Systemic ozone therapy and its immunomodulatory effects in COVID-19 patients
Objective: To evaluate the effect of systemic ozone therapy (OT) on the concentration of pro-inflammatory and anti-inflammatory cytokines in the blood in the complex treatment of COVID-19 patients.Fedorova T.A., Krechetova L.V., Bakuridze E.M., Rogachevsky O.V., Zaitsev V.Ya., Strelnikova E.V., Pyregov A.V., Kozachenko I.F., Esayan R.M., Khamidulina K.G., Inviyaeva E.V., Vtorushina V.V., Chudzhaeva E.B.
Materials and methods: The study included 65 patients with a confirmed diagnosis COVID-19 characterized by a moderate and severe course of the disease. The patients were admitted to the Infectious Disease Hospital of the V.I. Kulakov National Medical Research Centre for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. The patients’ age ranged from 29 to 78 years. All patients were treated in accordance with the Temporary Guidelines of the Ministry of Health of Russia “Prevention, Diagnosis and Treatment of Coronavirus Infection (COVID-19)”. Two groups of patients were randomly formed. The first group included 35 patients whose complex therapy included OT: intravenous administration of 400 ml of ozonated saline solution with an ozone concentration of 4 mg/L; a total course consisting of 6 procedures performed every other day. The second group included 30 patients who did not have OT. Clinical and laboratory parameters were evaluated on admission to the Infectious Disease Hospital and after two weeks of complex treatment; clinical, laboratory, special, statistical research methods were used. The content of cytokines GM-CSF, IFN-γ, TNF-α, IL-2, IL-4, IL-6, IL-8, IL-10 and their ratios were determined with a multiplex method on the Bioplex 200 analyzer (Bio-Rad, USA) using Bio-Plex Pro Human Cytokine 8-plex Panel (Bio-Rad, USA).
Results: On admission to the Infectious Disease Hospital 47/65 (72,3%) patients had a moderate course of the disease and 18/65 (27.7%) patients had a severe one. The length of hospital stay in the group of patients with OT averaged 12.2 (2.7) (8–17) days, and it was 17.9 (4.2) (12–26) days in the group of patients who did not have OT. On admission, all patients had an increase in the level of C-reactive protein in their blood serum; the cytokine content in patients of the groups differed from the initial cytokine level and it was different between groups after two weeks of therapy. The medians of the pro-inflammatory cytokines IL-2, IL-6, IL-8 were particularly different. The content of these cytokines remained elevated in the second group of patients who did not have OT, compared with the baseline data and compared with the first group. The study of anti-inflammatory cytokines showed that the patients of the first group who had OT demonstrated a significantly higher IL-10 content compared to IL-10 content in the patients of the second group. Ratios of pro- and anti-inflammatory cytokines IL-2/IL-10, IL-2/ IL-4, IL-6/IL-10, IL-6/IL-4, IL-8/IL-10, IL-8/IL-4, TNF-α/IL-4 in the first group of patients with OT significantly decreased compared to the baseline indicator, which can be suggestive of a marked decrease in the activity of the inflammatory process.
Conclusion: The positive effect of systemic OT on the clinical course of the disease has been revealed. Laboratory indicators in COVID-19 patients have shown a decrease in the severity of the inflammatory response. Besides having anti-inflammatory and immunomodulatory effect, OT helps to stop the process, improve the condition of patients and reduce the length of hospital stay. Systemic OT should be considered as an additional adjuvant method in the complex treatment of patients with SARS-CoV-2 infection.
Keywords
A new coronavirus infection (COVID-19) detected at the end of 2019 spread all over the world and led to a global pandemic. SARS-CoV-2 is known to be the causative agent of the disease; when it enters the human body, it begins to interact with angiotensin-converting enzyme 2 (ACE2), a receptor on the cell surface. Further, the virus penetrates into cells and triggers an infectious process with rapid endothelial damage, the development of a severe systemic inflammatory reaction, activation of intravascular coagulation with thrombotic complications, microangiopathic hemolytic anemia, and multiple organ failure. The immune response to the virus infection involves the activation of the synthesis and release of cytokines, chemokines, including interleukin (IL)-6, IL-2, IL-4, IL-6, IL-8, IL-10, tumor necrosis factor alpha (TNF-α), interferon gamma (IFN-γ), granulocyte-macrophage colony-stimulating factor (GM-CSF), macrophage inflammatory protein 1α (MIP1α), MIP-1β and MCP-1, and many others which contribute to the activation of adhesion molecules, migration of neutrophils, eosinophils, macrophages; the so-called “cytokine storm” takes place [1–3].
In recent years, there have been a number of works on the application and high efficiency of various methods of ozone therapy (OT) in the complex treatment of a new coronavirus infection (COVID-19) [4–6]. A marked immunomodulatory and anti-inflammatory effect of medical ozone has been demonstrated; it is known to stimulate both cellular and humoral immunity due to the induction of the synthesis of immunoactivating cytokines, transforming growth factor β1 and β2 (TGF-β1 and TGF-β2) and many others [7, 8].
The aim of this study was to evaluate the content of pro- and anti-inflammatory cytokines in the blood of patients with COVID-19 in systemic OT as part of the complex therapy for infection.
