Clinical and immunological characteristics of women in labor with COVID-19 and their newborn babies
Inviyaeva E.V., Tysyachnyi O.V., Kosolapova Yu.A., Vtorushina V.V., Baev O.R., Zubkov V.V., Krechetova L.V.
Objective: To characterize the course of pregnancy, immune status of women in labor with SARS-CoV-2, as well as health and immune status of their newborn babies.
Materials and methods: The study included 72 pregnant women. The main group (n=27) consisted of women in labor who tested positive for SARS-CoV-2 with a mild course of the disease during childbirth. The comparison group (n=45) comprised of women in labor without coronavirus infection during pregnancy and childbirth. Expression of T-cell markers CD3, CD3/CD4, CD3/CD8, CD19, CD3/CD56, CD16, CD19/CD5; as well as regulatory T (Treg) cells with the CD4+CD25+CD127low/- phenotype was analyzed by flow cytometry .
Results: Predominant diseases in women infected with SARS-CoV-2 during childbirth were gastrointestinal diseases, external genital endometriosis and ovarian cysts. Spontaneous pregnancy occurred in these women. The method of delivery was determined by obstetric factors. Timing and frequency of cesarean delivery were higher in the main group. Reduction in lymphocyte subpopulations of T cells and B cells, but not NK cells and NKT cells, was in women in the main group.
In the main group, five babies were born at 246 (1.1) days [35 weeks 1 day] (p=0.006). In the comparison group, all babies were born at full term. Anthropometric parameters of newborns were comparable between the groups.
In babies born to mothers in the main group, the lower number of leukocytes, absolute content of lymphocytes, neutrophils and their phagocytic activity were found, while the values of these indicators in newborns in both groups remained within the reference values.
Conclusion: No differences were found in the course of pregnancy in puerperant women with SARS-CoV-2, and not differences were found in their babies’ anthropometric indicators and Apgar scores. The changes identified in immune status of mothers and their newborn babies were within the reference range, and cannot be associated with SARS-CoV-2 infection in women in labor.
Authors' contributions: Krechetova L.V., Zubkov V.V. – the concept and design of the study; Inviyaeva E.V., Vtorushina V.V., Kosolapova Yu.A., Tysyachnyi O.V. – material collection and processing; Inviyaeva E.V., Krechetova L.V. – statistical data processing; Krechetova L.V., Inviyaeva E.V. – writing the text of the article; Baev O.R., Krechetova L.V. – text editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The study was conducted without any sponsorship.
Ethical Approval: The study was approved by the Local Ethics Committee of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
Patient Consent for Publication: The patients have signed informed consent for publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Inviyaeva E.V., Tysyachnyi O.V., Kosolapova Yu.A., Vtorushina V.V., Baev O.R., Zubkov V.V., Krechetova L.V. Clinical and immunological characteristics of women in labor with COVID-19 and their newborn babies.
Akusherstvo i Gynecologia/Obstetrics and Gynecology. 2024; (3): 63-72 (in Russian)
https://dx.doi.org/10.18565/aig.2024.42
Keywords
Coronavirus disease (COVID-19) is an infectious respiratory disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which was first identified in December 2019 Wuhan (China). On March 2020, the World Health Organization (WHO) has declared the coronavirus outbreak a global pandemic, as the virus infected a large number of people around the world. Currently, more than 4 million deaths have been reported worldwide [1]. The emergence of SARS-CoV-2 as a novel, rapidly spreading infection has posed a challenge to the healthcare system and medical practitioners to determine the impact of coronavirus infection on pregnancy and, accordingly, to assess the risk of complex adverse obstetric and neonatal outcomes that would require to review management of pregnant women infected with SARS-CoV-2. Physiological changes of the immune system during pregnancy, such as decreased cell-mediated cytotoxicity, cytokine response and lymphocyte proliferative response in case of SARS-CoV-2 infection in pregnant women, especially in the first trimester or in labor may complicate the cause of pregnancy and, accordingly, affect perinatal outcomes in women [2].
Over the years, many studies investigated the impact of several recent epidemics, such as influenza A (H1N1), SARS-CoV, Middle East respiratory syndrome (MERS), and respiratory syncytial virus (RSV) on maternal and neonatal outcomes. It is well known that infectious pneumonia is a common cause of morbidity and mortality among pregnant women due to a number of physiological factors, such as smaller lung volume and increased oxygen consumption [2, 3]. Indeed, it is estimated that a quarter of pregnancies complicated by pneumonia, require admission to intensive care unit and mechanical ventilation [4]. Therefore, the emergence of the SARS-CoV-2 pandemic has raised the issues of perinatal and obstetric management of patients.
