Application of noninvasive prenatal screening for aneuploidies in multiple pregnancies
Barkov I.Yu., Bolshakova A.S., Tetruashvili N.K., Shubina Je., Goltsov A.Yu., Trofimov D.Yu.
Relevance: Noninvasive prenatal screening (NIPS) for aneuploidies in maternal blood is a highly sensitive and specific method for screening trisomies 21, 18, and 13, as well as sex chromosome aneuploidies in singleton pregnancies. Although the use of NIPS in multiple pregnancies appears promising, the number of published studies on this subject is limited.
Objective: This study aimed to investigate the feasibility of detecting trisomy 21 using NIPS in multiple pregnancies.
Materials and methods: This study included 89 pregnant women with twins and one pregnant woman with triplets who underwent NIPS between May 2018 and December 2023 using high-throughput sequencing to identify common aneuploidies in their fetuses.
Results: The study identified a high risk of trisomy 21 in 6 cases, including 4 cases in the fetuses of dichorionic twins, one case possibly in a fetus of monochorionic twins, and one case in a fetus of trichorionic triplets. In five cases, the results were confirmed, and in one case there was the death of one of the fetuses in a twin pregnancy. Seventy-three patients with low risk according to the NIPS gave birth to phenotypically healthy children. The study showed that NIPS had almost 100% sensitivity and specificity for the detection of trisomy 21 in multiple pregnancies. The risk for major aneuploidies could not be assessed in only six patients (6.7%) due to low fetal fractions at the time of repeat blood sampling.
Conclusion: This study demonstrated the high accuracy of NIPS for trisomy 21, suggesting that NIPS could be offered to all pregnant women with twins. The introduction of this technology into clinical practice will likely improve the management of pregnant women with multiple gestations and, consequently, enhance perinatal outcomes in this group of patients.
Authors' contributions: Barkov I.Yu., Bolshakova A.S., Shubina Je., Trofimov D. Yu. – conception and design of the study; Barkov I.Yu., Bolshakova A.S., Tetruashvili N.K. – drafting of the manuscript; Barkov I.Yu., Shubina Je., Goltsov A.Yu. – data collection and analysis; Trofimov D.Yu. – editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The investigation has been supported by the Russian Foundation for Basic Research within the framework of Research. Project No. 121040600434-3.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P (Ref. No: 13 of 2015-12-10).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Barkov I.Yu., Bolshakova A.S., Tetruashvili N.K., Shubina Je., Goltsov A.Yu., Trofimov D.Yu. Application of noninvasive prenatal screening for aneuploidies in multiple pregnancies.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (2): 44-50 (in Russian)
https://dx.doi.org/10.18565/aig.2024.26
Keywords
Chromosomal aneuploidy refers to the presence of an abnormal number of chromosomes in a cell, making a significant contribution to childhood morbidity and mortality. Following sex chromosome aneuploidies, the most frequently encountered are trisomies 21, 18, and 13 [1].
Enhancing the quality of medical care for individuals with multifetal pregnancies is an extremely pressing task that requires immediate attention. In recent decades alone, the rate of twin births has increased by about a third, from 9.1 to 12.0 cases per 1000 births [2]. This rapid increase is mainly associated with an increase in the number of births of dizygotic twins owing to the use of assisted reproductive technologies (ART) [2, 3].
Chorionicity in twins is a crucial factor for determining the risk of aneuploidy. For instance, in dichorionic twins, the theoretical risk of aneuploidy for each fetus is twice that of a singleton pregnancy [4, 5]. Twin pregnancies consist of dizygotic (fraternal) twins in 60-70% of cases and monozygotic (identical) twins in 20-30% of cases [6]. Statistical data may vary depending on the mother's age, method of conception, and population, especially in the case of dizygotic twins. Dichorionicity is a obligatory feature of dizygotic twins; however, up to 25% of monozygotic twins have two separate placentas [7, 8].