Materials and methods
The study included 65 patients with a confirmed diagnosis COVID-19 characterized by a moderate and severe course of the disease. The patients were admitted to the Infectious Disease Hospital of the National Medical Research Centre for Obstetrics, Gynecology and Perinatology, Moscow, Russia, from April 23, 2020 to June 20, 2020. The patients’ age ranged from 29 to 78 years. All patients were treated in accordance with the Temporary Guidelines of the Ministry of Health of Russia “Prevention, Diagnosis and Treatment of Coronavirus Infection (COVID-19)” Variant 6 [9]. Two groups of patients were randomly formed using the MedCalc v.16.8 program group generator. On admission to the hospital the patient was referred to the group by opening an envelope marked with the patient’s serial number in the study, which contained a card indicating the group number assigned to this serial number during randomization. The first group included 35 patients whose complex therapy included OT. The second group consisted of 30 patients who received therapy according to the recommendations of the Ministry of Health but without OT. The severity of the condition of a patient suffering from COVID-19 was assessed according to the NEWS evaluation scale [10], the duration of hospital stay was assessed as well.
The complex therapy of 35 patients in the first group included systemic OT administered as an intravenous injection of 400 ml of ozonated saline solution with an ozone concentration in a solution of 4 mg/L, which was prepared immediately before injection using ozone generator “YOTA 60-01” (Medozon, Russia). The infusion rate was 20–25 ml per minute, a total course consisted of 6 procedures every other day. The criterion for inclusion of patients in the study was the consent of a patient with a confirmed diagnosis of COVID-19 to take part in the study; the exclusion criteria were an unconfirmed diagnosis of COVID-19 and pregnancy. Clinical and laboratory parameters were evaluated on admission to hospital and after two weeks of complex treatment. Blood sample was collected in the morning on an empty stomach from the peripheral vein using systems for taking venous blood S-Monovette (Sarstedt, Germany). The level of serum C-reactive protein (CRP) was measured using the turbidimetric method with an automatic biochemical analyzer VA-400 (Biosystems, Spain). The content of cytokines GM-CSF, IFN-γ, TNF-α, IL-2, IL-4, IL-6, IL-8, IL-10 and their ratios were determined with a multiplex method on the Bioplex 200 analyzer (Bio-Rad, USA) using Bio-Plex Pro Human Cytokine 8-plex Panel (Bio-Rad, USA). According to the manufacturer’s instructions, EDTA plasma samples were prepared with double centrifugation of peripheral blood samples at 1000g for 15 minutes at 4°C and 10000g for complete removal of platelets and sediments. Plasma samples were frozen and stored until the analysis was performed at a temperature of -80°C.
Statistical analysis
Statistical analysis of the data was carried out using the Microsoft Office Excel 2007 software package and the MedCalc v.16.8 program. The normality of the data distribution was determined using the Kolmogorov–Smirnov and Shapiro–Wilk tests. If the distribution of parameters was different from normal, the data were presented as median and interquartile range (Me (Q1; Q3); in the case of normal distribution of the studied parameters, the data were presented as mean (standard deviation) or M(SD). Statistical hypotheses about the absence of intergroup differences in quantitative variables in several groups were tested using the Kruskal–Wallis and Mann–Whitney U tests when compared in two groups with subsequent a posteriori analysis. The significance of changes in parameters during patients’ hospital stay was assessed using the Wilcoxon criterion. During the analysis of quantitative variables in two groups, the differences were considered significant at p<0.05; they were considered significant in three groups at p<0.017 taking into account the Bonferroni correction. During the analysis of differences in qualitative variables, the χ2 independence test was used, the differences were considered significant at p<0.05.
Results
The detailed clinical characteristics of all patients who received treatment in the Infectious Disease Hospital of the Center were presented previously [11]. The analysis of clinical parameters of patients in the study groups showed that all 65/65 (100%) patients complained of cough; 44/65 (67.9%) noted severe fatigue, sore throat, shortness of breath; 39/65 (60%) had an increase in temperature above 37.5°C; 35/65 (53.8%) had a loss of smell, 34/65 (52.3%) had intestinal disorders, 32/65 (49.2%) had tachycardia, 27/65 (41.5%) had headache, 7/65 (10.8%) had muscle pain. According to the computer tomography (CT) of the lungs, 38/65 (58.5%) patients had a moderate degree of lung tissue damage (CT2) and 27/65 (41.5%) patients had a severe degree (CT3) [12]. The moderate course of the disease was observed in 47/65 (72.3%) patients and severe one in 18/65 (27.7%). Decreased oxygen saturation <95% was noted in 38/65 (58.4%) patients. The average age was 43.6 (3.2) years ranging from 29 to 78 years. There were the following comorbid conditions that complicated the course of the disease: hypertension was noted in 18/65 (27.7%) patients, obesity and diabetes mellitus were in 12/65 (18.4%) patients, chronic bronchitis, emphysema of the lungs and bronchial asthma were in 10/65 (15.4%) patients, ischemic heart disease and myocardial infarction were in 9/65 (13.8%) patients, varicose veins in the legs were in 6/65 (9%) patients, autoimmune thyroiditis was in 5/65 (7.7%) patients. All 65 patients started to receive therapy for an infectious disease. The patients of the first group (n=35) received complex therapy including systemic OT, and on average each patient had 6.4 (1.3) procedures of OT. All patients of the first group underwent systemic OT without any complications.
