Fetal lymphangiomas of various locations. The Center’s 14-year experience
Aim. To improve the intrauterine diagnosis of fetal lymphangiomas at various locations and assess postnatal outcomes.Mashinets N.V., Demidov V.N., Dorofeeva E.I., Podurovskaya Yu.L., Panin A.P., Nikiforov D.V., Filippova E.A., Kulabukhova E.A.
Materials and methods. Between 2006 and 2019, 54 fetal lymphangiomas were diagnosed. The most common were lymphangiomas of the neck (51.8%), abdomen (11.1%), mediastinum (11.1%), axillary region (9.3%), and thigh and lower leg (9.3%). Less common were lymphangiomas of the chest and anterior abdominal wall (5.6%) and multiple lymphangiomas (1.9%).
Results. Ultrasound visualization of lymphangiomas showed masses of various sizes with multiple septations filled with fluid and without vascularization zones. The pregnancy resulted in a live birth in 50 cases; 49 infants were referred to the neonatal surgery for further treatment, and in one fetus the tumor disappeared before birth. Three patients underwent termination of pregnancy. There was one intrapartum fetal death. Thirty-three infants underwent surgery with favorable outcomes.
Conclusion. Ultrasound imaging is useful for the in utero diagnosis of such a rare pathology as fetal lymphangioma. In the absence of concomitant pathology, the prognosis for the newborn is favorable.
Keywords
Fetal lymphangioma is an uncommon congenital malformation of the lymphatic system characterized by thin-walled cystic dilations of various sizes, lined with endothelium and filled with chyle. It is believed that lymphangiomas result from complete obstruction of the lymphatic sacs, preventing communication with the venous system and causing large multilocular cysts [1–4].
Epidemiological studies have reported the prevalence of fetal lymphangiomas to be 1 per 6000 newborns and account for 10-12% of all benign tumors in children [1-6]. However, if pregnancy terminations are included, the prevalence is 1: 750–875 [2, 7, 8].
Histologically, lymphangiomas are divided into three types like simple, cavernous, and cystic lymphangiomas. Simple lymphangioma is formed from lymphatic capillaries and located in limited skin and subcutaneous tissue areas. Cavernous lymphangiomas consist of larger lymphatic vessels. They are unevenly filled with lymph and formed from a connective tissue spongy base containing smooth muscle fibers, an elastic frame, and small lymphatic vessels lined with epithelium. Cystic lymphangiomas are composed of cysts of few millimeters to several centimeters in diameter, which can communicate with each other. Their inner surface is lined with endothelium, and their walls contain dense connective tissue. Cavernous and cystic lymphangiomas are most common, and both of these types can occur in the same tumor [2, 7–11].
Lymphangioma can have different locations. However, 75-80% of the fetal lymphangiomas are located in the neck, axillary region (20%), abdominal cavity and retroperitoneum (2%), limbs and bones (2%), and only 1% in mediastinum [2, 5, 6, 8-10].
Fetal lymphangiomas located in the neck are also known as cervical hygromas. Cervical hygromas develop in the early stages of pregnancy, and in more than 50% of cases, are associated with chromosomal and structural abnormalities. Most often, cervical hygroma co-occurs with Trisomy 21, Turner's syndrome, trisomy 18, trisomy 13, triploidy, and Noonan's syndrome [2].
In 77.8% of cases, cervical hygroma has adverse pregnancy outcomes [2, 7]. Only in 16.7–22.2% of cases, the tumors are resolved, and infants born without congenital anomalies. The development of hydrops fetalis also associated with an adverse outcome [2, 7].
Ultrasound findings of cystic hygroma include thin-walled and serpiginous or multilocular intradermal fluid collections, which are often found at posterior to and lateral cervical regions. Doppler mapping shows no blood flow. The tumor size can vary from several millimeters to several centimeters [8, 12]. Differential diagnosis should include encephalocele, neck teratoma, epidermal cyst of the head's posterior surface, and cystic heterotopy of the brain [8, 12].
For the management of fetal cystic hygroma, the first common step is a cytogenetic study for suspected aneuploidy. Detailed and serial ultrasound examination for follow-up of the tumor's growth is necessary because massive cystic hygroma may cause dystocia, neonatal airway compression, or feeding problems.
