Adaptation of maternal central hemodynamics in the third trimester of single and multiple pregnancies
Objective. To compare central hemodynamics in non-pregnant women with that in women with singleton and multiple pregnancies, late in the third trimester before delivery.Makarov R.A., Kinzhalova S.V., Mamovich N.V., Markova T.V.
Materials and methods. This study used non-invasive bioimpedance analysis to examine central hemodynamics in 20 non-pregnant somatically healthy women and 100 patients in the third trimester of pregnancy including 60 patients with singleton pregnancies and 40 women with twin pregnancies.
Results. A comparative analysis of the study findings across the groups showed significant differences in the main parameters of central hemodynamics. Patients with multiple pregnancies had significantly lower stroke volume, cardiac index, oxygen delivery index and higher total peripheral vascular resistance than non-pregnant women and women with singleton pregnancies.
Conclusion. When choosing anesthesia and preoperative care for the prevention of intraoperative arterial hypotension and disorders of uteroplacental perfusion, incomplete removal of aortocaval compression in patients with multiple pregnancies should be taken into account.
Keywords
During normal pregnancy, maternal central hemodynamic undergoes changes aimed at increasing the delivery of oxygenated blood to peripheral tissues and the fetus [1, 2]. Understanding maternal physiology is important for selecting the optimal method of anesthesia and preoperative care. It is now widely accepted that cardiac output increases during singleton pregnancy, but the extent and timing of this increase remain the subject of debate [1, 3, 4]. Most studies have shown an increase in maternal cardiac output from early pregnancy to the third trimester [5], but hemodynamic changes during the third trimester are not well understood. Some studies have demonstrated an increase in cardiac output [5], some have found no changes [6], and some studies reported a decrease in cardiac output [7]. Maternal anatomical and physiological changes are believed to be more pronounced in multiple than in singleton pregnancies [8, 9].
In recent decades, the problem of multiple gestations has acquired particular relevance, due to the significant increase in multiple pregnancy rates. So, from 1980 to 2009, the number of multiple births in the United States almost doubled (from 18.9 to 33.2 per 1000 births). The rates of multiple pregnancies in most European countries range from 0.7 to 1.5%. In Russia, it also increased from 2000 to 2012 by 48.6% from 0.7 to 1.1% [10, 11].
Numerous studies suggest that a multiple pregnancy may be considered as a risk factor for adverse maternal and fetal outcomes. It is associated with higher incidence of preterm birth, pre-eclampsia, placental abruption, anemia, fetal growth restriction, fetal demise resulting in higher rates of perinatal morbidity and mortality compared with singleton pregnancies [10, 12–14].
Multiple gestation pregnancy can pose increased demands on the woman’s body, since all organs and systems in this state function with greater stress than during a singleton pregnancy. Besides, multiple pregnancy may contribute to developing aortocaval compression syndrome, which also increases the potential risk of severe arterial hypotension during neuraxial anesthesia [15].
Given the limited data on changes in the maternal cardiovascular system in multiple and singleton pregnancies and that in non-pregnant women, a comparative study of maternal hemodynamics in both singleton and twin pregnancies before delivery is necessary.
Material and methods
This study examined central hemodynamics in 20 non-pregnant somatically healthy women and 100 patients in the third trimester of pregnancy. All pregnant women underwent cesarean section at the USRIMCC of Minzdrav of Russia from 2016 to 2017. Group I (control group) comprised 20 somatically healthy, non-pregnant women to determine the basic hemodynamic parameters for subsequent assessment of changes in hemodynamics associated with singleton and multiple pregnancies. Group II included 60 patients with a normal singleton pregnancy, who gave birth at 39.19 ± 0.2 weeks’ gestation (hereafter, all mean values are given as M ± m). Group III included 40 pregnant women with twin pregnancies at 37.57 ± 0.7 weeks’ gestation. The mean age of women in groups I, II and III did not differ (30.8 ± 1.08, 29.86 ± 1.08, and 31.23 ± 2.25, respectively). The cesarean delivery was performed under spinal anesthesia.