Currently, the existing data on the impact of SARS-CoV-2 on obstetric and neonatal outcomes, as well as on the likelihood of intrauterine transmission, are conflicting [3, 5–9]. During the pandemic, SARS-CoV-2 repeatedly mutated and caused new outbreaks with a diverse range of clinical courses of infection, from asymptomatic to severe with fatal outcome. Continued circulation of the virus in environment, lack of methods that can guarantee protection of humanity from the influence of emerging new strains with unpredictable epidemiology, and gaps in knowledge about the impact of COVID-19 during pregnancy and in labor on obstetric and neonatal outcomes determined the relevance of this study.
The objective of the study was to characterize the course of pregnancy, immune status of women in labor with SARS-CoV-2, as well as health and immune status of their newborn infants.
Materials and methods
The study included 72 pregnant women, who were divided into 2 groups. The main group (n=27) consisted of pregnant women with the signs of acute infectious and inflammatory disease, who were admitted for delivery to the temporarily formed COVID hospital at the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia in April–July, 2020. Infection with SARS-CoV-2 was confirmed at the time of hospitalization by detection of SARS-CoV-2 RNA in oro- and nasopharyngeal swabs using reverse transcription-polymerase chain reaction (RT-PCR). All women enrolled in the study had a mild course of the disease. Mild symptoms of COVID-19 were: temperature <38°C, cough, fatigue, sore throat and absence of the symptoms of severe and moderate to severe course of the disease [10].
Elimination of the virus was defined, when there were two negative PCR tests within 24 hours. The diagnosis, management and treatment of pregnant women with COVID-19 was performed in accordance with temporary guidelines “Organization of medical care for women with novel coronavirus infection COVID-19 during pregnancy, childbirth and the postpartum period”, version 1.
The comparison group (n=45) included the patients without coronavirus infection, who gave birth at the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia in the period from November, 2019 to July, 2020. Absence of SARS-CoV-2 infection at the time of hospitalization during the COVID-19 pandemic was confirmed by RT-PCR tests using oropharyngeal and nasopharyngeal specimens. At that time, vaccination against SARS-CoV-2 was not carried out in Russia. SARS-CoV-2 antibodies were not found in patients, who underwent testing.
Inclusion criteria in the main group: the signed informed voluntary consent to participate in the study, laboratory confirmation of coronavirus infection during childbirth.
Inclusion criteria in the comparison group: absence of COVID-19 during childbirth, the signed informed voluntary consent to participate in the study.
Exclusion criteria: the patients, who were wanted to discontinue participation in the study.
Non-inclusion criteria: multiple pregnancy, positive results of testing for the presence of HIV-1/HIV-2 antigen and antibody, hepatitis B antigen, antibodies to hepatitis C, severe somatic, autoimmune and oncological diseases in pregnant women, organ transplantation in medical history.
Peripheral venous blood samples were taken from the patients to identify subpopulation composition of immune cells at the time of admission to the delivery unit. Evaluation of total leukocytes and lymphocytes count in peripheral blood was performed using System XS 800i hematology analyzer.
Phenotyping of peripheral blood lymphocytes was done using flow cytometry with labeled FITC, PE, and APC, monoclonal antibodies (MAbs) produced by Becton Dickinson and eBioscience (USA). The lymphocyte gate was set to exclude other blood cells from the analysis using mAbs to CD45 (Dako, Denmark). Expression of the following markers was assessed: CD3, CD3/CD4, CD3/CD8, CD19, CD3/CD56, CD16, CD19/CD5; CD4+CD25+CD127low/- were used as phenotype markers to identify regulatory T (TReg) cells with Navios Flow Cytometer and Kaluza Analysis Software (Beckman Coulter, Inc., USA).
Statistical analysis
Statistical analysis of obtained data was conducted using the tables in Microsoft Excel and MedCalc, version 16.8. Distribution of the quantitative data in the comparison group was tested using the Shapiro–Wilk W-statistic. The data were presented as the median (Me) between the upper quartile and the lower quartile (Q1; Q3). The differences between the groups were evaluated using the Mann–Whitney U test. The differences were considered to be statistically significant at p<0.05. The qualitative variables were represented as absolute and relative values (abs., %). Fisher’s exact test was used to assess the differences between the qualitative variables. The differences were considered to be statistically significant at p<0.05. The study was approved by the Ethics Commission of the Academician V.I. Kulakov National Medical Research Center, Ministry of Health of Russia (Protocol No. 4 of April 23, 2020).
