Неинвазивный пренатальный тест (НИПТ), основанный на выделении свободной ДНК плода из крови беременной для выявления наиболее клинически значимых и часто встречающихся генетически детерминированных синдромов (Дауна, Эдвардса, Патау, аномалии половых хромосом), сегодня широко применяется во всем мире. НИПТ впервые был внедрен в клиническую практику в Гонконге в августе 2011 г., с октября 2011 г. тест появился в США и далее начал быстро распространяться в странах Европы. На сегодняшний день уже в 60 странах на 6 континентах проводится НИПТ [1]. В 2013 г. НИПТ стал доступен и в России. В частности, данные исследования активно ведутся в отделе клинической и молекулярной генетики Национального медицинского исследовательского центра акушерства, гинекологии и перинатологии под руководством профессора Д.Ю. Трофимова.
Многие исследования показали высокую чувствительность и специфичность НИПТ и более высокую его эффективность по сравнению со стандартным комбинированным пренатальным скринингом [2–4]. Еще в 2012 г. Nicolaides K. предположил, что существующий комбинированный пренатальный скрининг может быть заменен на НИПТ в 10 недель с последующим ультразвуковым исследованием (УЗИ) в 12 недель [5]. В рекомендациях Американской коллегии акушеров-гинекологов (ACOG) отмечено, что данный метод имеет огромный потенциал [6, 7]. Причем, если поначалу это была исключительно коммерческая услуга, то на сегодняшний день все больше стран включают НИПТ в государственные программы по ведению беременности. Например, в Голландии на сегодняшний день НИПТ стал стандартом скрининга для всех беременных женщин [8]. Другие страны внедрили НИПТ в качестве «контингентного» скрининга, то есть на это исследование направляются не все женщины, а лишь группа высокого риска. Например, в Италии было показано, что при направлении пациенток с уровнем риска >1:1000 по результатам стандартного пренатального скрининга I триместра на НИПТ выявляемость основных трисомий увеличилась на 3%, а количество инвазивных диагностических вмешательств при этом снизилось на 71%. Благодаря этому, общие расходы на ведение беременностей снизились за год на 19 млн евро [9].
Все чаще тема необходимости включения НИПТ в программу государственных гарантий в рамках обязательного медицинского страхования звучит и в России. При этом неизбежно возникают вопросы правового регулирования генетических исследований [10]. На данный момент НИПТ в нашей стране доступен для пациенток только в качестве платной услуги. При этом отечественными авторами неоднократно отмечалось, что при нынешней процедуре пренатального скрининга выявляется лишь около 85% плодов с синдромом Дауна и требуется усовершенствование диагностических мероприятий [2, 11].
Целью нашего исследования была оценка популяционной эффективности НИПТ по выявлению основных анеуплоидий в Российской Федерации с момента его появления в нашей стране.
Материалы и методы
Исследование проводили на базе медико-генетического центра «Геномед» и кафедры акушерства и гинекологии ФПК и ПП и ПФ Уральского государственного медицинского университета. В исследование были включены пациентки, которые прошли процедуру неинвазивного пренатального тестирования в России в период с 01.01.2013 по 31.12.2018 г. (всего 27 845 пациенток). Всем пациенткам, у которых был выявлен высокий риск хромосомных аномалий у плода по данным НИПТ, была рекомендована инвазивная пренатальная диагностика.
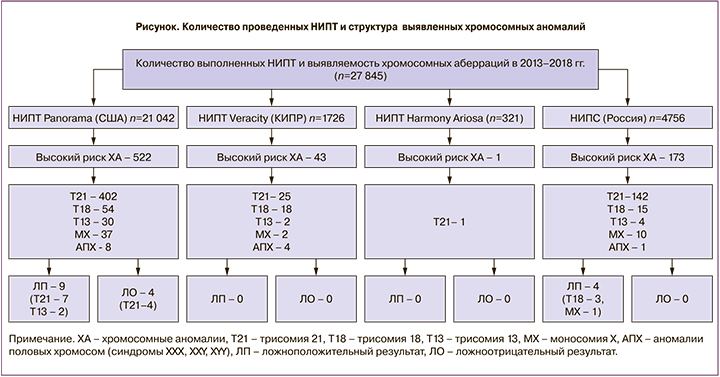
У 21 042 пациенток был сделан тест Panorama (лаборатория Natera, США), у 1726 – тест Veracity (NIPD Genetics, Кипр), у 321 – тест Harmony (Roche, США), у 4756 – НИПС («Геномед», Россия) (см. рисунок).