Because of the lower accuracy of identifying the risk of trisomy 21, 18, and 13 using combined first-trimester screening in twins compared to singleton pregnancies [9], it seems advisable to introduce more reliable methods, such as non-invasive prenatal DNA screening of aneuploidies in the fetus by maternal blood (NIPS). Currently, NIPS is the most sensitive method for screening aneuploidies of chromosomes 21, 18, 13, X, and Y [10, 11]. Previous studies have demonstrated the effectiveness of NIPS in multiple pregnancies [12, 13]. Huang X. et al., in their article, reported 100% sensitivity and specificity of NIPS for trisomy 21, but for trisomy 18, the sensitivity was 50% [14]. A meta-analysis conducted by Russian researchers in 2021 showed that the detection rates of trisomy 21 and 18 in twin pregnancies were comparable to those in singleton pregnancies [15].
In our study, we also demonstrated the high sensitivity and specificity of detecting trisomy 21 in twin pregnancies, and in some cases, the feasibility of using NIPS in triple pregnancies.
Materials and methods
Study design
This prospective observational study included 90 pregnant women with multiple pregnancies who underwent NIPS in V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia between May 2018 and December 2023.
The inclusion criteria were (1) twin pregnancy, (2) informed consent of the patient to participate in the study, and (3) gestational age ≥ 10 weeks (in exceptional cases ≥ 8 weeks). In one case, NIPS was performed in a patient with trichorionic triamniotic triplets.
The exclusion criteria were (1) maternal cancer and (2) no consent for participation in the study.
Genetic counseling was performed if a high risk of chromosomal aneuploidy was identified. In four cases, invasive prenatal diagnosis was performed by amniocentesis. In a pregnant woman with triplets, blood sampling for NIPS was performed simultaneously with chorionic villus sampling of three fetuses. In cases where a low risk of chromosomal abnormalities was identified according to the NIPS, patients underwent standard monitoring. Pregnancy outcomes were collected through delivery case notes and telephone interviews.
Chorionicity study
The main method for determining chorionicity is ultrasound diagnosis [16]. In some cases, chorionicity was confirmed by histologic examination of the placenta after birth.
Conducting NIPS and analyzing results
Whole maternal venous blood (9 mL) was collected in tubes containing EDTA. For DNA extraction, Applied Biosystems MagMAX kits (Thermo Fisher, USA) and the VAHTS Serum/Plasma Circulating DNA Kit (Vazyme, China) were used. Centrifugation was performed twice, immediately after the blood collection. At 2000 g for 10 min at 4°C, the supernatant was collected and centrifuged at 10,000g for 20 min at the same temperature. Cell-free DNA was isolated using Dynabeads technology immediately after blood collection or from plasma centrifuged and frozen at -20°C. High-throughput sequencing was performed using a NextSeq550 system (Illumina, USA).
Bioinformatic processing of the results was performed using AneuScreen software (DNA-Technology, Russia).
The study was reviewed and approved by the Research Ethics Committee of V.I. Kulakov NMRC for OG&P (Ref. No: 13 of 2015-12-10).
Results
During the study, which was conducted from May 2018 to December 2023, NIPS was performed on 89 pregnant women with twins and one pregnant woman with triplets. The mean age of the participants was 34 years (range – 22–46 years). The gestational age at blood collection ranged from 8 to 26 weeks (average, 13 weeks, 6 days). If a fetal DNA fraction of less than 8% was detected, blood was drawn again after 2 weeks. The mean fetal DNA fraction in pregnant women with twins was 11.2% (range 2.3% to 28.6%). In 5 patients, the level of extracellular fetal DNA was sufficient to draw a conclusion after repeated sampling. In six patients, the fetal DNA fraction was less than 8% after repeated sampling, and genetic counseling was recommended. In one patient, repeat blood sampling was not performed.
Dichorionic diamniotic twins were diagnosed in 61 cases, and monochorionic diamniotic twins in 28 cases. Pregnancy occurred spontaneously in 46.6% (42/90) of patients and with ART in 53.3% (48/90) of patients.