Results
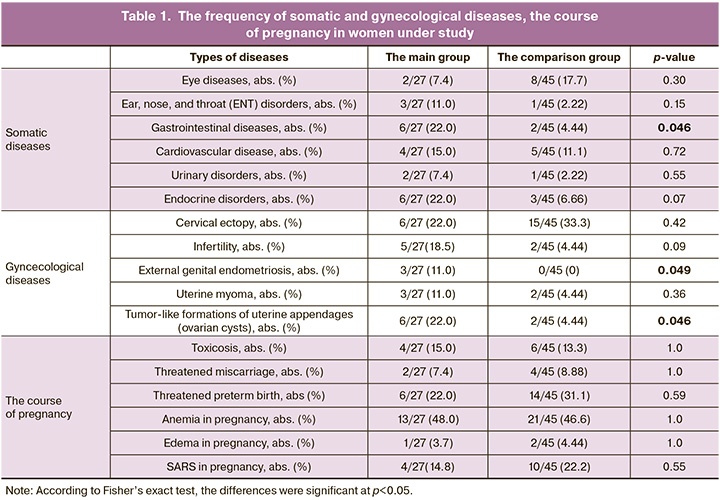
The mean age of women in the main group was 30.7 (4.8) years, and 31.3 (4.3) years in the comparison group (р>0.05). At the time of childbirth, pregnancy length was 268 (1.75) days [38 weeks 2 days] in the main group, and 280 (1.0) days [40 weeks] in the comparison group, respectively (р<0.0001). Assessment of somatic diseases in both groups showed prevalence of gastrointestinal diseases, and assessment of gynecological diseases showed prevalence of external genital endometriosis and ovarian cysts in women in the main group. The data are represented in Table 1.
Assessment of the number of primiparous and multiparous women – 44% (12/27)/56% (15/27) in the main group and 58% (26/45)/42% (19/45) in the comparison group did not show statistically significant difference (р>0.05).
Spontaneous pregnancies were in most women – in 23/27 (85.2%) women in the main group, and in 41/45 (91.1%) women in the comparison group (р>0.05); 4/27 (14.8%) women in the main group and 4/45 (8.9%) women in the comparison group became pregnant using assisted reproductive technologies (р>0.05).
There was difference in the timing and frequency of cesarean delivery between pregnant women in the main group and in the comparison group.
The reasons for preterm births in 5 pregnant women in the main group were placenta previa and placenta accreta, myometrium thinning at the scar area after three cesarean sections; isthmic-cervical insufficiency, threatening preterm birth; non-immune hydrops fetalis; impaired uteroplacental blood flow, stage 1, one of them was in each case; and in one case there were multiple fetal abnormalities (Chiari malformation, spina bifida, hydrocephalus, aortic arch and isthmus hypoplasia).
Spontaneous vaginal delivery was in 52% (14/27) of women with COVID-19 and in 82% (37/45) of women in the comparison group (р=0.008). Cesarean delivery rate was 48% (13/27) in the main group, and 18% (8/45) in the comparison group (р=0.008).
Indications for cesarean section were: clinically narrow pelvis in the main group (n=13) – 1/13, in the comparison group (n=8) – 2/8 (р=0.53); uterine scar after cesarean section in the main group – 4/13 women, 0/8 in the comparison group (р=0.13), obstetrical complications in medical history in the main group – 3/13, in the comparison group – 2/8 (p=0.55), fetal pathology in the comparison group – 3/13, in the comparison group – 4/8 (p=0.35), the total number of relative indications – 2/13 in the main group, and 0/8 in the comparison group (р=0.51).
All patients participating in the study did not require hospitalization to the department of resuscitation and intensive care. Before delivery, the analysis of the composition of lymphocyte subpopulations was performed in all women. The results are represented in Table 2.
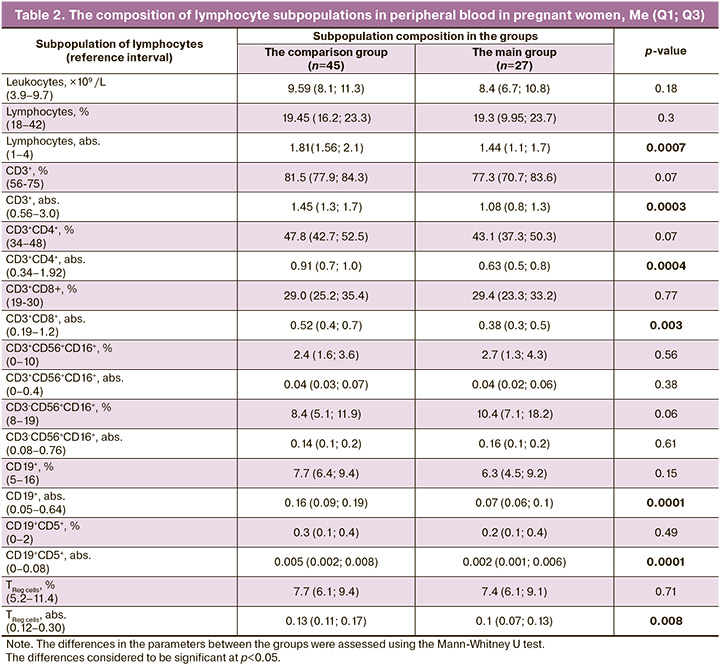
The results represented in Table 3, show decreased absolute lymphocyte count, which reflected reduction of absolute values of all assessed subpopulations, which did fall outside the reference intervals, with the exception of NK and NKT cells.
