В 2011 г. для определения рисков хромосомных аномалий (ХА) плода в клиническую практику включен новый метод исследования – неинвазивный пренатальный тест (НИПТ); также часто используется термин «неинвазивный пренатальный скрининг» (НИПС), подразумевающий скрининговый характер исследования [1]. В его основе лежит анализ внеклеточной фетоплацентарной ДНК (вфДНК). ВфДНК свободно циркулирует в крови беременной женщины, начиная с 5-й недели беременности [2], однако достаточной для проведения исследования концентрации – 3,5% и более – достигает на 10-й неделе беременности [3].
НИПТ возможно осуществлять с помощью двух технологий секвенирования: полногеномного или таргетного, основанного на анализе SNP (single nucleotide polymorphism) [4]. При полногеномном подходе секвенируется каждая хромосома, и кроме частых анеуплоидий и аномалий половых хромосом возможно выявление микроструктурных нарушений и анеуплоидий других хромосом. При таргетном НИПТ оцениваются только целевые фрагменты генома (однонуклеотидные полиморфизмы – SNP), возможно узнать риск частых анеуплоидий и аномалий половых хромосом, а также некоторые микроструктурные нарушения (Ди Джорджи, делеции 1p36, кошачьего крика, Ангельмана, Прадера–Вилли, Вольфа–Хиршхорна).
Результаты валидационных исследований продемонстрировали высокую чувствительность и специфичность НИПТ, высокий уровень выявляемости рисков ХА [5], что позволило профессиональным сообществам, в частности Американскому колледжу акушеров и гинекологов и Обществу медицины матери и плода, опубликовать в 2012 г. рекомендации по применению НИПТ у беременных женщин с высоким риском ХА плода. Под группой высокого риска подразумевались:
- беременные женщины с высоким риском ХА плода по результатам пренатального скрининга I триместра беременности;
- беременные женщины старше 35 лет;
- беременные женщины, у которых обнаружены ультразвуковые маркеры ХА плода;
- беременные женщины, имеющие в анамнезе случаи ХА плода;
- беременные женщины, у которых диагностирована сбалансированная робертсоновская транслокация, связанная с 13 или 21 хромосомами, или у супруга которых диагностирована сбалансированная робертсоновская транслокация.
В указанных рекомендациях спектр исследуемых анеуплоидий ограничивался трисомиями 13, 18 и 21 хромосом [6].
В 2015 г. расширен спектр исследуемых ХА в рамках НИПТ: к частым анеуплоидиям (трисомиям 13, 18 и 21 хромосом) в рекомендации добавлены нарушения по половым хромосомам [7].
В настоящее время актуальны рекомендации 2020 г. – проведение НИПТ для оценки риска у плода трисомий 13, 18 и 21 хромосом и нарушения по половым хромосомам у всех беременных женщин вне зависимости от риска, полученного по результатам стандартного пренатального скрининга, и/или других факторов риска, например, возраста беременной женщины и т.д. [8]. В Российской Федерации клинические рекомендации по применению НИПС были опубликованы в 2016 г. [9].
В мировой практике включения НИПТ в алгоритм пренатальной диагностики существует несколько подходов. Наиболее часто применяют НИПТ в качестве теста второй линии – проведение пренатального скрининга с формированием групп высокого, среднего и низкого риска с последующим проведением НИПТ в группе высокого и/или среднего риска. Основное преимущество этой модели – относительно низкие затраты на НИПТ. Среди стран, применяющих НИПТ как тест второй линии, – большинство стран Европы [10], Австралия, США [11], Канада [12, 13].
Несмотря на высокую цену, также практикуется применение НИПТ в качестве теста первой линии – проведение его всем беременным женщинам [10]. Реализация этой модели в каждой стране имеет свои особенности [11]. Например, в Нидерландах НИПТ предлагается всем беременным женщинам вместо традиционного пренатального скрининга, тогда как в Бельгии предлагается проведение НИПТ в сочетании с ультразвуковым исследованием (УЗИ) в I триместре беременности. В связи с этим популярность НИПТ в Бельгии значительно выше, чем в Нидерландах, так как УЗИ позволяет не пропустить пороки развития плода, которые могут быть не связаны с ХА плода.