Z. Mikovic et al. [13] reported successful intrauterine treatment of two patients with large fetal neck lymphangioma, which was performed at 28 weeks of gestation. Aspiration of fluid in cystic hygroma and injection of the same volume of OK-432 (Picibanil) were performed. No treatment was needed after birth. If there is a risk of airway obstruction after birth, an ex utero intrapartum treatment is recommended [14].
Postnatal management includes surgical excision and direct injection of sclerosing agents.
Axillary lymphangioma accounts for 20% of cases. Unlike neck lymphangioma, less common tumor locations manifest in the middle of pregnancy. Some authors believe that they are not associated with chromosomal abnormalities [4, 6, 11, 15]. However, given the small number of observations, some researchers [8, 16] recommend karyotyping because the risk of chromosomal abnormalities has not been precisely established. A. Reichler and M. Bronshtein [16] note that 3 out of 5 cases of axillary lymphangioma had a pathological karyotype.
Some literature reports isolated clinical observations of large axillary lymphangiomas measuring 6–14 cm, some of which extend to the thoracoabdominal region. In all cases, the fetal karyotype was normal [2–4, 15, 17]. In 6 out of 9 cases, there was successful postnatal surgery. In two cases, pregnancies were terminated at the parents' request, and in one case, pregnancy ended in intrauterine fetal death.
Prenatal diagnosis of axillary lymphangioma is possible in the II–III trimester of pregnancy. It is based on detecting hypoechoic multilocular cysts with thin septa in the axillary region and no flow on Doppler mapping. The size of the masses can vary from a few millimeters to gigantic sizes. Differential diagnoses should include hemangioma, teratoma, and Klippel-Trenone syndrome [2, 6, 11, 18].
The delivery mode depends on the tumor size. While small lymphangiomas do not affect the delivery mode, large masses can result in complicated deliveries involving dystocia, trauma, and infection. Therefore, this may be an indication for delivery by cesarean section. It should also be noted that delivery should be performed in a maternity hospital with the ability to provide adequate intrapartum and postnatal newborn resuscitation [3, 4, 6, 15].
The prognosis of lymphangiomas depends, first of all, on the presence of co-occurring malformations and chromosomal abnormalities. In their absence, the prognosis is generally favorable. In axillary lymphangioma, the depth of invasion has an important prognostic role [3, 6, 11]. Postnatal surgical resection of the tumors is indicated. As a rule, axillary lymphangiomas are well isolated from the surrounding tissues by surgery, compared with cervical localization. Surgery is not associated with significant risks of damage to critical anatomical structures. The recurrence rate is 10-15%, which is associated with the technical possibility of complete resection of pathological tissue.
Abdominal lymphangioma occurs in 2% of cases. Its diagnosis becomes possible at the end of the II and the beginning of the III trimester of pregnancy. On ultrasound, it is usually visualized as a multilocular cystic formation not involving abdominal organs. Most often, abdominal lymphangiomas arise from the small intestine mesentery and retroperitoneal space. The prognosis for abdominal lymphangioma is favorable in most cases. However, in cases of a combination of lymphangioma with ascites or when it spreads to other parts of the body, the prognosis is poor. Therefore, in cases of abdominal lymphangioma, a thorough examination of the fetus is necessary to exclude concomitant diseases and malformations [12, 19, 20].
P. Deshpande et al. [20] reported that left-sided abdominal lymphangioma was most common. Possible prenatal complications of this pathology include hydrops fetalis, ascites, and polyhydramnios.
Solitary lymphangiomas, even of large sizes, can be removed entirely. The surgical treatment of multiple lesions is rather complicated and is associated with a high mortality rate [20].
Due to this pathology's rarity, currently, only individual cases of lymphangiomas are described in the literature.
Observations presented in the literature indicate that there are three possible outcomes for fetal abdominal lymphangiomas. The first is the postnatal surgical removal of the lesion, even of large sizes (up to 9.0 cm), which has a favorable outcome [21]. The second involves the rapid growth of lymphangioma and its spread to other parts of the body and the development of multiple lesions [20, 22]. In such cases, the prognosis of pregnancy is usually poor.
In sporadic cases, spontaneous intrauterine resolution of lymphangioma is possible [23, 24].
Some authors believe that abdominal lymphangioma is a consequence of lymphatic obstruction resulting from intestinal ileus. Others suggest that intestinal ileus, impaired vascularization, and ascites develop due to intestinal displacement by a bulky mass [25].