The next step was comparison of neonatal outcomes between the study groups.
Table 3 represents anthropometric measurements of the newborns.
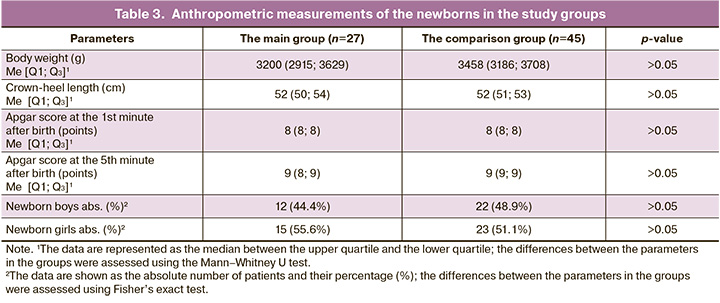
The results represented in Table 3 show that Apgar scores at the 1st minute and at the 5th minute, body weight and crown-heel length of the newborns were comparable between the main group and the comparison group.
PCR tests did not detect SARS-CoV-2 in the newborns at birth in the main group.
In the main group, 5/27 (18.5%) preterm infants were born at 246 (1,1) days [35 weeks 1 day] gestation. In the comparison group, all infants were born at full term (0/45, p=0.006). Three preterm infants (3/5, 60%) in the main group needed care in Neonatal Intensive Care Unit (NICU) due respiratory disorders of non-infectious etiology (in particular, transient tachypnea of newborns), and were subsequently transferred to the Department of Pathology of Newborns and Premature Babies. Two infants were transferred to the Department of Pathology of Newborns and Premature Babies immediately after birth.
In the comparison group, 2/45 (4%) infants needed special health care (3/27 (11%) in the main group) (р>0.05). One infant with severe birth asphyxia and diagnosed congenital pneumonia in the comparison group, in the absence of spontaneous breathing, was transferred from delivery room to the NICU, where mechanical ventilation was used for the infant. On the 4th day after birth, the newborn was extubated, and non-invasive Biphasic ventilation was used, further continuous positive airway pressure (CPAP) was used for the infant. From the 6th day, the infant did not need respiratory therapy, and was transferred to the Department of Pathology of Newborns and Premature Babies for further examination and treatment. In addition to congenital pneumonia, the infant was diagnosed with concomitant diseases: intestinal candidiasis (IC), motor impairment syndrome, left-sided torticollis, rotational subluxation of the C1 and C2 vertebrae, central nervous system depression at the stage of convalescence, disseminated intravascular coagulation (bleeding into the skin), occipital cephalohematoma.
Another infant in the comparison group had confirmed diagnosis of “infection specific for the perinatal period”, that is right-sided acute catarrhal otitis media. Neonatal neurosonography showed focal changes in the periventricular region on the right side, and congenital venous malformation in the right frontal lobe. The infant was transferred to NICU, where episodes of bradycardia were observed. On the 5th day of life, the newborn was transferred to the Department of Pathology of Newborns and Premature Babies for further follow up, examination and treatment. From the 8th day of life, convalescence of infectious process was observed, and the baby’s condition stabilized.
The infants in the main group had longer length of hospitalization – 10 (7.2; 17.0) days, and 3 (3; 3) days in the comparison group (р<0.0001). After making the conclusion that the infants were practically healthy (Z00.1 Routine examination of health status of newborns), 28/45 (62.2%) infants in the comparison group and 9/27 (33.3%) infants in the main group (p=0.028) were discharged from hospital.
The list of diseases in newborns in the main group, due to which there was prolonged hospital stay included congenital malformations: heart defects (critical aortic valve stenosis, ventricular septal and/or atrial septal defect, myocardial hypertrophy, cardiac arrhythmia), Chiari malformation, nasolacrimal duct obstruction; retroperitoneal pulmonary sequestration; intestinal dyskinesia; congenital anemia; transient tachypnea of the newborn; respiratory distress syndrome; hyperbilirubinemia. In one case, a clavicle fracture was diagnosed during birth. Infectious diseases of unknown etiology included infantile cephalic pustulosis, urinary tract infection and acute conjunctivitis. Among infectious diseases of unknown origin, neonatal cephalic pustulosis, urinary tract infection and acute conjunctivitis were diagnosed.
The diseases in newborns in the comparison group, that did not require prolonged hospital stay, included pyelectasis of the left kidney, anal atresia, congenital anemia, congenital nevus, occipital cephalohematoma, and intraventricular hemorrhage, grade 1. The condition of two infants in this group, that required special health care, was described above.