Математическая обработка данных проводилась с помощью пакета прикладных программ Microsoft Excel 7.0. Качественные показатели представлены в абсолютных и относительных величинах (%). Анализ качественных признаков проводили с помощью таблиц сопряженности с применением критерия χ2. Статистически значимыми считали различия при р<0,05. Для оценки результатов НИПТ рассчитаны чувствительность, специфичность, прогностическая ценность положительного (PPV) и отрицательного (NPV) результатов с указанием 95% доверительного интервала (ДИ).
Результаты и обсуждение
Средний возраст пациенток, направленных на НИПТ, составил 34,2 (3,63) года (M (SD)). Обращает на себя внимание более высокий средний возраст по сравнению с общей популяцией – по данным Росстата, средний возраст рожающих женщин в России составляет 28,4 года. Причем в других странах была отмечена аналогичная тенденция – НИПТ проходят женщины более старшего возраста [12]. Это объяснимо, поскольку в настоящее время, несмотря на то, что ведущими экспертами признано, что НИПТ показан всем беременным женщинам, в основном на него направляются женщины, у которых риск хромосомных аномалий у плода изначально повышен, а этот риск зависит, в том числе, от возраста матери [13].
Средний срок беременности, в котором женщины проходили НИПТ, составил 15 недель 2 дня. Только 6849 (24,6%) пациенток сделали НИПТ в сроке беременности менее 11 недель. Это объясняется тем, что чаще всего в России пациентки изъявляют желание пройти НИПТ после проведения стандартного комбинированного пренатального скрининга I триместра при получении неудовлетворительного или сомнительного результата и нежелании проходить инвазивную пренатальную диагностику – большинство пациенток сдали НИПТ именно в декретированные сроки скрининга I триместра – 11–14 недель (11 506 (41,32%) пациенток). Ряд пациенток информируется о возможности пройти НИПТ при поздней постановке на учет, когда сроки проведения пренатального скрининга I триместра упущены. К сожалению, некоторые пациентки направляются на НИПТ после проведения УЗИ-скрининга II триместра и выявления морфологических маркеров хромосомных аномалий. В данном случае предлагать пациентке НИПТ является ошибкой, поскольку при наличии УЗ-маркеров хромосомных аномалий либо пороков развития пациентка должна быть направлена на инвазивную пренатальную диагностику, и НИПТ не может в данном случае быть заменой регламентированных Минздравом России процедур [2, 6]. Ряд пациенток были направлены на НИПТ даже в III триместре – 52 (0,18%) пациенткам НИПТ был проведен в сроке беременности более 28 недель.
На рисунке показано, какие неинвазивные пренатальные тесты прошли женщины исследуемой группы и какое количество хромосомных аномалий было выявлено.
При использовании различных вариантов НИПТ в России в 2013–2018 гг. было выявлено 739 хромосомных аномалий (2,6% среди всех пациенток). Больший удельный вес хромосомных аномалий в этой группе (по сравнению с общей популяцией) объясняется тем, что часто на НИПТ направляют пациенток, у которых уже был выявлен высокий риск по результатам комплекса пренатальной диагностики I триместра (о чем было сказано выше), либо пациенток, у которых риск изначально оценивался как высокий, например, из-за возраста или рождения детей с хромосомными аномалиями в анамнезе. Только 8867 (31,8%) пациенток выбрали НИПТ в качестве теста первой линии.
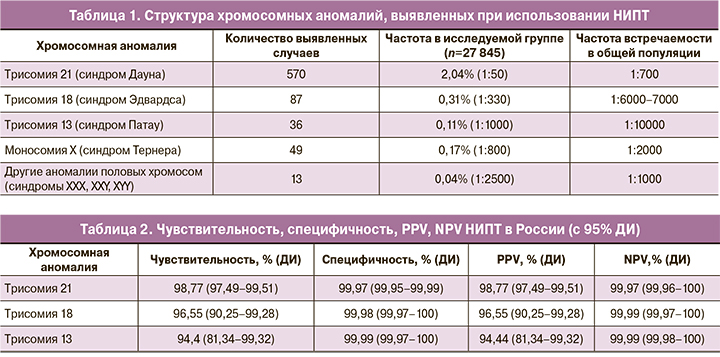
Структура выявленных хромосомных аномалий представлена в табл. 1.