Таким образом, в мире используются различные модели применения НИПТ в зависимости от особенностей страны и стоимости медицинской помощи.
В Российской Федерации впервые НИПТ был включен в структуру пренатальной диагностики в г. Москве и в Ямало-Ненецком автономном округе (ЯНАО) в рамках пилотных проектов в 2020 г. В Москве оценивается эффективность применения НИПТ в качестве теста второй линии [14]. В ЯНАО НИПТ проводится в качестве теста первой линии всем беременным вне зависимости от группы риска по результатам пренатального скрининга I триместра [15–17].
Целью нашего исследования является оценка клинической эффективности применения НИПТ у беременных женщин ЯНАО в сравнении с пренатальным скринингом I триместра беременности.
Материалы и методы
Первый этап исследования проведен в 2020 г. и предусматривал проведение НИПТ всем беременным женщинам, состоящим на учете по беременности в медицинских организациях, подведомственных Департаменту здравоохранения ЯНАО, направленных на пренатальный скрининг I триместра беременности [15]. Проанализировано 550 образцов крови беременных женщин.
Второй этап исследования проведен в 2021 г. [16, 17], проанализировано 1500 образцов крови беременных женщин, прошедших пренатальный скрининг I триместра беременности в государственных учреждениях здравоохранения ЯНАО, оказывающих помощь по профилю «акушерство и гинекология», вне зависимости от группы риска ХА плода, включая беременных женщин, проживающих в сельских местностях, отдаленных территориях и женщин кочевой культуры.
Общее количество проанализированных образцов за период с 01.08.2020 по 30.11.2021 составило 2050.
Средний возраст обследованных пациенток 30,2 (4,6) года, в 23,7% случаев возраст беременных женщин – 35 лет и старше. Средний индекс массы тела (ИМТ) пациенток – 25,3 кг/м2, в 42,3% случаев ИМТ составил 25 кг/м2 и более. Большинство беременностей – спонтанные (1975/2050, 96,3%) и одноплодные (2018/2050, 98,4%) (табл. 1).
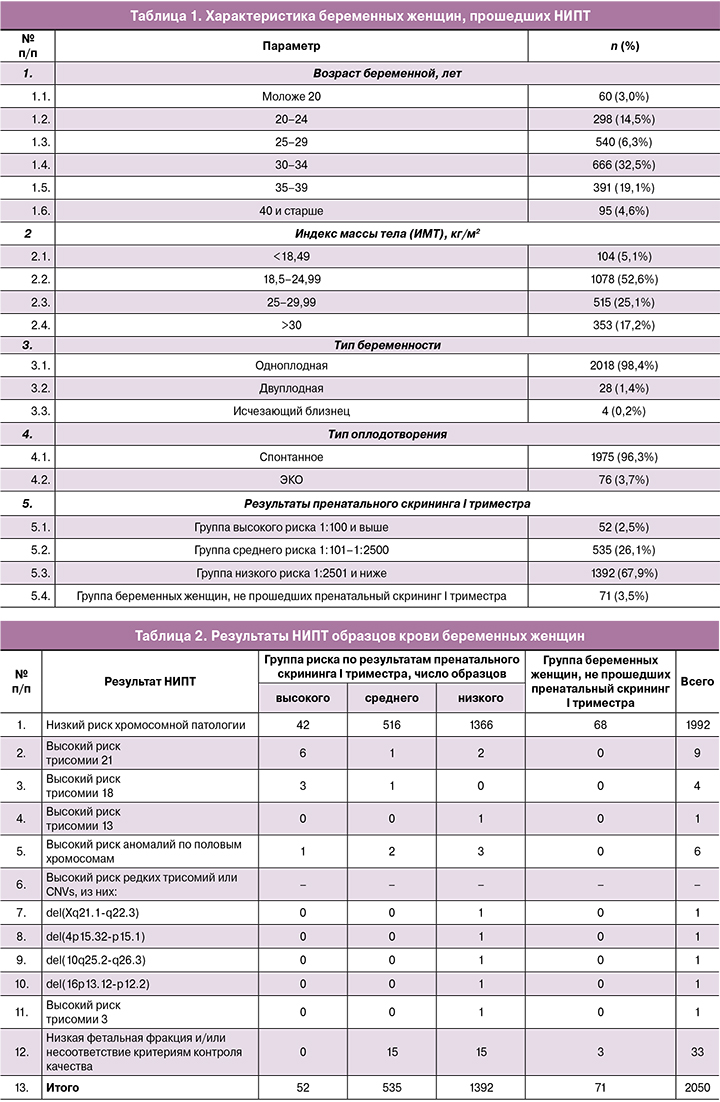
Беременные женщины разделены на группы:
- группа риска 1:100 и выше (группа высокого риска) по результатам пренатального скрининга I триместра – 52 беременные женщины;
- группа риска 1:101–1:2500 (группа среднего риска) по результатам пренатального скрининга I триместра – 535 беременных женщин;
