.org/10.1111/aji.12234.
- Машкина Е.В., Коваленко К.А., Миктадова А.В., Волосовцова Г.И., Сараев К.Н. Анализ экспрессии гена Il-10 в децидуальной и хорионической тканях при невынашивании беременности. Научное обозрение. Биологические науки. 2014; 1: 86-7. Доступно по: https://science-biology.ru/ru/article/view?id=109. [Mashkina E.V., Kovalenko K.A., Miktadova A.V., Volosovtsova G.I., Saraev K.N. Analysis of gene expression of il-10 in decidual and chorionic tissues during miscarriage // Scientific Review. Biological sciences. - 2014. 1: 86-87; Available at: https://science-biology.ru/ru/article/view?id=109 (in Russian)].
- Gonzalez-Galarza F.F., McCabe A., Melo Dos Santos E.J., Takeshita L., Ghattaoraya G., Jones A.R., Middleton D. Allele frequency net database. Methods Mol. Biol. 2018; 1802: 49-62.
- O’Garra A., Barrat F.J., Castro A.G., Vicari A., Hawrylowicz C. Strategies for use of IL-10 or its antagonists in human disease. Immunol. Rev. 2008; 223(1): 114-31.
- Нефедова Д.Д., Линде В.А., Левкович М.А. Иммунологические аспекты беременности (обзор литературы). Медицинский вестник Юга России. 2013; 4: 16-21. [Nefedova DD, Linde V.A., Levkovich M.A. Immunological aspects of pregnancy (literature review). Medical Bulletin of the South of Russia. 2013; 4: 16-21 (in Russian)].
- Чистякова Г.Н., Газиева И.А., Ремизова И.И., Черданцева Г.А. Оценка продукции цитокинов при беременности, осложненной угрозой прерывания в первом триместре. Фундаментальные исследования. 2005; 5: 96-8. [Chistyakova G.N., Gazieva I.A., Remizova I.I., Cherdantseva G.A. Evaluation of cytokine production during pregnancy complicated by the threat of termination in the first trimester Basic Research. 2005; 5: 96-98. (in Russian)].
- Lee Y.H., Kim J.H., Song G.G. Meta-analyses of associations between interleukin-10 polymorphisms and susceptibility to recurrent pregnancy loss. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016; 200: 51-7. https://dx.doi.org/10.1016/j.ejogrb.2016.02.032.
- Shi X., Xie X., Jia Y., Li S. Maternal genetic polymorphisms and unexplained recurrent miscarriage: a systematic review and meta‐analysis. Clin. Genet. 2017; 91(2): 265-84. https://dx.doi.org/10.1111/cge.12910.
- Rijhsinghani A.G., Thompson K., Tygrette L., Bhatia S.K. Inhibition of interleukin-10 during pregnancy results in neonatal growth retardation. Am. J. Reprod. Immunol. 1997; 37(3): 232-5.
- Cheng S.B., Sharma S. Interleukin‐10: a pleiotropic regulator in pregnancy. Am. J. Reprod. Immunol. 2014; 73(6): 487-500. https://dx.doi.org/10.1111/aji.12329.
Received 29.01.2019
Accepted 22.02.2019
About the Authors
Malyshkina Anna Ivanovna - doctor of medical sciences, professor, director of the Federal State Institution “Ivanovo Research Institute of Maternity and Childhood named V.N. Gorodkova” the Ministry of Health of the Russian Federation, 153045, Russia, Ivanovo, Pobedy str. 20 (4932) 33-62-63, fax (8-4932) 33-62-56, E-mail: ivniimid@inbox.ru.Boyko Yelena L’vovna – doctor of medical sciences, senior researcher, Department of Obstetrics and Gynecology, Federal State Institution “Ivanovo Research Institute of Maternity and Childhood named V.N. Gorodkova” the Ministry of Health of the Russian Federation,
153045, Russia, Ivanovo, Pobedy str. 20, (4932) 93-84-48, E-mail: Dr-Boyko@mail.ru.
Sotnikova Natal’ya Yur’yevna - doctor of medical sciences, Professor, head. laboratory of clinical immunology, Federal State Institution “Ivanovo Research Institute of Maternity and Childhood named V.N. Gorodkova” the Ministry of Health of the Russian Federation, department of pathophysiology and immunology, FGBOU VO “Ivanovo State Medical Academy” of the Ministry of Health of the Russian, honored doctor of the Russian Federation,
153045, Russia, Ivanovo, Pobedy str. 20,. tel. (8-4932) 33-83-20, E-mail: niimid.immune@mail.ru.
Panova Irina Aleksandrovna – doctor of medical sciences, assistant professor, head of Department of obstetrics and gynecology, Federal State Institution “Ivanovo Research Institute of Maternity and Childhood named V.N. Gorodkova” the Ministry of Health of the Russian Federation,
153045, Russia, Ivanovo, Pobedy str. 20, (4932)35-18-62, E-mail:ia_panova@mail.ru
Fetisova Irina Nikolayevna - doctor of medical sciences, Professor of the Department of Obstetrics and Gynecology, Medical Genetics, FGBOU VO “Ivanovo State Medical Academy” of the Ministry of Health of the Russian, Leading Researcher of the Laboratory of Clinical Biochemistry and Genetics, genetic doctor, Federal State Institution “Ivanovo Research Institute of Maternity and Childhood named V.N. Gorodkova” the Ministry of Health of the Russian Federation,
153045, Russia, Ivanovo, Pobedy str. 20, 8-910-985-06-06, E-mail: ivgenlab@gmail.com.
Voronin Dmitriy Nikolayevich - candidate of biological sciences, researcher of the clinical immunology laboratory, Federal State Institution “Ivanovo Research Institute of Maternity and Childhood named V.N. Gorodkova” the Ministry of Health of the Russian Federation,
153045, Russia, Ivanovo, Pobedy str. 20,. tel. (8-4932) 33-83-20, E-mail: niimid.immune@mail.ru.
Mileyeva Polina Leonidovna – obstetrician-gynecologist obstetric physiological department of the inpatient department of the clinic Federal State Institution “Ivanovo Research Institute of Maternity and Childhood named V.N. Gorodkova” the Ministry of Health of the Russian Federation,
153045, Russia, Ivanovo, Pobedy str. 20, tel. (8-4932) 33-83-20, E-mail: pvasilevskai@gmail.com.
For citation: Malyshkina A.I., Boyko E.L., Sotnikova N.Yu., Panova I.A., Fetisovа I.N., Voronin D.N., Mileeva P.L. Interleukin-10 production and secretion in blood in relation to Interleukin-10 A-1082G polymorphism in pregnant women with fetal growth restriction. Akusherstvo i Ginekologiya/Obstetrics and Gynecology.2019; (6):40-6 (in Russian)
https://dx.doi.org/10.18565/aig.2019.6.40-46