Все пациентки, у которых по результатам НИПТ был определен высокий риск хромосомной аномалии (ХА), были направлены на инвазивную пренатальную диагностику. В 4 (0,5%) случаях из 739 беременность прервалась до получения пациенткой результата теста, о 2 (0,25%) пациентках информации о результатах инвазивной пренатальной диагностики нет, 733 (99,18%) пациенткам была проведена инвазивная пренатальная диагностика, наличие ХА у плода не подтвердилось в 12 (1,63%) случаях. В 9 случаях ложноположительный результат был получен при использовании теста Panorama: 7 ложноположительных случаев трисомии 21 и 2 ложноположительных случая синдрома Патау. В 1 случае тест Panorama показал высокий риск по синдрому Патау (трисомия 13), а по результатам цитогенетического исследования биологического материала, полученного при амниоцентезе, был установлен синдром Эдвардса (трисомия 18). В 4 случаях ложноположительный результат был получен при использовании теста НИПС – в 3 случаях была определена трисомия 18 хромосомы, которая при проведении инвазивной диагностики не подтвердилась, в 1 случае тест показал высокий риск по синдрому Тернера, а по УЗИ во II триместре был выявлен мужской пол у плода (от проведения инвазивной диагностики пациентка отказалась). Кроме этого, при использовании теста Panorama в 4 случаях был получен ложноотрицательный результат, то есть тест продемонстрировал низкий риск по всем определяемым хромосомным аномалиям, на самом деле у плодов был аномальный кариотип. Все эти 4 случая были связаны с наличием трисомии 21 у плодов. В 2 случаях во II триместре по УЗИ были выявлены маркеры хромосомных аномалий у плода, в связи с чем пациентки, несмотря на результат НИПТ, были направлены на инвазивную пренатальную диагностику, в результате которой был выявлен синдром Дауна у плода, и беременность была прервана, в 2 случаях родился ребенок с синдромом Дауна. Ложноотрицательные результаты для теста Panorama составили 0,018% среди всех отрицательных результатов. Если рассчитывать долю ложноотрицательных результатов среди всех вариантов тестов, то количество их составило 0,014%.
Мы рассчитали общие показатели чувствительности, специфичности, прогностической ценности положительного результата (PPV) и прогностической ценности отрицательного результата (NPV) для всех тестов (рассчитать данные показатели для отдельных тестов мы не смогли, поскольку, если делить исследуемую группу на 4 части, выборка по каждому тесту окажется слишком мала, чтобы делать обоснованные выводы). Результаты представлены в табл. 2. Нам не удалось получить информацию об исходах беременности у пациенток, у которых НИПТ показал высокий риск аномалий половых хромосом, поэтому для этой группы рассчитать показатели эффективности теста мы также не смогли. Кроме того, неизвестно, у скольких родившихся детей на самом деле были аномалии половых хромосом, поскольку эти аномалии (за исключением синдрома Тернера) не имеют специфической клинической картины у новорожденных детей и диагностируются поздно, в подростковом возрасте или у взрослых, либо случайно выявляются при анализе кариотипа. В нашем исследовании частота аномалий половых хромосом (за исключением синдрома Тернера) у женщин, прошедших НИПТ, оказалась ниже, чем в общей популяции, что косвенно свидетельствует о достаточно низкой чувствительности НИПТ в отношении этой группы заболеваний.
В научной литературе описан ряд причин, по которым возможны ложные результаты НИПТ: недиагностированная двойня с гибелью одного из эмбрионов в раннем сроке беременности, плацентарный мозаицизм, наличие мозаицизма у матери, злокачественные новообразования у беременной женщины [2, 14–17].
Нам удалось расследовать 1 ложноотрицательный случай диагностики синдрома Тернера и 1 случай синдрома Патау.