Twelve pregnant women were referred to NIPS because of changes in the serum markers pregnancy-associated protein A (PAPP-A) and the beta-subunit of human chorionic gonadotropin (β-hCG) after prenatal screening in the first trimester. In eight pregnant women, one fetus showed an increase in nuchal translucency (above the 95th percentile). Five pregnant women were referred for NIPS because of other ultrasound markers of fetal chromosomal pathology (nasal bone hypoplasia, ductus venosus reflux, cyst, and single umbilical artery). Three pregnant women had a combination of ultrasound and biochemical markers, and two had structural abnormalities in the development of one fetus.
High-risk cases and pregnancy outcomes
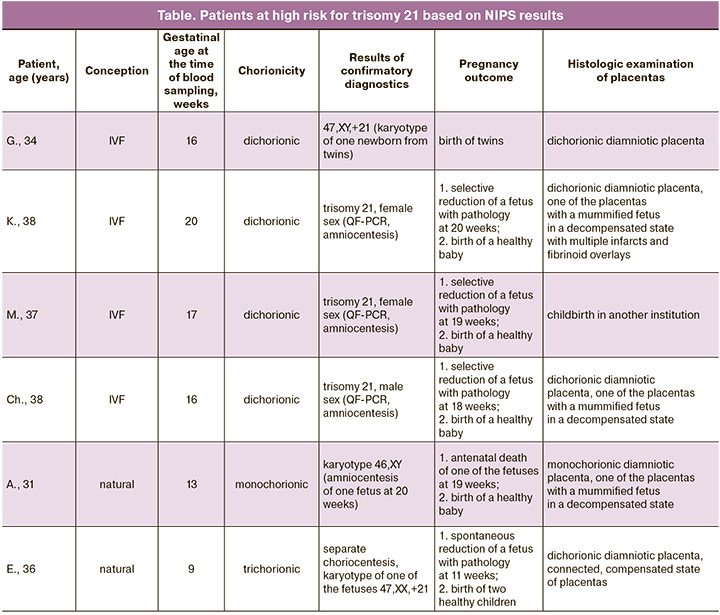
In three patients with dichorionic diamniotic twins, blood sampling for NIPS was performed prior to invasive diagnosis. The indication for separate amniocentesis was a high risk of trisomy 21 according to first-trimester prenatal screening. After examination of the amniotic fluid by quantitative fluorescent polymerase chain reaction, trisomy 21 was detected in one of the fetuses in all three cases; therefore, at the request of the patients, selective reduction of the fetus with chromosomal pathology was performed, and three phenotypically healthy children were born. In one case, NIPS was performed in a patient with dichorionic diamniotic twins due to increased nuchal thickness in one of the fetuses; one of the fetuses was found to be at a high risk for trisomy 21. The patient refused invasive diagnostic procedures. Subsequently, a phenotypically healthy child and child with trisomy 21, confirmed by cytogenetic analysis of the karyotype, were born. In one case, in a patient with monochorionic dizygotic twins and a high risk for trisomy 21 according to NIPS, one fetus died at 19 weeks; therefore, amniocentesis was performed in only one fetus of the twins. The fetus had a normal karyotype and a phenotypically healthy child was born.
In a patient with trichorionic triamniotic triplets, the first blood sample for NIPS was taken at 9 weeks and 4 days before chorionic villus sampling. Invasive prenatal diagnosis revealed a normal male karyotype in the 1st and 2nd fetuses (46, XY, 9ph heterochromatic region on the 9th chromosome – a variant of normal polymorphism), and trisomy on chromosome 21 (47,XX,+21) was detected in the 3rd fetus. According to NIPS, a high risk of trisomy of chromosome 21 was detected. During dynamic monitoring, ultrasound data at 12 weeks and 3 days of pregnancy showed progressive dichorionic diamniotic twins and spontaneous reduction of an abnormal fetus. Repeated blood sampling of the patient for NIPS at this stage of pregnancy also showed a high risk of trisomy 21. The results obtained suggest the presence of a "vanishing twin" effect, since at the time of repeated sampling, the pregnancy progressed with two healthy male fetuses. Pregnancy ended at 34 weeks and 4 days after the birth of phenotypically healthy boys (Table).