The analysis of the clinical course of the disease in patients of the first group (with OT) showed that after treatment only 7/35 (20%) patients complained of fatigue, cough, shortness of breath, sore throat and the need for oxygen support in comparison with 16/30 (53.3%) patients of the second group (p=0.055). The length of hospital stay in the group of patients with OT averaged 12.2 (2.7) (8–17) days, and it was 17.9 (4.2) (12–26) days in the group of patients who did not have OT (p=0.025). The parameters of the NEWS scale were 4.05 (0.36) scores before the therapy; after treatment, the parameters of the scale were 1.35 (0.54) scores in the first group, and 2.70 (0.14) scores in the second group (p=0.01).
All 65 patients admitted to the hospital had a level of serum CRP of 48.2 (9.1; 159.2) mg/L. After a two–week therapy this parameter decreased to 7.29 (1.7; 28.16) mg/L in patients of the first group (with OT), and to 7.98 (4.21; 35.67) mg/L in the second group (without OT), which is significant when compared with baseline values (p<0.05).
The level of serum cytokines in patients of both groups is presented in Table 1.
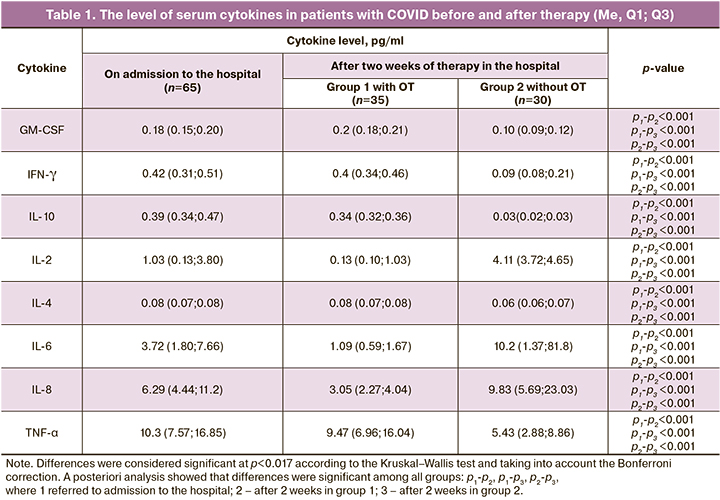
According to the results presented in Table 1, the level of serum cytokines measured two weeks after treatment in patients of both groups differed from the baseline level and between each other. The medians of the pro-inflammatory cytokines IL-2, IL-6, IL-8 were particularly different. The level of these cytokines remained elevated in the second group of patients who did not have OT, compared with the baseline data and compared with the first group. Thus, the level of serum IL-2 was initially 1.03 pg/ml, (0.13;3.80); it decreased to 0.13 pg/ml (0.13;1.03) in the first group after OT (p<0.001), and it became even higher in the second group and amounted to 4.11 pg/ml (3.72;4.65) (р<0.001). Similar trends were noted in the IL-6 level, which was 3.72 (1.80;7.66) pg/ml on admission; it decreased to 1.09 (0.59;1.67) pg/ml (p<0.001) in patients of the first group after OT, and the IL-6 level became higher in the second group and it was 10.2 (1.37;81.8) pg/ml (р<0.001). The level of cytokine IL-8 was initially 6.29 (4.44;11.2) pg/ml, then it significantly decreased in the first group of patients to 3.05 (2.27;4.04) pg/ml (p<0.001), and, on the contrary, the level of IL-8 became even higher in the second group of patients after treatment and amounted to 9.83 (5.69;23.03) pg/ml (p<0.001), which may be indicative of the persistence of an active systemic inflammatory response in the second group of patients.
The study of anti-inflammatory cytokines in groups of patients revealed particularly significant changes in the IL-10 parameters. Thus, the level of IL-10 was significantly higher in the first group of patients who had OT compared to the values in the second group (without OT): 0.34 (0.32;0.36) pg/ml and 0.03 (0.02;0.03) pg/ml, respectively, (p<0.001).
The ratio of pro- and anti-inflammatory cytokines in patients with COVID infection was analyzed. The results of the analysis are presented in Table 2.