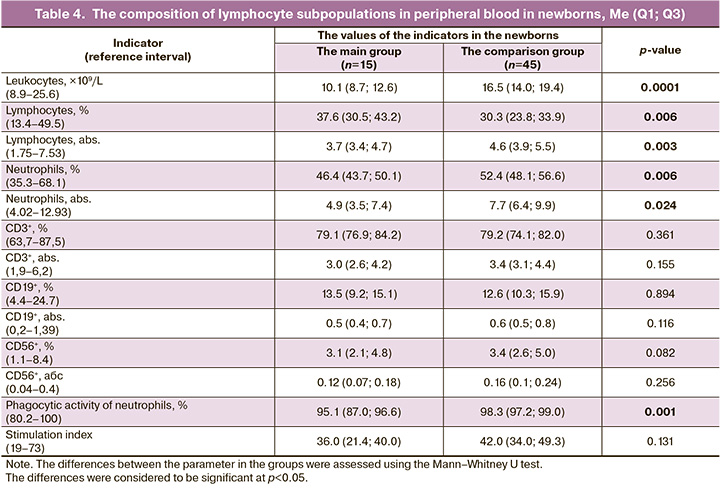
Immunological tests were performed for 15 babies, born to mothers in the main group. Table 4 represents the results of analysis of the composition of lymphocyte subpopulations on the 2nd–3rd day after being born to mothers with coronavirus infection in labor compared to relevant peripheral blood indicators in babies born to mothers in the comparison group.
The analysis of the data presented in Table 4 showed that lower levels of leukocytes, neutrophils and their phagocytic activity, lower absolute values, but higher lymphocyte count was in newborns born to mothers in the main group.
Discussion
The purpose of this study was to characterize the course of pregnancy, immune status of women in labor with SARS-CoV-2, as well as health and immune status of their newborn infants.
According to the data obtained by us, there were no differences in the course of pregnancy between the study groups. Analysis of anamnestic data showed prevalence of gastrointestinal diseases among somatic diseases; and prevalence of external genital endometriosis and ovarian cysts among gynecological diseases in women in the main group. There was significant difference in the number of primiparous and/or multiparous women between the main group and the comparison group.
Spontaneous pregnancies were in most women in the study groups.
The difference was found in the timing and frequency of cesarean delivery (р<0.008). Pregnancy length was 268 (1.75) days [38 weeks 2 days] in the main group and 280 (1.0) [40 weeks] days in the comparison group (р<0.0001). Cesarean delivery rate was 48% in the main group, and 18% in the comparison group (р=0.008).
In both groups, the choice of cesarean delivery was determined by obstetric indications and fetal pathology. Therefore, it is difficult to draw a conclusion about the influence of coronavirus infection on the frequency of cesarean sections and preterm births in the main group.
Analysis of the composition of lymphocyte subpopulations showed decreased absolute lymphocyte count in the main group versus the comparison group, that reflected reduction of absolute values of all assessed subpopulations (with the exception of NK and NKT cells), that can be associated with coronavirus infection in women in labor (Table 2).
In July 2020, a group of authors from Iran were the first to report on 7 cases of lethal outcome in pregnant women due to severe course of novel coronavirus infection [11]. The meta-analysis published in September 2020, showed that pregnant and women in labor with COVID-19 are more likely to be hospitalized to the intensive care unit or require mechanical ventilation, have premature delivery and increased risk of death [5]. Compared to known virus infections, the prognosis for pregnant women with SARS-CoV-2 is favorable, even when antiviral therapy is used [5].
In China, and in the countries with high standards of living, such as USA and Great Britain, the women with COVID-19 during pregnancy (but not in labor) significantly more often are hospitalized and require hospitalization to intensive care units, mechanical ventilation and extracorporeal membrane oxygenation compared to non-pregnant women with COVID-19, with adjustment for age, major diseases and race/ethnicity [12]. Nevertheless, mortality rate was low and similar, both in pregnant and in non-pregnant women of the same age with SARS-CoV-2 (0.1–0.2%) [5, 13].
The risk of premature delivery and cesarean section due to severity of the disease in mother is likely to be higher in pregnant women with COVID-19 compared to pregnant women without COVID-19. It is more likely that the newborns will be hospitalized to the NICU due to preterm birth and/or exclusively for follow up of babies infected with SARS-CoV-2 [14].
In our study, the women had mild COVID-10, this correlates with data reported by researchers from the USA, that in majority of pregnant women with COVID-19, disease course is asymptomatic or mild [15].
All neonates born to mothers in the comparison group were premature babies; 5 infants in the comparison group were born at 246 (1,1) days [35 weeks 1 day] (p=0.006).
According to PCR tests, SARS-CoV-2 was not detected in all newborns in the main group indicating that there was no coronavirus SARS-CoV-2 transmission from mother to newborn in our study. The anthropometric characteristic of all neonates born to mothers with coronavirus infection during delivery were comparable with the anthropometric characteristics and Apgar scores in neonates born to mothers in the comparison group.
In the main group, four out of five preterm babies were born through cesarean section. The need for hospitalization of 3 newborns from the delivery room to the NICU and 2 newborns to the Department of Pathology of Newborns and Premature Babies was determined primarily due to preterm birth and development of respiratory impairment, since SARS-CoV-2 was not identified in these infants. Due to this reason, the length of hospitalization was longer in the main group versus to the comparison group.