Cases with low risk of aneuploidy of chromosomes 21, 18, 13 according to NIPS and pregnancy outcomes
Of the 84 pregnant women with a low risk of aneuploidy 21, 18, 13 according to NIPS, seven pregnancies were prolonged at the time of writing. In two patients with dichorionic diamniotic twins, the NIPS result showed failed twins (at 11 and 14 weeks). In two pregnant women with dichorionic diamniotic twins, prenatal death was diagnosed in one of the fetuses at 16 and 18 weeks. In two pregnant women with monochorionic diamniotic twins, antenatal death of one of the fetuses was diagnosed at 22 weeks. One patient with dichorionic diamniotic twins had spontaneous abortion at 18 weeks. In four patients with monochorionic diamniotic twins, pregnancy was complicated by twin-to-twin transfusion syndrome, and termination of pregnancy occurred in two cases at 16 weeks and in one case at 21 weeks. In one case, after fetoscopy and coagulation of vascular anastomoses of the placenta, antenatal death of one of the fetuses occurred at 20 weeks of gestation. Seventy-three patients gave birth to children without phenotypic evidence of trisomy 21, 18, 13.
Discussion
Russian clinical guidelines for NIPS, published in 2016, state that the use of NIPS is contraindicated in multiple pregnancies [17]. However, the lower sensitivity and specificity for detecting trisomies 21, 18, and 13 in combined screening of twin pregnancies compared with singleton pregnancies [18, 19] necessitates the introduction of more accurate modern methods into practice. Additionally, the risk of miscarriage after invasive manipulation is higher in twin pregnancies than in singleton pregnancies [20, 21].
In 2020, the feasibility of using NIPS in multiple gestations was reflected in the clinical recommendations of several countries and international communities [22-24]. Our experience also demonstrates the possibility of using NIPS in multiple gestations, which requires a revision of the national recommendations.
A systematic review of 997 twin pregnancies published in 2019 showed high accuracy and low false-positive rates for trisomy 21 (98.2% and 0.05 %, respectively) [25]. Similar results have been reported in other laboratories [26, 27].
In our study, we demonstrated almost 100% sensitivity and specificity for detecting trisomy 21 using the NIPS results in multiple pregnancies, although the number of observations was limited. In three pregnant women with dichorionic diamniotic twins and trisomy 21 in one of the fetuses (confirmed by separate amniocentesis), NIPS revealed a high risk of trisomy 21. In addition, a high risk of trisomy 21 was identified in dichorionic diamniotic twins, resulting in the birth of a child with trisomy 21, confirmed by karyotyping of peripheral blood.
In one case of monochorionic diamniotic twins, antenatal death of one fetus occurred after identification of a high risk by NIPS. In this regard, amniocentesis was performed on only one of the co-twins and showed normal results. In this case, despite the supposed origin of the twins from the same zygote, the possible discordance in the number of chromosomes in the fetuses could be explained by mosaicism. Unfortunately, we were unable to confirm this assumption because of the unavailability of placenta after birth.
In a pregnant woman with trichorionic triamniotic triplets, trisomy 21 was detected in one of the fetuses during separate choriocentesis. In this case, NIPS performed before invasive intervention and after spontaneous affected fetal reduction revealed a high risk of trisomy 21.
According to the NIPS, 73 pregnant women with a low risk of aneuploidy of chromosomes 21, 18, and 13 gave birth to phenotypically healthy children.
According to our observations, the average proportion of fetal DNA in pregnant twins was 11.2%, that is, for most women, it exceeded 8.0%.