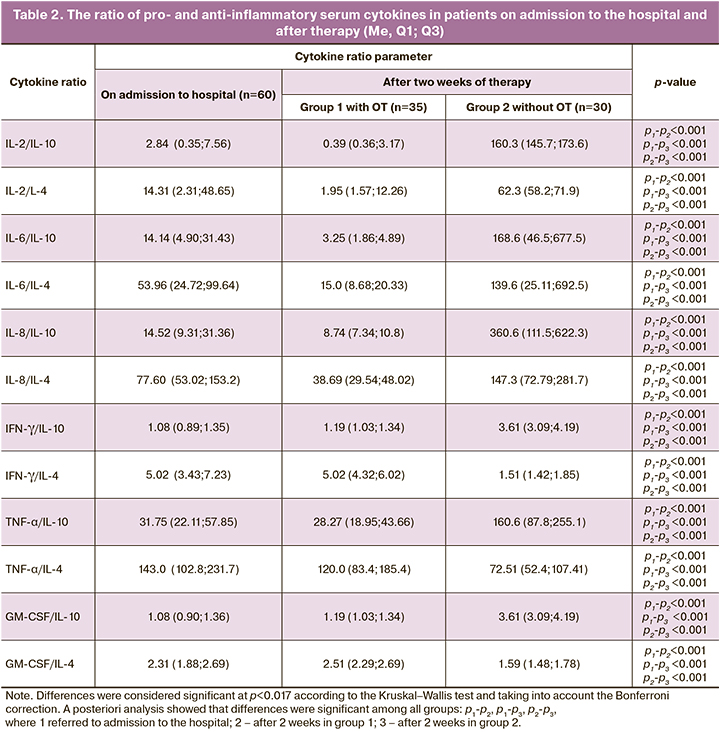
The analysis of the ratio of pro- and anti-inflammatory cytokines showed that the ratio IL-2/IL-10, IL-2/IL-4, IL-6/IL-10, IL-6/IL-4, IL-8/IL-10, IL-8/IL-4 significantly decreased in the first group of patients with OT compared to the baseline indicator, which can be suggestive of a marked decrease in the activity of the inflammatory process. On the contrary, the ratio of these cytokines increased several times in the second group of patients who did not have OT. Thus, the ratio of IL-2/IL-10 increased from 2.84 (0.35; 7.56) to 160.3 (145.7;173.6) (p<0.001) in the second group after treatment, and on the contrary, it decreased to 0.39 (0.36;3.17) (p<0.001) in the first group.
As for the ratio of other cytokines, for example, TNF-α/IL-4, it significantly decreased in both groups: from 143.0 (102.8;231.7) to 120.0 (83.4;185.4), (p<0.001) in the first group, and from 143.0 (102.8;231.7) to 72.51 (52.4;107.41), (p<0.001) in the second group. The ratio of GM-CSF/IL-4 increased from 2.31 (1.88;2.69) to 2.51 (2.29;2.69) (p<0.001) in the first group, and decreased to 1.59 (1.48;1.78) (p<0.001) in the second group. Such trends reflect complex correlations in the immunological response during the inflammatory process due to the activation of cells, both in innate and cellular immunity.
We also conducted a paired analysis of the serum cytokine levels before and after therapy in the same patients of the first group. The results are presented in Tables 3 and 4, respectively.
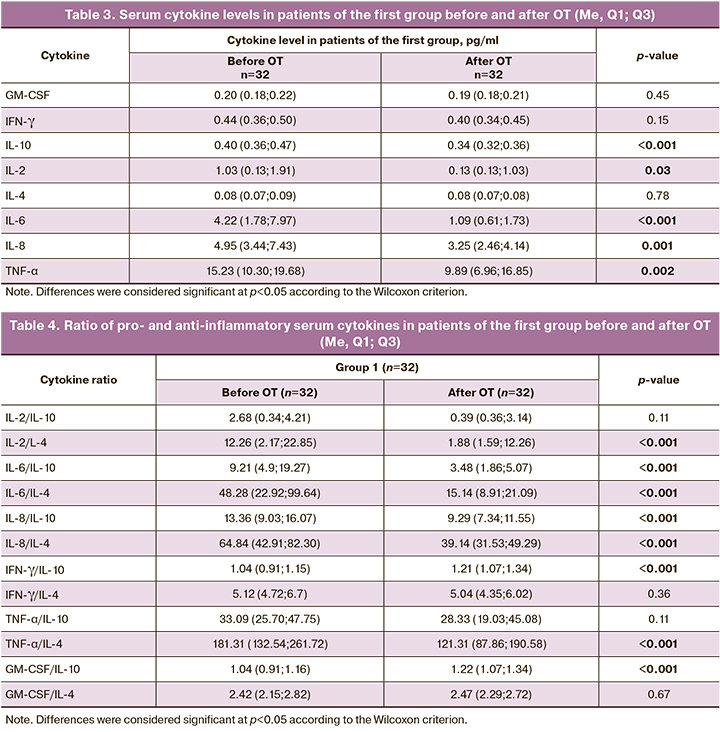
The analysis of the results presented in Table 3 showed that a significant decrease in IL-2, IL-6, IL-8, IL-10, and TNF-α was detected in patients with COVID-19 after two weeks of complex treatment with systemic OT.
The analysis of the parameters presented in Table 4 revealed a significant decrease in the ratios of pro- and anti-inflammatory cytokines, such as IL-2/IL-4, IL-6/IL-10, IL-6/IL-4, IL-8/IL-10, IL-8/IL-4 and TNF-α/IL-4. And the ratio of IFN-γ/IL-10 and GM-CSF/IL-10 increases slightly (p<0.05).
The results of the study on the serum cytokine concentration in 15 patients of the second group (without OT) are presented in Table 5.