Limb lymphangioma is extremely rare and accounts for 2% of all lymphangiomas. The literature contains only a few observations of this location of lymphangioma. E.R. Sung [26] presented the observation of a lymphangioma detected at 28 weeks of gestation and was located in the right half of the abdominal cavity with the right thigh and lower leg involvement. The pregnancy was terminated due to a poor prognosis.
Isolated mediastinal lymphangioma is an extremely rare pathology accounting for 0.7–4.5% of all tumor-like lesions of the mediastinum and 1% of all lymphangiomas [27]. Seventy-five percent of mediastinal lymphangiomas are diagnosed in adulthood. Although mediastinal lymphangioma is usually asymptomatic, symptoms can arise secondary to compression of adjacent tissues and organs [5, 27, 28].
Prenatal diagnosis of isolated mediastinal lymphangioma is extremely rare. Currently, only 12 case reports are presented in the English-language literature [5, 27–29]. Mediastinal lymphangiomas are diagnosed at 20–31 weeks of pregnancy. Lymphangiomas were located in the anterior (n=10) and posterior (n=2) mediastinum. The dimensions varied on average from 2.0 to 4.5 cm. In one case, the lesion underwent spontaneous resolution, in five sizes did not change during pregnancy. In six patients, lesion growth was observed, resulting in 3 instances of hydrops fetalis and one postnatal death. In 10 infants, complete or partial surgical resection of the tumor was performed before one year of age with a favorable outcome. One baby was treated with expectant management due to the absence of clinical symptoms.
There was no combination of genetic abnormalities and mediastinal lymphangioma [7].
Differential diagnosis of mediastinal lymphangioma should include congenital diaphragmatic hernia, teratoma, thymoma, neurogenic tumor, hemangioma, pulmonary sequestration, bronchogenic cysts, and cystic-adenomatous lung malformation [5, 27–29].
Prenatal diagnosis of mediastinal lymphangiomas becomes possible in the second trimester of pregnancy. This pathology's main ultrasound features include an asymmetrically located thin-walled chest lesions, consisting of many cysts of varying sizes filled with homogeneous anechoic contents and separated by thin septa with no blood flow [5, 10, 27–29].
By location, mediastinal lymphangiomas are classified as anterior and posterior, of which the most common are lymphangiomas of the anterior mediastinum [5, 28]. The dimensions of these lesions are extremely variable and range from a few millimeters to several centimeters. In most cases, lymphangiomas' growth is slow; an increase in growth rate may be caused by bleeding or infection [5].
Large lesions can cause pulmonary hypoplasia, displacement of the heart and mediastinum, and large vessels' compression, resulting in ascites and hydrops fetalis [5, 27].
Postnatal surgical resection of the lymphangioma is performed after additional examination, but in some cases, technical difficulties may arise due to the proximity of large vessels and airways [5, 7, 27, 28].
The prognosis for mediastinal lymphangioma depends on the location, size, and the presence of co-occurring anomalies [28].
Due to a lack of literature reports on lymphangiomas of various locations, we decided to present our data.
Materials and methods
We analyzed 54 cases of fetal lymphangiomas of various locations diagnosed at the Department of Functional Diagnostics, V.I. Kulakov NMRC for OG&P from 2006 to 2019. During this period, we published four articles about various locations of lymphangiomas [30–33].
Baseline examination included measuring biparietal and occipitofrontal diameter of the fetal head, the interhemispheric cerebellar size, mean abdominal circumference, mean transverse dimension of the heart, the length of the shoulder, thigh, tibia, and foot to establish the gestational age, weight, and growth of the fetus. Then the location and size of the placenta were determined. Particular attention was paid to the state of internal organs and other fetal anatomical structures. Upon detecting a lesion in the fetus, its location and size were determined, and the internal structure was assessed.
We also performed dynamic ultrasound monitoring of fetuses and changes in the size and structure of the lesions during the entire gestation and postnatally.
Ultrasound examination was performed with a Siemens SONOLINE Elegra ultrasound system (Germany) and Alfa 10, Aloka (Japan) using a transabdominal 3.5MHz convex probe and a 7.5 MHz linear probe. All patients also underwent Doppler sonography and cardiotocography using a fully automated cardiac monitor («Fetal Condition Analyzer») from Unicos (Moscow).