Cesarean section was performed mainly for obstetric indications. The leading indications for abdominal delivery were “combined” indications in 56.7% of cases in group II and 52.5% in group III, which included: traumatic (cervical scar) cervical deformity, complicated obstetric history (COH), pregnancy after IVF, prolonged infertility, large fetus, immature cervix, symphysiopathy, as well as the age of the pregnant women. Cesarean section was performed for the uterine scar (from previous cesarean section or conservative myomectomy) in 27.7% and 30% of cases in group II and III, respectively, and for abnormal fetal presentation (pelvic, foot, breech, transverse, unstable position) in 15.6% and 17.5% in group II in III, respectively.
The first-minute Apgar score among newborns in Group II and III was 7.54 ± 0.11 and 6.48 ± 0.13, respectively; the fifth-minute Apgar score among newborns in Group II and III was 8.22 ± 0.14 and 7.36 ± 0.11, respectively.
Patients with valvular heart disease, arrhythmias, hypertensive disorders, obesity class 2 (BMI ≥ 35) and more were excluded from the study.
Hemodynamic parameters were examined by non-invasive bioimpedance technology using a MARG 10-01 monitoring system (Microlux, Chelyabinsk), which is based on the tetrapolar impedance cardiography. The analyzed parameters included systolic blood pressure (SBP, mmHg), diastolic blood pressure (DBP, mm Hg), mean arterial pressure (MAP, mmHg), heart rate (HR, beats per min -bpm), stroke volume (SV, ml), left ventricular ejection fraction (EF, %), cardiac index (CI, l/min/m2); cardiac output (CO, l/min), oxygen delivery index (IDO2, ml/min/m2); total peripheral vascular resistance (TPVR, dyn/s*cm2). All bioimpedance measurements were taken with patients in a supine position on a roller underneath the right side, and the operating table in a 15–20˚ left lateral tilted position to prevent aortocaval compression syndrome in pregnant participants. The parameters were registered in the beat to beat mode with the calculation of mean of 500 beats.
Statistical analysis was performed using standard computer programs Microsoft Excel. The significance of differences between the values of the indicators was assessed by Student’s t-test. Differences were considered significant at p < 0.05.
Results and discussion
A comparative analysis of the study findings across the groups showed significant differences in the main parameters of central hemodynamics (table).
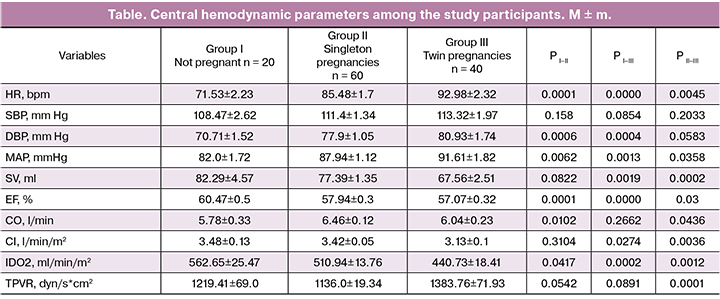
Among pregnant women, heart rate was significantly higher (Group II 85.48 ± 1.7 bpm, p < 0.01; Group III 92.98 ± 2.32 bpm, p <0.01), than in non-pregnant participants (71.53 ± 2.23 bpm. Heart rate was significantly higher (p < 0.01) in multiple pregnancies than in singleton pregnancies. Almost all studies [1–9] reported an increase in heart rate in the second half of pregnancy.
No significant differences between the study groups were observed in systolic blood pressure, trough diastolic blood pressure was significantly higher in pregnant patients, regardless of the number of fetuses. Mean arterial pressure was higher in pregnant women; women with twin pregnancies had a higher MAP than those having singleton pregnancies. Many studies have observed a trend towards lower blood pressure in the first half of pregnancy, followed by an increase to normal values at term [1, 2, 4–6, 7, 9].
Patients in group II and non-pregnant women had similar stroke volume (77.39 ± 1.35 ml and 82.29 ± 4.57 ml, respectively, p = 0.08), whereas it was significantly lower in women with twin pregnancies (67.56 ± 2.51 ml) than in non-pregnant women (p < 0.01) and women with singleton pregnancies (p < 0.01). Most studies have described an increase in stroke volume during pregnancy by 10–15% [1, 3–5]. In multiple pregnancies, this increase was greater than in singleton pregnancies [8, 9]. Reduced stroke volume in multiple pregnancies may be due to incompletely eliminated compression of the inferior vena cava. The ejection fraction was slightly, but significantly lower in the groups of pregnant patients (Group II 57.94 ± 0.3%, p < 0.01; Group III 57.07 ± 0.32%, p < 0.01), compared with non-pregnant women (60.47 ± 0.5%).