- группа риска 1:2501 и ниже (группа низкого риска) по результатам пренатального скрининга I триместра – 1392 беременные женщины;
- группа беременных женщин, не прошедших пренатальный скрининг I триместра беременности – 71 беременная женщина (табл. 1).
Для проведения НИПТ в рамках исследования забор венозной крови у беременных женщин осуществлялся в пробирки типа STRECK (РУ от 12.04.2021 №РЗН 2021/14009) с идентификационным номером пациентки. Транспортировка образцов крови в лабораторию осуществлялась в специальных контейнерах с соблюдением температурного режима (от +15 до +25°C).
После получения лабораторией пробирок с венозной кровью и их регистрации образцы крови подвергались процессу двухстадийного центрифугирования, в результате чего из цельной крови отделялась плазма, содержащая внеклеточную ДНК матери и плода. После выделения внеклеточной ДНК приступали к созданию ДНК-библиотек и проводили секвенирование. Полученные данные подвергались биоинформатической и клинической обработке, после чего формировалось заключение и выдавался результат.
Хранение пробирок с образцами плазмы осуществлялось при температуре -20°C для краткосрочного хранения (не более 1 недели) и при температуре -80°C для долгосрочного хранения.
Этапы пробоподготовки проводились согласно протоколу производителя BGI Genomics, этап секвенирования при проведении НИПТ в лаборатории осуществлялся секвенаторами BGISEQ-500 компании BGI Genomics. Для расчета результатов секвенирования использовался оригинальный программный пакет BGI HALOS NIFTY-2.3.2.1011. Окончательный отчет включал определение риска трисомии 21, 18, 13 (синдромов Дауна, Эдвардса и Патау), анеуплоидии по половым хромосомам, редким трисомиям и микроструктурным перестройкам.
Беременным женщинам с высоким риском ХА плода по результатам НИПТ рекомендовались консультация врача-генетика и инвазивная пренатальная диагностика (ИПД) – биопсия ворсин хориона, кордоцентез или амниоцентез. Направление на ИПД пациенток группы высокого риска по результатам пренатального скрининга I триместра беременности осуществлялось в установленном законодательством порядке [18]. На рисунке графически представлена маршрутизация беременных женщин в рамках НИР 2020–2021 гг.
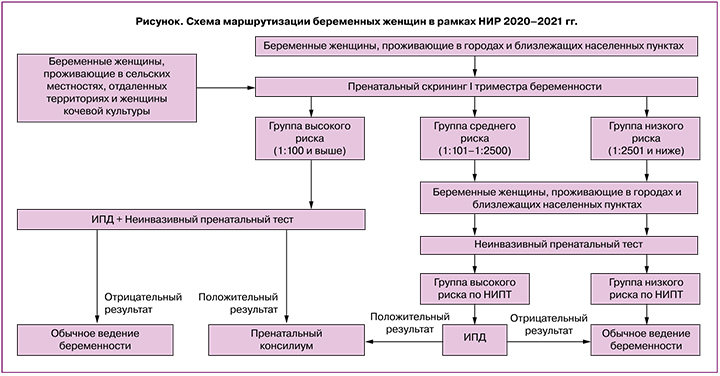
Все обследованные в рамках проекта подписали информированное добровольное согласие.