Results
We observed 54 fetuses with lymphangioma of various locations. After birth, 49 newborns were admitted to the Department of Neonatal Surgery for further examination and treatment. Surgery was performed in 33 infants (67%), of which three underwent sclerotherapy and one both surgery and sclerotherapy.
Three women underwent pregnancy termination for medical reasons, intrapartum fetal death occurred in one case, and in one case, the lesion underwent spontaneous resolution.
The diagnosis of fetal lymphangioma was correctly determined in all women at 18-37 weeks gestation during the first consultation at the Center.
Ultrasound findings of lymphangioma included thin-walled and multilocular fluid collections without zones of vascularization. By location, the most common was lymphangioma of the neck [n=28 (51.8%)], followed by abdominal [n=6 (11.1%)], mediastinum [6 (11.1%)], axillary [n=5 (9.3%] ), thighs and lower limbs [n=5 (9.3%)], chest and anterior abdominal wall [n=3 (5.6%)], and multiple hemlimphangiomas (Proteus syndrome) [n=1 (1.9%)].
As noted above, neck lymphangioma accounted for about half of all cases – 28 (51.8%). Of these, in 2 (7.1%) observations, the lesion also extended to the submandibular region, in 3 (10.7%) to the head, in 1 (3.6%) to the axillary region, in 3 (10.7%) ) on the chest wall, and 1 (3.6%) in the mediastinum. Two newborns had giant lymphangioma of the neck, measuring more than 10 cm in diameter (Fig. 1). In one of these observations, the child was also diagnosed with congenital anemia due to massive prenatal hemorrhage into the cystic cavity. All newborns were admitted to the Department of Neonatal Surgery and underwent additional examination.
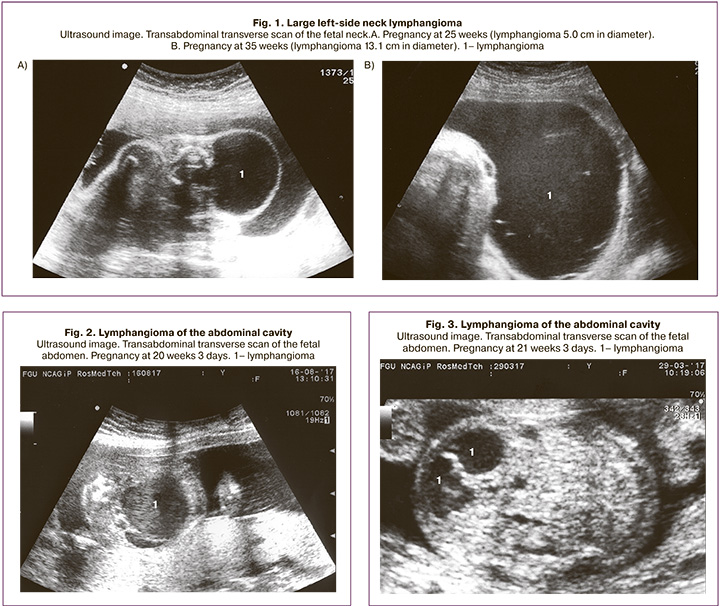
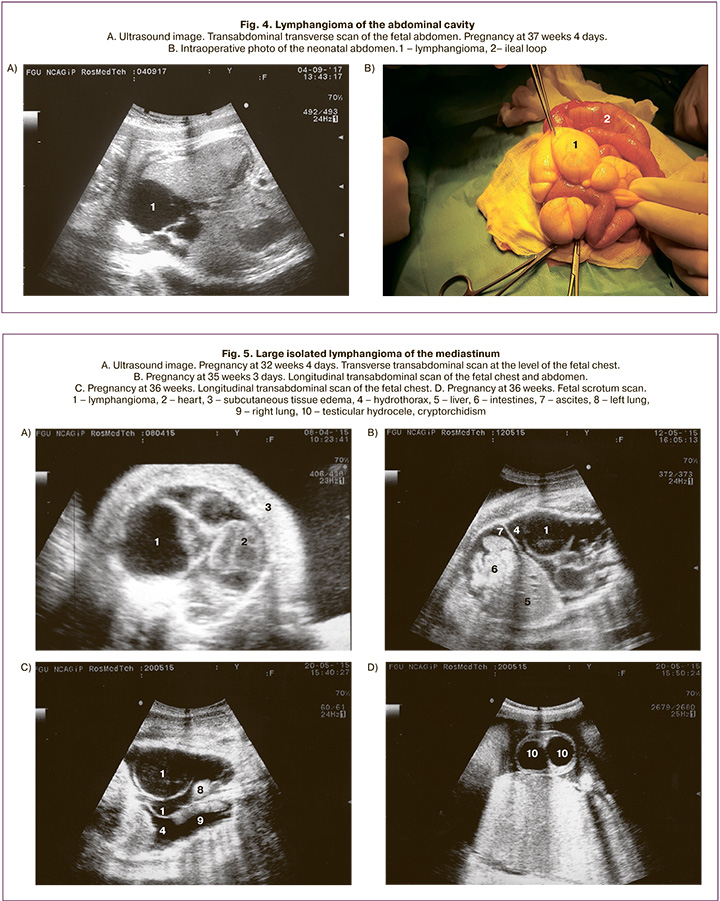