Among women with singleton pregnancies cardiac output (6.46 ± 0.12 l/min) was significantly higher than in non-pregnant patients (5.78 ± 0.33 l/min, p < 0.05); CO did not differ between women with twin pregnancies (6.04 ± 0.23 l / min) and non-pregnant women (p = 0.26) and was significantly lower than in women with singleton pregnancies (p < 0.05). An increase in cardiac output during pregnancy has been observed in most studies [1, 3–5], although in some cases it was similar to that in non-pregnant women [6], and some studies even reported a decrease in cardiac output during pregnancy [7]. Cardiac index was significantly lower in twin pregnancies (3.13 ± 0.1 l/min/m2) than in non-pregnant women (3.48 ± 0.13 l/min/m2, p <0.05) and singleton pregnancies (3.42 ± 0.05 l/min/m2, p < 0.01).
The oxygen delivery index decreased significantly among pregnant women (Group II 510.94 ± 13.76 ml/min/m2, p < 0.05, Group III 440.73 ± 18.41 ml/min/m2, p < 0.01) compared with the control (562.65 ± 25.47 ml/min/m2), and was the highest in twin pregnancies (p < 0.01).
Total peripheral vascular resistance decreased during singleton pregnancy (1136.0 ± 19.34 dyne / s*cm2), compared with non-pregnant women (1219.41 ± 69.0 dyne / s * cm2), but not significantly (p = 0.054). In twin pregnancies, TPVR (1383.76 ± 71.93 dyn/s * cm2) was significantly different from that in singleton pregnancies (p <0.01), but did not differ statistically significantly from TPVR in non-pregnant women (p = 0.08).
Conclusion
The findings of this study suggest that despite the measures taken to prevent aortocaval compression (patients in a supine position on a roller underneath the right side and the operating table in a 15–20˚ left laterally tilted position), those measures were insufficient, and in multiple pregnancies the inferior vena cava syndrome manifested by a decrease in stroke volume and cardiac index despite normal blood pressure.
This should be taken into account when choosing anesthesia and preoperative care for the prevention of intraoperative arterial hypotension and disorders of uteroplacental perfusion in patients with multiple pregnancies.
Further intraoperative studies of maternal central hemodynamics in multiple pregnancies are needed with different types of neuraxial blocks (epidural, spinal, combined spinal-epidural) to determine the most appropriate option for this category of patients.
References
- Sanghavi M., Rutherford J.D. Cardiovascular physiology of pregnancy. Circulation. 2014; 130(12): 1003-8. doi: 10.1161/CIRCULATIONAHA.114.009029.
- Ouzounian J.G., Elkayam U. Physiologic changes during normal pregnancy and delivery. Cardiol. Clin. 2012; 30(3): 317-29. doi: 10.1016/j.ccl.2012.05.004.
- Melchiorre K., Sharma R., Thilaganathan B. Cardiac structure and function in normal pregnancy. Curr. Opin. Obstet. Gynecol. 2012; 24(6): 413-21. doi: 10.1097/GCO.0b013e328359826f.
- Ram M., Lavie A., Lev S., Blecher Y., Amikam U., Shulman Y. et al. Cardiac hemodynamics before, during and after elective cesarean section under spinal anesthesia in low-risk women. J. Perinatol. 2017; 37(7): 793-9. doi: 10.1038/jp.2017.53.
- 5. Desai D.K., Moodley J., Naidoo D.P. Echocardiographic assessment of cardiovascular hemodynamics in normal pregnancy. Obstet. Gynecol. 2004; 104(1): 20-9. doi: 10.1097/01.AOG.0000128170.15161.1d
- Volman M.N.M., Rep A., Kadzinska I., Berkhof J., van Geijn H.P., Heethaar R.M., de Vries J.I. Haemodynamic changes in the second half of pregnancy: a longitudinal, noninvasive study with thoracic electrical bioimpedance. BJOG. 2007; 114(5): 576-81. doi: 10.1111/j.1471-0528.2007.01300.x
- San-Frutos L., Engels V., Zapardiel I., Perez-Medina T., Almagro-Martinez J., Fernandez R., Bajo-Arenas J.M. Hemodynamic changes during pregnancy and postpartum: a prospective study using thoracic electrical bioimpedance. J. Matern. Fetal Neonatal Med. 2011; 24(11): 1333-40. doi: 10.3109/14767058.2011.556203.