Обработка данных осуществлялась в программном пакете Microsoft Excel методами описательной статистики: количественные показатели представляли в виде среднего значения и стандартного отклонения; для показателей, характеризующих качественные признаки, указывали абсолютное число и долю в процентах.
Результаты
За период с 01.08.2020 г. по 30.11.2021 г. проанализировано 2050 образцов крови беременных женщин ЯНАО (n=2050).
Группа высокого риска по результатам пренатального скрининга I триместра. Из 52 образцов крови беременных женщин группы высокого риска в 10 (19,2%) методом НИПТ установлен высокий риск:
- синдрома Дауна (трисомии 21 хромосомы) – у 6 плодов;
- синдрома Эдвардса (трисомии 18 хромосомы) – у 3 плодов;
- нарушений по половым хромосомам – у 1 плода (табл. 2).
Низкий риск по результатам НИПТ установлен в 42 (80,8%) из 52 образцов крови беременных женщин группы высокого риска.
Группа среднего риска по результатам пренатального скрининга I триместра. Из 535 образцов крови беременных женщин группы среднего риска в 4 (0,8%) методом НИПТ установлен высокий риск:
- синдрома Дауна (трисомии 21 хромосомы) – у 1 плода;
- синдрома Эдвардса (трисомии 18 хромосомы) – у 1 плода;
- нарушений по половым хромосомам – у 2 плодов (табл. 2).
Низкий риск по результатам НИПТ установлен в 516 (96,4%) из 535 образцов крови беременных женщин группы среднего риска.
По 15 образцам крови (2,8%) из 535 результат НИПТ не получен ввиду низкой фетальной фракции (ниже 3,5%) и/или несоответствия образца крови критериям контроля качества.
Группа низкого риска по результатам пренатального скрининга I триместра. Из 1392 образцов крови беременных женщин группы низкого риска в 11 (0,8%) методом НИПТ установлен высокий риск:
- синдрома Дауна (трисомии 21 хромосомы) – у 2 плодов;
- синдрома Патау (трисомии 13 хромосомы) – у 1 плода;
- нарушений по половым хромосомам – у 3 плодов;
- нарушений по другим хромосомам – у 5 плодов, из них 1 высокий риск делеции длинного плеча X хромосомы, 1 – делеция короткого плеча 4 хромосомы, 1 – делеция длинного плеча 10 хромосомы, 1 – делеция короткого плеча 16 хромосомы и 1 случай высокого риска трисомии 3 хромосомы (табл. 2).
Низкий риск по результатам НИПТ установлен в 1366 (98,1%) из 1392 образцов крови беременных женщин группы низкого риска.
По 15 образцам крови (1,1%) из 1392 результат НИПТ не получен ввиду низкой фетальной фракции (ниже 3,5%) и/или несоответствия образца крови критериям контроля качества.
Группа беременных женщин, не прошедших пренатальный скрининг I триместра. Среди беременных женщин, не прошедших пренатальный скрининг I триместра беременности, в 68 (95,8%) из 71 образца крови методом НИПТ установлен низкий риск ХА плода. По 3 образцам крови (4,2%) из 71 результат НИПТ не получен ввиду низкой фетальной фракции (ниже 3,5%) и/или несоответствия образца крови критериям контроля качества.
В группе высокого риска по результатам пренатального скрининга I триместра инвазивная пренатальная диагностика (ИПД) проведена 7 (13,5%) из 52 беременных женщин, при этом в 3 (42,8%) случаях подтвержден синдром Дауна (трисомия 21 хромосомы) у плода, а в 4 (57,2%) получены нормальные кариотипы плодов, что соответствует результатам НИПТ.