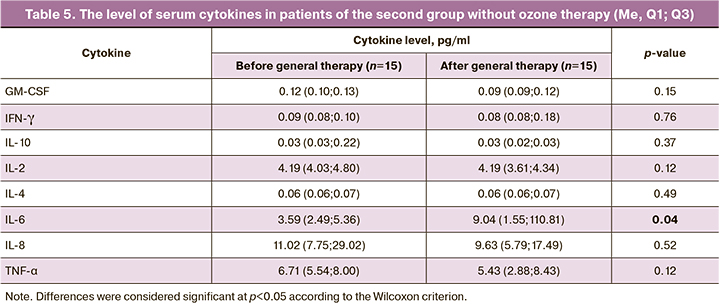
The results presented in Table 5 do not indicate any changes in the concentration of cytokines after standard treatment without OT, except for IL-6, the concentration of which increased significantly after two weeks of therapy: 3.59 (2.49;5.36) pg/ml before treatment and 9.04 (1.55;110.81) pg/ml after standard treatment (p=0.0413, p<0.05), which is reflective of the persistent inflammatory process.
The analysis of the ratios of serum pro- and anti-inflammatory cytokines (IL-2/IL-4, IL-6/IL-10, IL-6/IL-4, IL-8/IL-10, IL-8/IL-4, IFN-γ/IL-10 and GM-CSF/IL-4) before and after standard therapy in patients of the second group did not reveal any significant changes, except for the TNF-α/IL-4 ratio, which significantly decreased after treatment from 97.05 (79.40;133.61) to 72.06 (49.45;106.93) (p=0.02).
Discussion
SARS-CoV-2 which has spread rapidly around the world is a highly contagious respiratory virus that affects not only the lungs, but it may also cause systemic diseases. The coronavirus spike protein binds the ACE2 receptor, which is present in many tissues, for example, in the epithelium of the small intestine, in arterial and venous endothelial cells, smooth muscle cells of the arteries of the stomach, small intestine, colon, skin, lymph nodes, thymus, bone marrow, spleen, liver, kidneys and brain [13–15]. Numerous studies in recent years have shown that the severe course of COVID-19 is characterized by excessive inflammation, as well as a marked increase in the level of CRP, pro-inflammatory cytokines and chemokines in the blood serum, which has been called a “cytokine storm”. The production of pro-inflammatory cytokines IL-1ß, IL-2, IL-4, IL-6, IL-8 and TNF-α causes various effects including the release of other inflammatory and chemotactic factors that activate adhesion molecules and increase the migration of eosinophils and neutrophils, thereby activating the innate immune response [16]. In case of a sharp increase in the level of IL-6 and impaired regulation of some cytokines, for example, such as IFN-γ, GM-CSF and others, there is no balance between pro-inflammatory and anti-inflammatory cytokines [17]. The elevated level of systemic IL-6 in patients with COVID-19 is considered to be a significant parameter for predicting the most severe course of the disease and the need for intensive therapy [18]. The use of systemic OT administered as intravenous infusions of ozonated saline solution was proposed as a part of the complex therapy of patients with COVID-19. According to the experts of the Russian Association of Ozone Therapy and doctors from other countries, it is recommended to use medical ozone as a safe, inexpensive and effective method of therapy for the prevention and treatment of coronavirus infection [19–21].
During the examination of 65 patients with COVID-19 infection with a moderate and severe course of the disease, we studied the concentrations of a number of cytokines and chemokines in the dynamics of treatment. Differences in the dynamics of a number of serum pro- and anti-inflammatory cytokines in both groups have been demonstrated. The level of IFN-γ in the blood plasma in the group of patients who had OT remained at the same level as it was before treatment, and it may be indicative of the obvious need for further rehabilitation of patients with a coronavirus infection.
GM-CSF plays an important role in the process of inflammation, since this growth factor promotes the activation and prolonged survival of monocytes, macrophages and neutrophils, increases the pool of proinflammatory cytokines secreted by these cells, and promotes phagocytosis and the release/clearance of damaged tissues from infectious agents. In this regard, the absence of changes in the concentration of GM-CSF in the blood of patients after two weeks of complex therapy including OT should be considered as a factor preventing the inflammatory process [22–24].
The results of our study demonstrated the effect of OT on the concentration of the pro-inflammatory and anti-inflammatory cytokines in the dynamics of therapy. The mechanisms of OT effectiveness in coronavirus infection are diverse, including adaptation to oxidative stress and induction of pro- and anti-inflammatory cytokines. One of the OT effects is the formation of alkenals due to the oxidation of fatty acids in the blood, in particular, omega-6 and omega-3, carried by albumin. They act as signal transducers activating the cytoplasmic protein Nrf2 and consequently transcription of several antioxidant genes under the ARE (antioxidant-responsive element) promoter [25]. These gene products inhibit the cascade of inflammatory cytokines and the NLRP3 inflammasome, they also cause the synthesis of anti-inflammatory cytokines, such as IL-10 [26–28]. In addition, ozone and its derivatives act on viruses through capsid oxidation; they are more effective in viruses with a lipid coating than in viruses with a protein capsid, so the viral load can be dramatically reduced. Additional effects of OT are a decrease in the aggregation capacity of erythrocytes, decrease in viscosity and improvement of blood rheology in the macro- and microvascular systems [29].