It should be mentioned that despite the low rate of false-positive results, if a high risk of trisomy 21 is detected in a multiple pregnancy, as in the case of a singleton pregnancy, genetic counseling is indicated to decide on the issue of invasive diagnosis. In addition, genetic counselling is advisable for pregnant women with low fetal fractions, as invasive diagnostics may also be suggested in these cases [28].
Relatively little information is available in the literature regarding the effectiveness of NIPS for detecting trisomies 18 and 13 in multiple pregnancies, largely because of their lower prevalence. In our study, aneuploidy was not observed on chromosomes 18 and 13. However, both syndromes have more pronounced phenotypic features and are easily diagnosed using ultrasonography. Accounting for sex chromosome abnormalities during multiple pregnancies is not recommended in most countries.
It is noteworthy that due to increased obstetric risks in twin pregnancies, NIPS is advisable at early gestational ages (starting from 10 weeks).
References
- Nussbaum R.L., McInnes R.R., Willard H.F. Thompson & Thompson genetics in medicine. 7th ed. Philadelphia: Saunders/Elsevier; 2007.
- Monden C., Pison G., Smits J. Twin Peaks: more twinning in humans than ever before. Hum. Reprod. 2021; 36(6): 1666-73. https://dx.doi.org/10.1093/humrep/deab029.
- Калашников С.А., Сичинава Л.Г. Течение и исходы многоплодной беременности, наступившей при использовании вспомогательных репродуктивных технологий. Акушерство и гинекология. 2020; 10: 71-7. [Kalashnikov S.A., Sichinava L.G. The course and outcomes of multiple pregnancy followed assisted reproductive technology. Obstetrics and Gynecology. 2020; (10): 71-7. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.10.71-77.
- Jenkins T.M., Wapner R.J. The challenge of prenatal diagnosis in twin pregnancies. Curr. Opin. Obstet. Gynecol. 2000; 12(2): 87-92. https://dx.doi.org/10.1097/00001703-200004000-00006.
- Vayssière C., Benoist G., Blondel B., Deruelle P., Favre R., Gallot D. et al.; French College of Gynaecologists and Obstetricians. Twin pregnancies: guidelines for clinical practice from the French College of Gynaecologists and Obstetricians (CNGOF). Eur. J. Obstet. Gynecol. Reprod. Biol. 2011; 156(1): 12-7. https://dx.doi.org/10.1016/j.ejogrb.2010.12.045.
- Benn P., Rebarber A. Non-invasive prenatal testing in the management of twin pregnancies. Prenat. Diagn. 2021; 41(10): 1233-40. https://dx.doi.org/10.1002/ pd.5989.
- Peters H.E., König T.E., Verhoeven M.O., Schats R., Mijatovic V., Ket J.C., Lambalk C.B. Unusual twinning resulting in chimerism: a systematic review on monochorionic dizygotic twins. Twin Res. Hum. Genet. 2017; 20(2): 161-8. https://dx.doi.org/10.1017/thg.2017.4.
- Stevenson R.E., Hall J.G., eds. Human malformations and related anomalies. 2nd ed. Oxford University Press; 2006.
- Spencer K., Nicolaides K.H. Screening for trisomy 21 in twins using first trimester ultrasound and maternal serum biochemistry in a one-stop clinic: a review of three years experience. BJOG. 2003; 110(3): 276-80.
- Porreco R.P., Garite T.J., Maurel K., Marusiak B.; Obstetrix Collaborative Research Network; Ehrich M., van den Boom D., Deciu C., Bombard A. Noninvasive prenatal screening for fetal trisomies 21, 18, 13 and the common sex chromosome aneuploidies from maternal blood using massively parallel genomic sequencing of DNA. Am. J. Obstet. Gynecol. 2014; 211(4): 365.e1-12. https://dx.doi.org/10.1016/j.ajog.2014.03.042.
- Norton M.E., Jacobsson B., Swamy G.K., Laurent L.C., Ranzini A.C., Brar H. et al. Cell-free DNA analysis for noninvasive examination of trisomy. N. Engl. J. Med. 2015; 372(17): 1589-97. https://dx.doi.org/10.1056/NEJMoa1407349.