В группе высокого риска по результатам НИПТ ИПД проведена у 6 (24%) из 25 беременных женщин. Во всех 6 (100,0%) случаях подтверждена хромосомная патология у плода, из них:
- у 3 плодов (50,0%) – синдром Дауна (трисомия 21 хромосомы) среди беременных группы высокого риска по результатам пренатального скрининга I триместра;
- у 2 плодов (33,3%) – синдром Клайнфельтера (дисомия Х хромосомы у плода мужского пола) у пациенток из группы среднего и низкого риска по результатам пренатального скрининга I триместра;
- у 1 плода (16,7%) – синдром Джейкобс (дисомия Y хромосомы) среди пациенток из группы среднего риска по результатам пренатального скрининга I триместра.
У 4 пациенток, которым была показана ИПД по результатам НИПТ (из них у 3 ИПД показана и по результатам пренатального скрининга I триместра), беременности прерваны по медицинским показаниям без проведения ИПД, молекулярно-генетическое исследование абортивного материала проведено не было.
Таким образом, проведение НИПТ в рамках пренатального скрининга I триместра в ЯНАО позволило:
- дополнительно выявить 15 плодов с высокими рисками ХА среди беременных женщин групп среднего и низкого риска, из них: 5 плодов с высокими рисками частых анеуплоидий (3 – с синдромом Дауна, трисомией 21 хромосомы, 1 – с синдромом Эдвардса, трисомией 18 хромосомы и 1 – с синдромом Патау, трисомией 13 хромосомы); 5 плодов с высоким риском аномалий половых хромосом и 5 плодов с высоким риском редких анеуплоидий, включая микроструктурные перестройки;
- сократить число ИПД у беременных женщин на 51,9% (с 52 до 25).
Обсуждение
Развитие молекулярно-генетических технологий привело к включению НИПТ в алгоритм пренатальной диагностики. НИПТ является высокоэффективным методом определения риска хромосомной патологии плода у беременных и имеет высокую чувствительность и специфичность по сравнению с биохимическим исследованием, что доказано рядом международных исследований [19]. В Российской Федерации также оценивалась эффективность НИПТ – полученные в ходе исследований результаты продемонстрировали увеличение эффективности выявления ХА плода при применении НИПТ в качестве теста второй линии, а также уменьшение числа ИПД и ассоциированных с диагностикой прерываний беременности [20, 21]. Настоящее исследование демонстрирует клиническую эффективность и возможность включения НИПТ в алгоритм пренатальной диагностики в ЯНАО.
В рамках исследования по результатам пренатального скрининга I триместра у 2050 беременных женщин высокий риск ХА плода определен у 52, в том числе 42 случая высокого риска синдрома Дауна (трисомии 21 хромосомы). Согласно Порядку оказания медицинской помощи по профилю «акушерство и гинекология» [18], им показано проведение ИПД. По результатам НИПТ высокий риск ХА плода и показания для проведения ИПД были только у 10 (19,2%) из 52 беременных женщин группы высокого риска по результатам пренатального скрининга I триместра беременности; остальным 42 (80,8%) беременным женщинам проведение ИПД по результатам НИПТ не показано – определен низкий риск ХА плода. Таким образом, НИПТ позволяет значительно снизить долю ложноположительных результатов пренатального скрининга.
Средний и низкий риск ХА по результатам пренатального скрининга I триместра беременности определен у 1998 беременных женщин, включая женщин, не прошедших пренатальный скрининг I триместра беременности. Согласно Порядку оказания медицинской помощи по профилю «акушерство и гинекология» [18], им не показано проведение ИПД и дальнейшее медико-генетическое консультирование. В этой группе по результатам НИПТ установлен высокий риск ХА плода и определены показания для ИПД у 15 беременных женщин, в том числе 3 высоких риска синдрома Дауна (трисомии 21 хромосомы). Таким образом, проведение НИПТ позволяет выявить ХА плода, которые пропущены пренатальным скринингом I триместра беременности.