OT as a part of the complex therapy in patients with COVID-19 produced anti-inflammatory and immunomodulatory effects that contributed to the relief of the inflammatory process, improvement of the patients’ condition and reduced length of hospital stay. Therefore, systemic OT can be recommended for inclusion in the comprehensive treatment of patients with moderate to severe infection caused by SARS-CoV-2 [30, 31].
Conclusion
The results of the analysis of the serum cytokine content in patients infected with SARS-CoV-2 indicate a decrease in the activity of the so-called “cytokine storm”, which is also observed in infections caused by other SARS viruses. The obtained data demonstrate different dynamics of the cytokines in groups of patients receiving standard therapy and complex therapy with ozone. The patients who received complex therapy demonstrated a decrease in the concentration of pro-inflammatory cytokines, improvement in clinical parameters and a decrease in parameters of NEWS scale of severity assessment, as well as reduced length of hospital stay in comparison with the patients who received standard therapy.
References
- Hu B., Guo H., Zhou P., Shi Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021; 19(3): 141-54. https://dx.doi.org/10.1038/s41579-020-00459-7.
- Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020; 368(6490): 473-4. https://dx.doi.org/10.1126/science.abb8925.
- Conti P., Ronconi G., Caraffa A., Gallenga C.E., Ross R., Frydas I. et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents. 2020; 34(2): 327-31. https://dx.doi.org/10.23812/CONTI-E.
- Martínez-Sánchez G., Schwartz A., Donna V.D. Potential cytoprotective activity of ozone therapy in SARS-CoV-2/COVID-19. Antioxidants (Basel). 2020; 9(5): 389. https://dx.doi.org/10.3390/antiox9050389.
- Percivalle E., Clerici M., Cassaniti I., Vecchio Nepita E., Marchese P., Olivati D. et al. SARS-CoV-2 viability on different surfaces after gaseous ozone treatment: a preliminary evaluation. J. Hosp. Infect. 2021; 110: 33-6.https://dx.doi.org/10.1016/j. jhin.2021.01.014.
- Yano H., Nakano R., Suzuki Y., Nakano A., Kasahara K., Hosoi H. Inactivation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by gaseous ozone treatment. J. Hosp. Infect. 2020; 106(4): 837-8.https://dx.doi.org/10.1016/j.jhin.2020.10.004.
- Cenci A., Macchia I., La Sorsa V., Sbarigia C., Di Donna V., Pietraforte D. Mechanisms of action of ozone therapy in emerging viral diseases: immunomodulatory effects and therapeutic advantages with reference to SARS-CoV-2. Front. Microbiol. 2022; 13: 871645. https://dx.doi.org/10.3389/fmicb.2022.871645.
- Chirumbolo S., Valdenassi L., Simonetti V., Bertossi D., Ricevuti G., Franzini M. et al. Insights on the mechanisms of action of ozone in the medical therapy against COVID-19. Int. Immunopharmacol. 2021; 96: 107777. https://dx.doi.org/10.1016/j.intimp.2021.107777.
- Министерство здравоохранения Российской Федерации. Временные методические рекомендации. Профилактика, диагностика и лечение новой коронавирусной инфекции (covid-19). Версия 6 (28.04.2020). [Ministry of Health of the Russian Federation. Temporary guidelines. Prevention, diagnosis and treatment of new coronavirus infection (covid-19). Version 6 (28/04/2020). (in Russian)].
- Терапевтический профиль. Протокол оценки тяжести состояния пациента (NEWS). Доступно по: https://euat.ru/upload/images/ 1586077839.pdf?ysclid=l70h8s2eb9327079697 [Therapeutic profile. Protocol for assessing the severity of the patient's condition (NEWS). (in Russian)]. Available at: https://euat.ru/upload/images/ 1586077839.pdf?ysclid=l70h8s2eb9327079697
- Федорова Т.А., Бакуридзе Э.М., Пырегов А.В., Гаврилова Т.Ю., Козаченко И.Ф., Есаян Р.М., Хамидулина К.Г., Хачатрян Н.А., Иванец Т.Ю., Кречетова Л.В., Инвияева Е.В., Вторушина В.В., Безнощенко О.С., Нечипуренко Д.Ю., Береснева Е.А. Возможности применения системной озонотерапии у пациентов с СOVID-19 инфекцией. Биорадикалы и антиоксиданты. 2021; 8(2): 8-24. [Fedorova T.A. Bakuridze E.M., Pyregov A.V., Gavrilova T.Yu., Kozachenko I.F., Yesayan R.M., Khamidulina K.G. et al. Possibilities of using systemic ozone therapy in patients with COVID-19 infection. Bioradicals and Antioxidants. 2021; 8(2): 8-24. (in Russian)].
- Классификация степени тяжести вирусной/СОV-19 пневмонии по КТ. Доступно по: http://www.secondopinions.ru Дата обращения: 27.05.2020. [Classification of the severity of viral/COV-19 pneumonia by CT. URL:http://www.secondopinions.ru (Accessed: 05/27/20) (in Russian)].
- Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004; 203(2): 631-7. https://dx.doi.org/10.1002/path.1570.
- Hernández A., Papadakos P.J., Torres A., González D.A., Vives M., Ferrando C. et al. Two known therapies could be useful as adjuvant therapy in critical patients infected by COVID-19. Rev. Esp. Anestesiol. Reanim. (Engl. Ed.). 2020; 67(5): 245-52. https://dx.doi.org/10.1016/j.redar.2020.03.004.