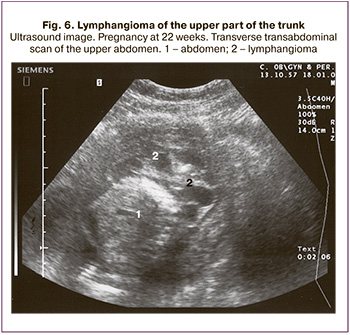
Surgery for giant and large lymphangiomas of the neck was performed in 23 infants (82%), of which two underwent sclerotherapy and one both surgery and sclerotherapy. According to follow-up data, cosmetic and functional treatment outcomes were favorable. Five newborns had no surgery due to small lesions and were sent for routine examinations to specialized institutions. Weobservedsixpatientswithabdominallymphangioma (11.1%). The gestational age at the time of detection ranged from 20 to 37 weeks. Of these, four women had full-term deliveries, and the newborns were admitted to the Department of Neonatal Surgery, where all of them underwent surgery with favorable outcomes. At the same time, one baby underwent laparoscopic surgery. One patient underwent pregnancy termination for medical reasons at 20 weeks of pregnancy (the diameter of the abdominal lymphangioma was 3.7 cm; the fetus was also diagnosed with left kidney cystic dysplasia) (Fig. 2). In one case, a lesion underwent spontaneous resolution at 25 weeks gestation (at 21 weeks, the diameter was 2.4 cm) (Fig. 3).
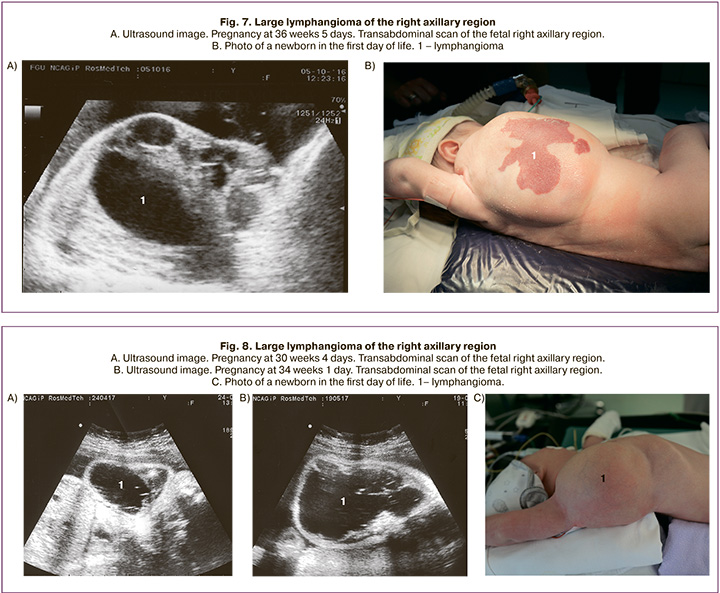
Large fetal abdominal lymphangioma was detected at 37 weeks of gestation. The tumor measuring 6 cm was found in the lower parts of the fetal abdominal cavity (Fig. 4). The pregnancy ended in spontaneous full-term delivery. On the 13th day of life, the newborn underwent surgery. Intraoperatively, the tumor measuring 8.5 cm was located at the base of the ileum's mesentery, which was in a state of ileus. The lymphangioma with a supporting loop of the ileum was resected, and direct ileo-ileal anastomosis was performed. The child was discharged home in satisfactory condition on the 16th postoperative day.