При проведении исследования обращает на себя внимание то, что показатель согласившихся на ИПД в группе высокого риска по результатам пренатального скрининга I триместра беременности составляет 13,5% – это на 49,9% ниже среднероссийского показателя (63,4%). В свою очередь, число согласившихся в группе высокого риска по результатам НИПТ составляет 28% – в результате применения НИПТ в ЯНАО доля согласившихся на проведение ИПД увеличилась на 14,5%. Подобная закономерность наблюдается и в ряде международных исследований: Bjerregaard L. et al. отметили, что доля женщин из группы высокого риска по результатам пренатального скрининга, которые отказываются от ИПД, сократилась на 23% (с 26 до 3%) в связи с включением НИПТ в качестве теста второй линии в алгоритм пренатальной диагностики [22]. В исследовании Chetty S. et al. включение НИПТ в алгоритм пренатальной диагностики значительно снизило вероятность отказа от дальнейшей подтверждающей диагностики в результате определения высокого риска ХА плода: 52,8% обследованных отказались от ИПД в результате определения высокого риска ХА плода по результатам стандартного пренатального скрининга, в то время как после НИПТ отказались всего 21,2% обследованных [23].
В рамках НИР 2020–2021 гг. в ЯНАО применялся полногеномный НИПТ. Применение полногеномного подхода не является общепринятым при проведении НИПТ [13], однако это позволило дополнительно выявить 5 плодов с риском частых анеуплоидий и 10 плодов с высокими рисками аномалий других хромосом, включая микроструктурные перестройки. Таким образом, в ЯНАО целесообразно в дальнейшем применение полногеномного НИПТ.
Для ЯНАО выбрана модель применения НИПТ в качестве первой линии – всем беременным женщинам, прошедшим пренатальный скрининг I триместра беременности, вне зависимости от группы риска ХА плода, включая беременных женщин, проживающих в сельских местностях, отдаленных территориях и женщин кочевой культуры. Выбор указанной модели обусловлен, в частности, территориальными особенностями субъекта: протяженность территории, длительное время в пути до медицинских учреждений, необходимость транспортировки пациентов посредством санитарной авиации – все это делает оказание специализированной помощи по профилю «акушерство и гинекология» труднодоступным, в том числе для беременных женщин, проживающих в сельских местностях, отдаленных территориях и женщин кочевой культуры. При модели первой линии жительницам труднодоступных территорий не требуется повторно приезжать для проведения НИПТ, так как взятие крови проводится в день проведения пренатального скрининга I триместра.
Таким образом, наиболее целесообразной моделью в ЯНАО является применение НИПТ в качестве теста первой линии. Это позволит повысить качество оказания медицинской помощи беременным женщинам, своевременно информировать семью о существовании патологии у плода, подготовиться к рождению ребенка с патологией или принять решение о прерывании беременности.
Выводы
В рамках НИР, проведенных в 2020–2021 гг., отмечена клиническая эффективность применения полногеномного варианта НИПТ в формате массового скрининга всем беременным женщинам – вне зависимости от результатов пренатального скрининга I триместра.
Включение НИПТ в алгоритм пренатальной диагностики ХА плода для беременных женщин в ЯНАО в сравнении с пренатальным скринингом I триместра беременности позволило:
- дополнительно к пренатальному скринингу I триместра выявить 15 плодов с высокими рисками ХА, из них 5 находок относятся к частым анеуплоидиям и 10 – к редким, включая микроструктурные перестройки;
- снизить долю ложноположительных результатов при проведении пренатальной диагностики, предотвращая проведение необоснованных инвазивных вмешательств, которые могут приводить к осложнениям, в том числе к прерыванию беременности, – высокая чувствительность и специфичность НИПТ позволили в 5 раз уменьшить количество ИПД среди беременных женщин группы высокого риска по результатам пренатального скрининга I триместра беременности (с 52 до 10);
- увеличить долю согласившихся на проведение ИПД на 14,5%.