- The ZOE Health Study. How long does COVID-19 last? UK: London; 2020. Available at: https://covid19.joinzoe.com/post/covid-long-term?fbclid=IwAR1RxIcmmdL-EFjh_aI-. Accessed 15.03.2021.
- Wang W., Ye L., Ye L., Li B., Gao B., Zeng Y. et al. Up-regulation of IL-6 and TNF-α induced by SARS-coronavirus spike protein in murine macrophages via NF-κappaB pathway. Virus Res. 2007; 128(1-2): 1-8. https://dx.doi.org/10.1016/j. virusres.2007.02.007.
- Olbei M., Hautefort I., Modos D., Treveil A., Poletti M., Gul L. et al. SARS-CoV-2 causes a different cytokine response compared to other cytokine storm-causing respiratory viruses in severely ill patients. Front. Immunol. 2021; 12: 629193. https://dx.doi.org/10.3389/fimmu.2021.629193.
- Gubernatorova E.O., Gorshkova E.A., Polinova A.I., Drutskaya M.S. IL-6: relevance for immunopathology of SARS-CoV-2. Cytokine Growth Factor Rev. 2020; 53: 13-24. https://dx.doi.org/10.1016/j.cytogfr.2020.05.009.
- Озон против COVID-19 – Ассоциация Российских Озонотерапевтов. Потенциальное использование озона при инфекции SARS-CoV-2/COVID-19. 2020. Доступно по: www.ozonetherapy.ru [Ozone vs COVID-19 – Association of Russian Ozone Therapists. Potential use of ozone in SARS-CoV-2/COVID-19 infection. www.ozonetherapy.ru. 2021.(in Russian)].
- Rowen R., Robins H. A plausible “penny” costing effective treatment for corona virus - ozone therapy. J. Infect. Dis. Epidemiol. 2020; 6(2): 113.https://dx.doi.org/10.23937/2474-3658/1510113.
- Franzini M., Valdenassi L., Ricevuti G., Chirumbolo S., Depfenhart M., Bertossi D., Tirelli U. Oxygen-ozone (O2-O3) immunoceutical therapy for patients with COVID-19. Preliminary evidence reported. Int. Immunopharmacol. 2020; 88: 106879. https://dx.doi.org/10.1016/j.intimp.2020.106879.
- Hamilton J.A., Whitty G.A., Stanton H., Meager A. Effects of macrophage-colony stimulating factor on human monocytes: induction of expression of urokinase-type plasminogen activator, but not of secreted prostaglandin E2, interleukin-6, interleukin-1, or tumour necrosis factor-alpha. J. Leukoc. Biol. 1993; 53(6): 707-14. https://dx.doi.org/10.1002/jlb.53.6.707.
- Takahashi G.W., Andrews D.F. 3rd, Lilly M.B., Singer J.W., Alderson M.R. Effect of granulocyte-macrophage colony-stimulating factor and interleukin-3 on interleukin-8 production by human neutrophils and monocytes. Blood. 1993; 81(2): 357-64.
- Martinez-Sanchez G. Mechanisms of action of O3. Genomic pathways. Ozone Ther. Glob. J. 2019; 9(1): 21-2.
- Martinez-Sanchez G., Delgado-Roche L. Up-date on the mechanisms of action of ozone through the modification of cellular signaling pathways. Role of Nrf2 and NFkb. Rev. Esp. Ozonoterapia. 2017; 7: 17-8.
- Li W., Khor T.O., Xu C., Shen G., Jeong W.S., Yu S. et al. Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochem. Pharmacol. 2008; 76(11): 1485-9.https://dx.doi.org/10.1016/j.bcp.2008.07.017.
- Li Q., Verma I.M. NF-κappaB regulation in the immune system. Nat. Rev. Immunol. 2002; 2(10): 725-34. https://dx.doi.org/10.1038/nri910.
- Araimo F., Imperiale C., Tordiglione P., Ceccarelli G., Borrazzo C., Alessandri F.et al. Ozone as adjuvant support in the treatment of COVID-19: a preliminary report of probiozovid trial. J. Med. Virol. 2021; 93(4): 2210-20.https://dx.doi.org/10.1002/jmv.26636.
- Хаммад Е.В., Никитин И.Г., Федорова К.В. Применение озонотерапии у пациентов с новой коронавирусной инфекцией COVID-19. Вестник восстановительной медицины. 2020; 5(99): 94-100. [Hammad E.V., Nikitin I.G., Fedorova K.V. Ozone Therapy in Patients with the New Coronavirus Infection COVID-19. Bulletin of Restorative Medicine. 2020; 5(99): 94-100. (in Russian)]. https://dx.doi.org/10.38025/2078-1962-2020-99-5-94-100.
Received 15.06.2022
Accepted 26.07.2022
About the Authors
Tatiana A. Fedorova, Dr. Med. Sci., Professor, Head of Transfusional Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics,Gynecology and Perinatology, Ministry of Healthcare of Russian Federation, +7(495)438-71-35, t_fyodorova@oparina4.ru, https://orcid.org/0000-0001-6714-6344,
117997, Russia, Moscow, Ас. Oparinа str., 4.