- Fosler L., Winters P., Jones K.W., Curnow K.J., Sehnert A.J., Bhatt S., Platt L.D. Aneuploidy screening by non-invasive prenatal testing in twin pregnancy. Ultrasound Obstet. Gynecol. 2017; 49(4): 470-7. https://dx.doi.org/10.1002/uog.15964.
- Chen M., Jiang F., Guo Y., Yan H., Wang J., Zhang L. et al. Validation of fetal DNA fraction estimation and its application in noninvasive prenatal testing for aneuploidy detection in multiple pregnancies. Prenat. Diagn. 2019;39(13):1273-82. https://dx.doi.org/10.1002/pd.5597.
- Huang X., Zheng J., Chen M., Zhao Y., Zhang C., Liu L. et al. Noninvasive prenatal testing of trisomies 21 and 18 by massively parallel sequencing of maternal plasma DNA in twin pregnancies. Prenat. Diagn. 2014; 34(4): 335-40. https://dx.doi.org/10.1002/pd.4303.
- Беспалова О.Н., Бутенко М.Г., Пачулия О.В., Глотов А.С., Коган И.Ю. Эффективность неинвазивного пренатального тестирования при многоплодии: систематический обзор и метаанализ. Акушерство и гинекология. 2021; 7: 10-8. [Bespalova O.N., Butenko M.G., Pachulia O.V., Glotov A.S., Kogan I.Yu. The effectiveness of non-invasive prenatal testing in multiple pregnancy: a systematic review and a meta-analysis. Obstetrics and Gynecology. 2021; (7): 10-8. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.7.10-18.
- Khalil A., Rodgers M., Baschat A., Bhide A., Gratacos E., Hecher K. et al. ISUOG Practice Guidelines: role of ultrasound in twin pregnancy. Ultrasound Obstet. Gynecol. 2016; 47(2): 247-63. https://dx.doi.org/10.1002/uog.15821.
- Неинвазивный пренатальный ДНК-скрининг анеуплоидий плода по крови матери методом высокопроизводительного секвенирования. Клинические рекомендации. Акушерство и гинекология. 2016; 6 (Приложение). [Non-invasive prenatal DNA screening of fetal aneuploidies by maternal blood by high-throughput sequencing. Clinical guidelines. Obstetrics and Gynecology. 2016; 6 (Suppl). (in Russian)]. https://dx.doi.org/10.18565/aig.2016.6.recomendations.
- Prats P., Rodríguez I., Comas C., Puerto B. Systematic review of screening for trisomy 21 in twin pregnancies in first trimester combining nuchal translucency and biochemical markers: a meta-analysis. Prenat. Diagn. 2014; 34(11): 1077-83. https://dx.doi.org/10.1002/pd.4431.
- Garchet-Beaudron A., Dreux S., Leporrier N., Oury J.F., Muller F.; ABA Study Group; Clinical Study Group. Second-trimester Down syndrome maternal serum marker screening: a prospective study of 11 040 twin pregnancies. Prenat. Diagn. 2008; 28(12):1105-9. https://dx.doi.org/10.1002/pd.2145.
- Hansen M., Kurinczuk J.J., Milne E., de Klerk N., Bower C. Assisted reproductive technology and birth defects: a systematic review and meta-analysis. Hum. Reprod. Update. 2013; 19(4): 330-53. https://dx.doi.org/10.1093/humupd/dmt006.
- Yukobowich E., Anteby E.Y., Cohen S.M., Lavy Y., Granat M., Yagel S. Risk of fetal loss in twin pregnancies undergoing second trimester amniocentesis (1). Obstet. Gynecol. 2001; 98(2): 231-4. https://dx.doi.org/10.1016/s0029-7844(01)01416-8.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics; Committee on Genetics; Society for Maternal-Fetal Medicine. Screening for Fetal Chromosomal Abnormalities: ACOG Practice Bulletin, Number 226. Obstet. Gynecol. 2020; 136(4): e48-e69. https://dx.doi.org/10.1097/AOG.0000000000004084.