In general, with the exception of preterm birth complications, the anthropometric characteristics of infants born to mothers with COVID-19 were comparable with the anthropometric characteristics of infants in the comparison group (Table 3). Although immunological tests for infants born to mothers in the main group showed lower leukocyte count, lower neutrophil and neutrophil counts, and lower phagocytic activity of leukocytes and neutrophils, lower absolute, but higher relative lymphocyte count, it should be noted that the values of the indicators in newborns in both groups remained within the reference values (Table 4), that is consistent with the absence of SARS-CoV-2 infection in infants.
It is believed that severe infections are most common in high-risk pregnancies compared to low-risk pregnancies and may lead to more severe outcomes [16]. Some studies have shown that pregnant women with serious medical conditions are more likely to have preterm birth [17–21]. Their newborns have lower Apgar scores, require NICU hospitalization more often [19], and mortality rate among them is higher [22]. However, recent systematic reviews did not confirm such associations [19, 23, 24]. According to Liu D. et al., pregnancy and childbirth did not increase the severity of SARS-CoV-2 disease in the study patients. Pregnant women are not at increased risk of severe disease or mortality due to COVID-19 compared with the general population [25]. Neonatal death due to COVID-19 has been rarely reported [26, 27].
Our previously published results demonstrated the absence of significant adverse neonatal outcomes in infants born to mothers infected with COVID-19 at different terms of gestation, that showed feasibility of pregnancy maintenance and continuation in these women [7, 28].
The results of this study showed that the rate of cesarean section in women with laboratory-confirmed SARS-CoV-2 infection during delivery is higher (р<0008), and the length of neonatal hospitalization is longer, compared with women without SARS-CoV-2 (р<0.0001). To assess the impact of COVID-19 in mother during delivery on newborn’s health requires collection of catamnestic data, and this should be research priority trend in the future.
Conclusion
Based on the obtained results, the following conclusions can be drawn:
- no differences were found in the cause of pregnancy between women in labor with SARS-CoV-2 и the women in the comparison group;
- in puerperant women with SARS-CoV-2 immunological parameters were within reference values most likely due to mild course of COVID-19;
- the identified decreased absolute number of major lymphocyte populations and no changes of relative values of their composition in women’s immune status in the main group was due to total lymphocyte count reduction, that apparently reflects the presence of viral infection in these women:
- in the group of women in labor with SARS-CoV-2 the rates of preterm birth and cesarean section were higher due to obstetric indications;
- no differences were found in newborns’ anthropometric indicators and Apgar scores;
- the identified changes in immune status of infants born to mothers in the main group were within the reference range, and in the absence of SARS-CoV-2 infection in infants cannot be definitely associated with SARS-CoV-2 infection in mothers.
References
- World Health Organization. COVID-19 weekly epidemiological update, edition 58. 2021. 23p.
- Berkowitz K., LaSala A. Risk factors associated with the increasing prevalence of pneumonia during pregnancy. Am. J. Obstet. Gynecol. 1990; 163(3): 981-5. https://dx.doi.org/10.1016/0002-9378(90)91109-p.
- Brito V., Niederman M.S. Pneumonia complicating pregnancy. Clin. Chest Med. 2011; 32(1): 121-32. https://dx.doi.org/10.1016/j.ccm.2010.10.004.
- Madinger N.E., Greenspoon J.S., Ellrodt A.G. Pneumonia during pregnancy: has modern technology improved maternal and fetal outcome? Am. J. Obstet. Gynecol. 1989; 161(3): 657-62. https://dx.doi.org/10.1016/0002-9378(89)90373-6.
- Allotey J., Stallings E., Bonet M. Yap M., Chatterjee S., Kew T. et al.; for PregCOV-19 Living Systematic Review Consortium. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. B.M.J. 2020; 370: m3320. https://dx.doi.org/10.1136/bmj.m3320.
- La Verde M., Riemma G., Torella M., Cianci S., Savoia F., Licciardi F. et al. Maternal death related to COVID-19: a systematic review and meta-analysis focused on maternal co-morbidities and clinical characteristics. Int. J. Gynaecol. Obstet. 2021; 154(2): 212-9. https://dx.doi.org/10.1002/ijgo.13726.
- Инвияева Е.В., Косолапова Ю.А., Кречетова Л.В., Вторушина В.В., Макиева М.И., Зубков В.В. Особенности субпопуляционного состава лимфоцитов новорожденных детей, рожденных у матерей, перенесших COVID-19 на разных сроках беременности. Инфекция и иммунитет. 2023; 13(1): 46-54. [Inviyaeva E.V., Kosolapova Yu.A., Krechetova L.V., Vtorushina V.V., Makieva M.I., Zubkov V.V. Features of the subpopulation composition of lymphocytes in newborns born to mothers who had COVID-19 at different stages of pregnancy. Infection and Immunity. 2023; 13(1): 46-54. (in Russian)]. https://dx.doi.org/10.15789/2220-7619-FOL-2098.