Mediastinal lymphangioma was detected in 6 fetuses (11.1%). Five of them were delivered at full term, and the newborns were transferred to the Department of Pediatric Surgery. Two of them underwent thoracoscopic surgery with favorable outcomes.
One case of large lymphangioma ended in intrapartum fetal death. In this case, the diagnosis was made at 32 weeks gestation. Ultrasound findings in the fetus’s chest on the left side included thin-walled and multilocular anechoic fluid collections without blood flow. The largest chambers were 6.0x4.9x5.7 cm and 2.4x2.7x2.8 cm. The heart was displaced to the right. The lungs were hypoplastic, measuring 2.0x1.2x1.5 cm and 3.1x1.4x1.9 cm on the right and left side, respectively. The fetus also had hydrothorax, mild ascites, subcutaneous fat edema of the trunk, and the stomach was reduced in size (Fig. 5). This patient was monitored with serial ultrasound scans at 34, 35, and 36 weeks of pregnancy. The ultrasound image and size of the lymphangioma did not change. Starting from 35 weeks of pregnancy, along with the above changes, cardiomegaly, edema of the head, subcutaneous fat of the trunk and limbs, testicular hydrocele, and cryptorchidism were revealed. Starting from 36 weeks of pregnancy, Doppler sonography showed impaired fetoplacental blood perfusion; the uteroplacental blood flow was not changed. According to the automated antenatal cardiotocography data, carried out at 36 weeks of 2 days of pregnancy, the fetal status indicator was 4.0, which corresponded to a critical condition. Immediately after cardiotocography, the patient underwent cesarean delivery. A dead preterm boy weighing 5094 g and a height of 53 cm was born. Ultrasound findings were consistent with the pathological diagnosis.
 Axillary lymphangioma was diagnosed in 5 fetuses (9.3%) at 22 to 37 weeks of gestation. Pregnancy ended in full-term delivery in 4 cases; the children were admitted to the Department of Neonatal Surgery and underwent surgery with favorable outcomes. In one case, pregnancy was terminated at 22 weeks of gestation (the lymphangioma was located in the upper chest and spread to the right arm and abdomen of the fetus; its diameter was 6.1 cm, and a 2.0 cm abdominal lymphangioma was also detected) (Fig. 6).
Axillary lymphangioma was diagnosed in 5 fetuses (9.3%) at 22 to 37 weeks of gestation. Pregnancy ended in full-term delivery in 4 cases; the children were admitted to the Department of Neonatal Surgery and underwent surgery with favorable outcomes. In one case, pregnancy was terminated at 22 weeks of gestation (the lymphangioma was located in the upper chest and spread to the right arm and abdomen of the fetus; its diameter was 6.1 cm, and a 2.0 cm abdominal lymphangioma was also detected) (Fig. 6).
Two patients had giant fetal axillary lymphangioma detected at 37 and 31 weeks of gestation. Ultrasound showed a mass filled with fluid with multiple septations of various thickness located in the fetal right axillary region. Its upper pole was found under the neck, and the lower one reached the lumbar area. The dimensions of the formation were 10.1x8.3x8.4 cm (volume 368.3 cm3) (Fig. 7). In the second case, lymphangioma was seen as an anechoic lesion with several thin septa measuring 6.0x6.0x3.3 cm (volume 62.1 cm3) located from the right upper cervical region to the lower edge of the chest (Fig. 8). At 39 weeks gestation, a rapid increase in lymphangioma growth rate was observed, which amounted to 11.4x9.4x9.4 cm (volume 526.8 cm3).
Pregnancy in both cases ended in cesarean delivery at term. After birth, the newborns were admitted to the Department of Surgery and Neonatal IC for further examination and treatment.
The newborns underwent radical resection of right axillary lymphangioma of the region on the 18th and 11th day of their lives.
The postoperative period in both cases was uneventful. Children on the 15th postoperative day (33rd day of life) and 13th postoperative day (24th day of life) were discharged home in a satisfactory condition.