- Palomaki G.E., Chiu R.W.K., Pertile M.D., Sistermans E.A., Yaron Y., Vermeesch J.R. et al. International Society for Prenatal Diagnosis Position Statement: cell free (cf)DNA screening for Down syndrome in multiple pregnancies. Prenat. Diagn. 2020; 41: 1222-32. https://dx.doi.org/10.1002/pd.5832.
- Dungan J.S., Klugman S., Darilek S., Malinowski J., Akkari Y.M.N., Monaghan K.G., Erwin A., Best R.G.; ACMG Board of Directors. Electronic address: documents@acmg.net. Noninvasive prenatal screening (NIPS) for fetal chromosome abnormalities in a general-risk population: An evidence-based clinical guideline of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2023; 25(2):100336. https://dx.doi.org/10.1016/j.gim.2022.11.004.
- Gil M.M., Galeva S., Jani J., Konstantinidou L., Akolekar R., Plana M.N., Nicolaides K.H. Screening for trisomies by cfDNA testing of maternal blood in twin pregnancy: update of The Fetal Medicine Foundation results and meta-analysis. Ultrasound Obstet. Gynecol. 2019; 53(6):734-42. https://dx.doi.org/10.1002/uog.20284.
- Bai T., Liu S., Liu J., Jing X., Deng C., Xia T. et al. Performance of noninvasive prenatal screening in twin pregnancies: a retrospective study of 5469 twin pregnancies. J. Matern. Fetal Neonatal Med. 2022; 35(25): 5999-6007. https://dx.doi.org/10.1080/14767058.2021.1903860.
- Eiben B., Glaubitz R., Winkler T., Teubert A., Borth H. Clinical experience with noninvasive prenatal testing in twin pregnancy samples at a Single Center in Germany. J. Lab. Physicians. 2023; 15(4): 590-5. https://dx.doi.org/10.1055/s-0043-1770066.
- Gregg A.R., Skotko B.G., Benkendorf J.L., Monaghan K.G., Bajaj K., Best R.G. et al. Noninvasive prenatal screening for fetal aneuploidy, 2016 update: a position statement of the American College of Medical Genetics and Genomics. Genet. Med. 2016; 18(10): 1056-65. https://dx.doi.org/10.1038/gim.2016.97.
Received 07.02.2024
Accepted 19.02.2024
About the Authors
Ilya Yu. Barkov, MD, PhD, Head of the Laboratory of Prenatal DNA Screening, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia, 4, Acad. Oparin str., Moscow, Russia, 117997, +7(495)438-24-10, i_barkov@oparina4.ruAnna S. Bolshakova, MD, Geneticist, Department of Clinical Genetics, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia, 4, Acad. Oparin str., Moscow, Russia, 117997, +7(495)438-24-11, a_bolshakova@oparina4.ru
Nana K. Tetruashvili, Dr. Med. Sci., Head of the Department of Pregnancy Loss Prevention and Therapy, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia, 4, Acad. Oparin str., Moscow, Russia, 117997, +7(495)438-14-77, tetrauly@mail.ru
Jekaterina Shubina, PhD in Biology, Head of the Laboratory of Genomic Data Analysis, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia, 4, Acad. Oparin str., Moscow, Russia, 117997, +7(495)531-44-44, e_shubina@oparina4.ru
Andrey Yu. Goltsov, Researcher at the Clinical and Molecular Genetics Department, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia, 4, Acad. Oparin str., Moscow, Russia, 117997, +7(495)531-44-44, a_goltsov@oparina4.ru
Dmitry Yu. Trofimov, Dr. Bio. Sci., Corresponding member of the RAS, Head of the Clinical and Molecular Genetics Department, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia, 4, Acad. Oparin str., Moscow, Russia, 117997, +7(495)438-49-51,
d_trofimov@oparina4.ru