В первом случае пациентка А., 24 года, прошла комплекс комбинированной пренатальной диагностики I триместра в сроке беременности 13 недель 6 дней. По результатам УЗИ выявлено: срок беременности 13 недель 6 дней, толщина воротникового пространства 2,45 мм, яремные мешки расширены до 2,3 мм, носовая кость визуализируется, сердцебиение плода 169 в минуту, пороков развития не обнаружено. Уровни хорионического гонадотропина человека (ХГЧ) и ассоциированного с беременностью протеина-А плазмы (РАРР-а) соответствовали 1,75 Мом и 0,29 Мом. По синдрому Дауна индивидуальный риск был определен 1:101, по синдрому Эдвардса – 1:12 224, по синдрому Патау – 1:63. От проведения инвазивной диагностики пациентка отказалась, и через несколько дней после проведения стандартного пренатального скрининга I триместра сделала НИПТ (НИПС, Россия). Был выявлен высокий риск по синдрому Тернера, пол плода – женский, уровень фетальной фракции – 7,11%. Поскольку серьезных пороков развития по УЗИ выявлено не было, а прогноз для жизни и здоровья при синдроме Тернера относительно благоприятный, к вопросу о проведении инвазивной пренатальной диагностики пациентка решила вернуться после УЗ-скрининга II триместра. При УЗ-скрининге II триместра в 19 недель был обнаружен плод мужского пола, пороков развития не выявлено, размеры плода соответствуют сроку гестации. При проведении УЗ-скрининга III триместра, в 31–32 недели беременности, также визуализировался плод мужского пола, без видимых аномалий развития, но был выявлен синдром задержки роста плода (ЗРП) 1 степени. В 38–39 недель пациентка самостоятельно родоразрешилась живым доношенным мальчиком весом 2600 г, с оценкой по школе Апгар 7/8 баллов. При рождении были выявлены врожденные пороки развития наружных половых органов у плода: гипоспадия полового члена, яички в мошонке, нормальных размеров. В роддоме был взят кариотип, результат – 46,Х,r(Y)/45,X. Далее было проведено УЗИ брюшной полости, почек, сердца ребенка – иных пороков развития не выявлено.
Во 2 случае пациентка Б., 39 лет, в сроке беременности 10 недель 4 дня сдала НИПТ (НИПС, Россия). По результатам исследования был выявлен высокий риск трисомии 13 (синдрома Патау) у плода, пол плода – мужской, фетальная фракция – 5%. В 11 недель 5 дней был проведен УЗ-скрининг I триместра, отклонений от нормы не выявлено (плод соответствует сроку беременности, толщина воротникового пространства 1,9 мм, носовая кость визуализируется, данных за наличие пороков развития не получено). В 18 недель беременности пациентка была направлена на амниоцентез, определен кариотип плода – 46,XY – нормальный мужской кариотип. В сроке беременности 39–40 недель пациентка самостоятельно родоразрешилась живым доношенным мальчиком без видимых внешних аномалий, с весом 3420 г, с оценкой по шкале Апгар 8/9 баллов. Ребенку был проведен хромосомный микроматричный анализ – выявлена мозаичная дупликация длинного плеча 20 хромосомы, размером 197 896 п.н. с неопределенным клиническим значением (молекулярный кариотип – 46,XY, arr[hg19] 20q13.33(62032988_62230974)x1). Для уточнения происхождения данной перестройки и ее клинического значения было рекомендовано обследование родителей, от которого они отказались. Плацента также была направлена на хромосомный микроматричный анализ. Обнаружена мозаичная трисомия 13 хромосомы, уровень мозаицизма около 80%.
У 1322 (4,7%) пациенток не удалось получить результат НИПТ при первичном исследовании по причине низкой фетальной фракции. Согласно данным других исследователей, повторный забор анализа требуется в 3–6% случаев [2, 3, 6, 18, 19].
В данном исследовании при использовании НИПС частота нерезультативных исследований составила 3,7% (157 пациенток). Это существенно меньше, чем при использовании тестов Panorama, Veracity и Harmony Ariosa, для которых данный показатель составил соответственно 4,9% (n=1024), 5,4% (n=94), 8,4% (n=27) – различия статистически достоверны для всех тестов (р<0,05). Наиболее высокая частота повторного забора анализа потребовалась у пациенток, которым был проведен тест Harmony Ariosa.
На повторную сдачу анализа согласились 1136 пациенток. Из них у 909 (80,1%) удалось со 2-го раза получить результат. Исходя из полученных данных, мы считаем, что в случае, если при первой сдаче НИПТ у пациентки определяется низкий уровень фетальной фракции и тест нерезультативный, ей следует предложить сдать НИПТ повторно.
Заключение
В России НИПТ чаще всего используется в качестве теста второй линии у пациенток с высоким риском хромосомных аномалий по результатам пренатального скрининга I триместра, поэтому количество хромосомных аномалий у плодов пациенток, прошедших НИПТ, превышает таковое в общей популяции.
НИПТ является более точным методом исследования, чем стандартный комбинированный пренатальный скрининг I триместра, и обладает высокой чувствительностью и специфичностью в отношении трисомий 13, 18 и 21 хромосом.
У 3,7–8,4% пациенток не удается получить результат НИПТ при первичном исследовании по причине низкой фетальной фракции, однако у 80,1% из них повторное исследование будет результативным.