- Li Y., Zhao R., Zheng S., Chen X., Wang J., Sheng X. et al. Lack of vertical transmission of severe acute respiratory syndrome coronavirus 2, China. Emerg. Infect. Dis. 2020; 26(6): 1335-6. https://dx.doi.org/10.3201/eid2606.200287.
- Chamseddine R.S., Wahbeh F., Chervenak F., Salomon L.J., Ahmed B., Rafii A. Pregnancy and neonatal outcomes in SARS-CoV-2 infection: a systematic review. J. Pregnancy. 2020; 2020: 4592450. https://dx.doi.org/10.1155/2020/4592450.
- Министерство здравоохранения Российской Федерации. Временные методические рекомендации. Профилактика, диагностика и лечение новой коронавирусной инфекции (COVID-19). Версия 6 (28.04.2020г.). [Ministry of Health of the Russian Federation. Temporary guidelines. Prevention, diagnosis and treatment of new coronavirus infection (COVID-19). Version 6 (28.04.2020). (in Russian)].
- Hantoushzadeh S., Shamshirsaz A.A., Aleyasin A., Seferovic M.D., Aski S.K., Arian S.E. et al. Maternal death due to COVID-19. Am. J. Obstet. Gynecol. 2020; 223(1): 109.e1-e16. https://dx.doi.org/10.1016/j.ajog.2020.04.030.
- Yu N., Li W., Kang Q., Xiong Z., Wang S., Lin X. et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect. Dis. 2020; 20(5): 559-64. https://dx.doi.org/10.1016/S1473-3099(20)30176-6.
- Zambrano L.D., Ellington S., Strid P., Galang R.R., Oduyebo T., Tong V.T. et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22 - October 3, 2020. M.M.W.R. Morb. Mortal. Wkly. Rep. 2020; 69(44): 1641-7. https://dx.doi.org/10.15585/mmwr.mm6944e3.
- Khalil A., Kalafat E., Benlioglu C., O'Brien P., Morris E., Draycott T. et al. SARS-CoV-2 infection in pregnancy: a systematic review and meta-analysis of clinical features and pregnancy outcomes. EClinicalMedicine. 2020; 25: 100446. https://dx.doi.org/10.1016/j.eclinm.2020.100446.
- Samadi P., Alipour Z., Ghaedrahmati M., Ahangari R. The severity of COVID‐19 among pregnant women and the risk of adverse maternal outcomes. Int. J. Gynaecol. Obstet. 2021; 154(1): 92-9. https://dx.doi.org/10.1002/ijgo.13700.
- D'Antonio F., Sen C., Di Mascio D.D., Galindo A., Villalain C., Herraiz I. et al.; On the behalf of the World Association of Perinatal Medicine working group on coronavirus disease 2019. Maternal and perinatal outcomes in high compared to low-risk pregnancies complicated by severe acute respiratory syndrome coronavirus 2 infection (phase 2): the World Association of Perinatal Medicine working group on coronavirus disease 2019. Am. J. Obstet. Gynecol. M.F.M. 2021; 3(4): 100329. https://dx.doi.org/10.1016/j.ajogmf.2021.100329.
- Crovetto F., Crispi F., Llurba E., Pascal R., Larroya M., Trilla C. et al.; KidsCorona Pregnancy COVID-19 Group. Impact of severe acute respiratory syndrome coronavirus 2 infection on pregnancy outcomes: a population-based study. Clin. Infect. Dis. 2021; 73(10): 1768-75. https://dx.doi.org/10.1093/cid/ciab104.
- Wei S.Q., Bilodeau-Bertrand M., Liu S., Auger N. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. C.M.A.J. 2021; 193(16): E540-8. https://dx.doi.org/10.1503/cmaj.202604.
- Gurol-Urganci I., Jardine J.E., Carroll F., Draycott T., Dunn G., Fremeaux A. et al. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection at the time of birth in England: national cohort study. Am. J. Obstet. Gynecol. 2021; 225(5): 522.e1-e11. https://dx.doi.org/10.1016/j.ajog.2021.05.016.
- Metz T.D., Clifton R.G., Hughes B.L., Sandoval G., Saade G.R., Grobman W.A. et al.; for the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Disease severity and perinatal outcomes of pregnant patients with coronavirus disease 2019 (COVID-19). Obstet. Gynecol. 2021; 137(4): 571-80. https://dx.doi.org/10.1097/AOG.0000000000004339.
- Li W., Yu N., Kang Q., Zeng W., Deng D., Chen S. et al. Clinical manifestations and maternal and perinatal outcomes with COVID-19. Am. J. Reprod. Immunol. 2020; 84(5): e13340. https://dx.doi.org/10.1111/aji.13340.