- Kametas N.A., McAuliffe F., Krampl E., Chambers J., Nicolaides K.H. Maternal cardiac function in twin pregnancy. Obstet. Gynecol. 2003; 102(4): 806-15.
- Kuleva M., Youssef A., Maroni E., Nanni M., Pilu G., Rizzo N. et al. Maternal cardiac function in normal twin pregnancy: a longitudinal study. Ultrasound Obstet. Gynecol. 2011; 38(5): 575-80. doi: 10.1002/uog.8936.
- Сичинава Л.Г. Многоплодие. Современные подходы к тактике ведения беременности. Акушерство, гинекология и репродукция. 2014; 8(2): 131-8. [Sichinava L.G. Multiple fertility. Modern approaches to the tactics of pregnancy. Obstetrics, gynecology and reproduction. 2014; 8 (2): 131-8. (in Russian)]
- Martin J.A., Hamilton B.E., Osterman M.J., Curtin S.C., Matthews T.J. Births: final data for 2013. Natl. Vital Stat. Rep. 2015; 64(1): 1-65.
- Краснопольский В.И., Новикова С.В., Цивцивадзе Е.Б., Жарова А.А. Ведение беременности и родов при многоплодной беременности. Альманах клинической медицины. 2015; 37: 32-40. [Krasnopolsky V.I., Novikova S.V., Tsivtsivadze E.B., Zharova A.A. Conducting pregnancy and childbirth in multiple pregnancies. Almanac of clinical medicine. 2015; 37: 32-40. (in Russian)]
- Fox N.S., Roman A.S., Hastings J., Saltzman D.H., Hourizadeh T., Rebarber A. Blood pressure changes across gestation in patients with twin pregnancies. J. Matern. Fetal Neonatal Med. 2014; 27(9): 898-903. doi: 10.3109/14767058.2013.845660.
- Campbell D.M., Templeton A. Maternal complications of twin pregnancy. Int. J. Gynaecol. Obstet. 2004; 84(1): 71-3.
- Филиппович Г.В., Шифман Е.М. Анестезия операции кесарева сечения при многоплодной беременности. [Filippovich G.V., Shifman E.M. Anesthesia cesarean section for multiple pregnancies. (in Russian)] Available at: http://www.critical.ru/actual/anest/multifruit.htm
Received 17.05.2018
Accepted 22.06.2018
About the Authors
Makarov, Roman A., PhD, senior researcher at the Department of Intensive Care and Emergency Medicine, USRIMCC of Minzdrav of Russia.620028, Russia, Ekaterinburg, Repina str.1. Tel.: +73433598878.
E-mail: r_makarov_ekb@mail.ru. http://orcid.org/0000-0002-8067-5643
Kinzhalova, Svetlana V., MD, associate professor, head of the Department of Intensive Care and Emergency Medicine, USRIMCC of Minzdrav of Russia.
620028, Russia, Ekaterinburg, Repina str.1. Tel.: +73433598878. E-mail: sveking@mail.ru. http://orcid.org/0000-0003-2576-6742
Mamovich, Natalya V., junior researcher at the Department of Intensive Care and Emergency Medicine, USRIMCC of Minzdrav of Russia.
620028, Russia, Ekaterinburg, Repina str. 1. Tel.: +73433598878. E-mail: mamoshka_n@mail.ru. http://orcid.org/0000-0002-1966-2835
Markova, Tatyana V., PhD, senior researcher at the Department of Ecology and Reproduction, USRIMCC of Minzdrav of Russia.
620028, Russia, Ekaterinburg, Repina str. 1. Tel.: +73433598878. E-mail: ta.ma.vl@mail.ru.
For citations: Makarov R.A., Kinzhalova S.V., Mamovich N.V., Markova T.V. Adaptation of maternal central hemodynamics in the third trimester of single and multiple pregnancies. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; (2): 74-7. (in Russian)
http://dx.doi.org/10.18565/aig.2019.2.74-77