The thigh and lower leg's lymphangioma was detected in 5 fetuses (9.3%) at gestational ages from 21 to 34 weeks. Four pregnancies ended in full-term delivery. Abortion was performed in one case at 22 weeks gestation (6.3 cm lymphangioma occupied the lower abdomen and small pelvis, extended to the perineum, genitals, and right thigh) (Fig. 9).
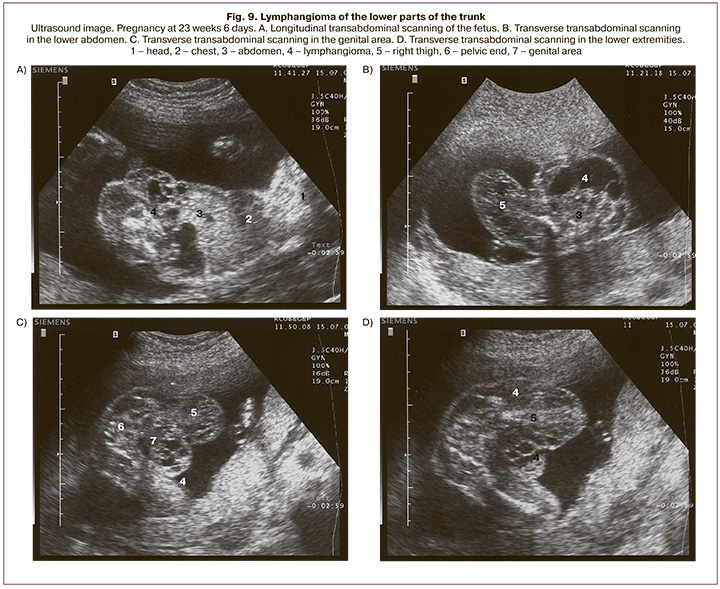
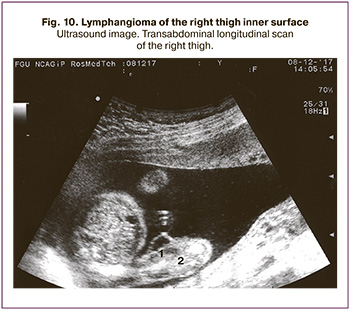
Small femur lymphangioma was found in one observation at 21 weeks gestation. Ultrasound showed a 1.2 cm fluid-filled mass without visible septations located in the right thigh's inner surface. By the end of pregnancy, the diameter of the lymphangioma was 4.0. The pregnancy ended in full-term delivery with a favorable outcome (Fig. 10).
 Discussion
Discussion
Fetal lymphangioma is a rare congenital anomaly of the lymphatic system characterized by the proliferation of lymphatic vessels filled with chyle. Lymphangioma results from complete obstruction of the lymphatic sacs, preventing communication with the venous system and causing large multilocular cysts. According to the literature, lymphangioma occurs in 1 case per 6000 newborns [1–6].
This study analyzed 54 cases of fetal lymphangioma of various locations observed over 14 years.
The diagnosis of fetal lymphangioma was based on detecting hypoechoic multilocular cysts with thin septa and a lack of blood flow on Doppler mapping. The size of a conglomerate of cysts can vary from a few millimeters to gigantic.
According to literature, most often, lymphangioma is located in the neck (75%) and axillary region (20%). In rare cases, they are found in the abdominal cavity and retroperitoneally, in the limbs and bones, and mediastinum – 1–2% each.
The location of the lymphangioma in our observations was somewhat different. The most common were lymphangiomas of the neck (51.8%), abdominal cavity (11.1%), and mediastinum (11.1%), followed by axillary region (9.3%), thigh and lower leg (9.3%), chest and anterior abdominal wall (5.6%), and multiple hemlimphangiomas (Proteus syndrome) – 1.9%.
Pregnancy ended in full-term delivery in 50 cases, of which 49 infants were referred to the Department of Neonatal Surgery for further examination and treatment. In one fetus, lymphangioma underwent a complete resolution in utero. Three women underwent termination of pregnancy for medical reasons. There was one intranatal fetal death. Thirty-three infants (67%) underwent surgery with favorable outcomes.
Some authors believe that most cases of fetal lymphangiomas diagnosed in the second trimester of pregnancy are not associated with chromosomal abnormalities. However, some observations do not entirely exclude this possibility. In our study, lymphangiomas were diagnosed after 18 weeks of pregnancy, and none of the fetuses had chromosomal abnormalities.