- Caniglia E.C., Magosi L.E., Zash R., Diseko M., Mayondi G., Mabuta J. et al. Modest reduction in adverse birth outcomes following the COVID-19 lockdown. Am. J. Obstet. Gynecol. 2021; 224(6): 615.e1-e12. https://dx.doi.org/10.1016/j.ajog.2020.12.1198.
- Chmielewska B., Barratt I., Townsend R., Kalafat E., van der Meulen J., Gurol-Urganci I. et al. Effects of the COVID-19 pandemic on maternal and perinatal outcomes: a systematic review and meta-analysis. Lancet Glob. Health. 2021; 9(6): e759-2. https://dx.doi.org/10.1016/S2214-109X(21)00079-6.
- Mirbeyk M., Saghazadeh A., Rezaei N. A systematic review of pregnant women with COVID-19 and their neonates. Arch. Gynecol. Obstet. 2021; 304(1): 5-38. https://dx.doi.org/10.1007/s00404-021-06049-z.
- Liu D., Li L., Wu X., Zheng D., Wang J., Yang L. et al. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. A.J.R. Am. J. Roentgenol. 2020; 215(1): 127-32. https://dx.doi.org/10.2214/AJR.20.23072.
- Khoury R., Bernstein P.S., Debolt C., Stone J., Sutton D.M., Simpson L.L. et al. Characteristics and outcomes of 241 births to women with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection at five New York City medical centers. Obstet. Gynecol. 2020; 136(2): 273-82. https://dx.doi.org/10.1097/AOG.0000000000004025.
- Woodworth K.R., Olsen E.O., Neelam V., Lewis E.L., Galang R.R., Oduyebo T. et al.; CDC COVID-19 Response Pregnancy and Infant Linked Outcomes Team; COVID-19 Pregnancy and Infant Linked Outcomes Team (PILOT). Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 infection in pregnancy - SET-NET, 16 jurisdictions, March 29-October 14, 2020. N.M.W.R. Morb. Mortal. Wkly. Rep. 2020; 69(44): 1635-40. https://dx.doi.org/10.15585/mmwr.mm6944e2.
- Косолапова Ю.А., Борис Д.А., Полуденко Н.Д., Макиева М.И., Никитина И.В., Инвияева Е.В., Вторушина В.В., Кречетова Л.В., Миханошина Н.В., Зубков В.В., Дегтярев Д.Н. Влияние новой коронавирусной инфекции COVID-19, перенесенной женщинами во время беременности, на состояние здоровья новорожденных детей. Акушерство и гинекология. 2022; 11: 90-8. [Kosolapova Yu.A., Boris D.A., Poludenko N.D., Makieva M.I., Nikitina I.V., Inviyaeva E.V., Vtorushina V.V., Krechetova L.V., Mikhanoshina N.V., Zubkov V.V., Degtyarev D.N. The impact of the new coronavirus infection COVID-19, suffered by women during pregnancy, on the health of newborn children. Obstetrics and Gynecology. 2022; (11): 90-8. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.11.90-98.
Received 29.02.2024
Accepted 13.03.2024
About the Authors
Evgeniya V. Inviyaeva, PhD (Bio), Senior Researcher at the Laboratory of Clinical Immunology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(495)438-11-83, e_inviyaeva@oparina4.ru,https://orcid.org/0000-0001-9878-3637
Oleg V. Tysyachnyi, PhD, Researcher at the1st Maternity Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, o_tysyachny@oparina4.ru, https://orcid.org/0000-0001-9282-9817
Yulia A. Kosolapova, neonatologist, Junior Researcher at the Neonatal Department No. 2, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(999)987-82-55, yu_kosolapova@oparina4.ru,
https://orcid.org/0000-0001-8180-3275
Valentina V. Vtorushina, PhD, clinical laboratory diagnostics doctor at the Laboratory of Clinical Immunology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4., +7(495) 438-11-83, v_vtorushina@oparina4.ru,
https://orcid.org/0000-0002-8406-3206
Oleg R. Baev, Dr. Med. Sci., Professor, Head of the 1st Maternity Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(495)438-11-88, o_baev@oparina4.ru, https://orcid.org/0000-0001-8572-1971
Victor V. Zubkov, Dr. Med. Sci., Director of the Institute of Neonatology and Pediatrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4; Professor at Neonatal Department of Pediatric Faculty, I.M.Sechenov First State Medical University, Ministry of Health of Russia, 119991, Russia, Moscow, Trubetskaya str., 8-2, victor.zubkov@mail.ru, https://orcid.org/0000-0002-9697-9596
Lubov V. Krechetova, Dr. Med. Sci., Head of The Laboratory of Clinical Immunology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(495)438-11-83, l_krechetova@oparina4.ru,
https://orcid.org/0000-0001-5023-3476117997
Corresponding author: Evgeniya V. Inviyaeva, e_inviyaeva@oparina4.ru