Lyubov V. Krechetova, Dr. Med. Sci., Head of the Laboratory of Clinical Immunology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of Russian Federation, +7(495)438-11-83, l_krechetova@oparina4.ru, https://orcid.org/0000-0001-5023-3476,
117997, Russia, Moscow, Ас. Oparinа str., 4.
Eteri M. Bakuridze, PhD, Head of the Clinic at the Department of Transfusion and Extracorporeal Hemocorrection, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of Russian Federation, +7(495)438-71-35, e_bakuridze@oparina4.ru,
117997, Russia, Moscow, Ас. Oparinа str., 4.
Oleg V. Rogachevsky, Dr. Med. Sci., Head of the Department of Extracorporal Methods of Treatment and Detoxification, Professor at the Department of Anesthesiology and Resuscitation, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of Russian Federation, +7(495)438-71-35, о_rogachevskiy@oparina4.ru, https://orcid.org/0000-0002-4332-430X, 117997, Russia, Moscow, Ас. Oparinа str., 4.
Vladimir Ya. Zaitsev, PhD (Chemistry), Director, "Medozon", +7(495)420-56-30, +7(495)663-73-50, zaitsev@medozone.ru,
117647, Russia, Moscow, Profsoyuznaya str., 113, bldg. 3, apt. 425.
Elena V. Strelnikova, PhD, doctor at the Department of Extracorporal Methods of Treatment and Detoxification, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of Russian Federation, +7(495)438-71-35, e_strelnikova@oparina4.ru,
https://orcid.org/0000-0002-6926-8414, 117997, Russia, Moscow, Ас. Oparinа str., 4.
Aleksey V. Pyregov, Dr. Med. Sci., Professor, Director of the Institute of Anesthesiology and Resuscitation, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of Russian Federation, +7(495)438-77-77, a_pyregov@oparina4.ru, https://orcid.org/0000-0001-8382-9671, 117997, Russia, Moscow, Ас. Oparinа str., 4.
Irena F. Kozachenko, PhD, Leading Researcher at the Gynecological Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of Russian Federation, +7(495)438-77-83, i_kozachenko@oparina4.ru, https://orcid.org/0000-0003-1822-9164,
117997, Russia, Moscow, Ас. Oparinа str., 4.
Rosa M. Esayan, PhD, endocrinologist, Head of the Therapeutic Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of Russian Federation, +7(495)438-26-33, r_esayan@oparina4.ru, https://orcid.org/ 0000-0002-6808-113X,
117997, Russia, Moscow, Ас. Oparinа str., 4.
Ksenia G. Khamidulina, PhD, Researcher at the Department of Extracorporeal Methods of Treatment and Detoxification, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of Russian Federation, +7(495)438-71-35, k_bykova@oparina4.ru,
https://orcid.org/ 0000-0001-5394-1910, 117997, Russia, Moscow, Ас. Oparinа str., 4.
Elena V. Inviyaeva, PhD, Senior Researcher at the Laboratory of Clinical Immunology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of Russian Federation, +7(495)438-11-83, e_inviyaeva@oparina4.ru, 117997, Russia, Moscow, Ас. Oparinа str., 4.
Valentina V. Vtorushina, PhD, clinical laboratory diagnostics doctor, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of Russian Federation, +7(495)438-11-83, v_vtorushina@oparina4.ru,
https://orcid.org/0000-0002-8406-3206, 117997, Russia, Moscow, Ас. Oparinа str., 4.
Elzyata B. Chudzhaeva, the nurse at the Therapeutic Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of Russian Federation; student; I.M. Sechenov First Moscow State Medical University, Ministry of Healthcare of Russian Federation
(Sechenov University), +7(495)438-26-33, chudzhaeva@mail.ru, 117997, Russia, Moscow, Ас. Oparinа str., 4.
Authors’ contributions: Fedorova T.A., Krechetova L.V., Rogachevsky O.V., Zaitsev V.Ya. – developing the concept and design of the study; Bakuridze E.M., Strelnikova E.V., Pyregov A.V., Kozachenko I.F., Esayan R.M. – collecting and processing the material; Inviyaeva E.V., Vtorushina V.V., Chudzhaeva E.B. – statistical processing; Khamidulina K.G., Inviyaeva E.V. – writing the text; Fedorova T.A., Krechetova L.V. – editing the text.
Conflicts of interest: The authors declare that they have no competing interests.
Funding: The study was sponsored by the National Association of Specialists in Patient Blood Management (Russia, Moscow) and LLC Firm Medozon (Russia, Moscow).
Ethical Approval: The study was approved by the Ethical Review Board of Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Fedorova T.A., Krechetova L.V., Bakuridze E.M.,
Rogachevsky O.V., Zaitsev V.Ya., Strelnikova E.V., Pyregov A.V., Kozachenko I.F., Esayan R.M., Khamidulina K.G., Inviyaeva E.V., Vtorushina V.V., Chudzhaeva E.B. Systemic ozone therapy and its immunomodulatory effects in COVID-19 patients.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 8: 85-94 (in Russian)
https://dx.doi.org/10.18565/aig.2022.8.85-94