Conclusion
The present study's findings suggest that the prognosis for lymphangioma depends on the presence of concomitant malformations and chromosomal abnormalities. In their absence, the outcomes of the disease are generally favorable.
References
- Перельман М.И., Юсупов И.А., Седова Т.Н. Хирургия грудного протока. М.: Медицина; 1984: 136 с. [Perelman M.I., Yusupov I.A. Sedova T.N. Thoracic duct surgery. M.: Medicine; 1984: 136 p. (in Russian)].
- Lu D., Wang Y., Zeng W., Peng B. Giant fetal lymphangioma at chest wall and prognosis: case report and literature review. Taiwan. J. Obstet. Gynecol. 2015; 54(1): 62-5. https://dx.doi.org/10.1016/j.tjog.2014.11.009.
- Masood S.N., Masood M.F. Case report of fetal aхillo-thoraco-abdominal cystic hygroma. Arch. Gynecol. Obstet. 2010; 281(1): 111-5. https://dx.doi.org/10.1007/s00404-009-1068-2.
- Temizkan O., Abike F., Ayvaci H., Demigar E., Görücü Y., Isik E. Fetal axillary cystic hygroma: a case report and review. Rare Tumors. 2011; 3(4): e39. https://dx.doi.org/10.4081/rt.2011.e39.
- Tanaka H., Masumoto K., Aoyama T., Sanmoto Y., Ono K., Sakamoto N. et al. Prenatally diagnosed large mediastinal lymphangioma: a case report. Clin. Case Rep. 2018; 6(9): 1880-4. https://dx.doi.org/10.1002/ccr3.1760.
- Tongsong Th., Luewan S., Khorana J., Sirilert S., Charoenratana C. Natural course of fetal axillary lymphangioma based on prenatal ultrasound studies. J. Ultrasound Med. 2018; 37(5): 1273-81. https://dx.doi.org/10.1002/jum.14473.
- Исаков Ю.Ф., Володин Н.Н., Гераськин А.В., ред. Неонатальная хирургия. М.: Династия; 2011. 680 с. [Isakov Yu.F., VolodinN.N., Geraskin A.V., ed. Neonatal Surgery. M.: Publishing house «Dynasty»; 2011. 680 p. (in Russian)].
- Chen Y.N., Chen Ch.P., Lin Ch.J., Chen S.W. Prenatal ultrasound evaluation and outcome of pregnancy with fetal cystic hygromas and lymphangiomas. J. Med. Ultrasound. 2017; 25(1): 12-5. https://dx.doi.org/10.1016/j.jmu.2017.02.001.
- Adaletli I., Towbin A.J., Ozbayrak M., Madazli R. Anterior mediastinal limphangioma: pre- and postnatal sonographic findings. J. Clin. Ultrasound. 2013; 41(6): 383-5. https://dx.doi.org/10.1002/jcu.21960.
- Дженти П., Гонсалвес Л.Ф. Аномалии развития органов шеи и грудной полости. В кн.: Флейшер А., Мэнинг Ф., Дженти П., Ромеро Р., ред. Эхография в акушерстве и гинекологии. Теория и практика. ч. 1. Пер. с англ. М.: Издательский дом Видар-М; 2005: 423-44. [Jeanty Ph., Goncalves L.F. Anomalies in the development of the organs of the neck and chest cavity. Echography in obstetrics and gynecology. Theory and practice. Part One. Ed. A. Fleischer, F. Maning, P. Genti, R. Romero: Trans. from English. M.: Publishing house Vidar-M; 2005: 423-44. (in Russian)].
- Ersoy A.O., Oztas E., Saridogan E., Ozler S., Danisman N. An unusual origin of fetal lymphangioma filling right axilla. J. Clin. Diagn. Res. 2016; 10(3): QD09-11. https://dx.doi.org/10.7860/JCDR/2016/18516.7513.
- Медведев М.В. Пренатальная эхография. Дифференциальный диагноз и прогноз. М.: Реал Тайм; 2009. 368 c. [Medvedev M.V. Prenatal echography. Differential diagnosis and prognosis. Ed. M.V. Medvedev. M.: Real Time; 2009. 368 p. (in Russian)].
- Mikovic Z., Simic R., Egic A., Opincal T.S., Koprivsek K., Stanojevic D. et al. Intrauterine treatment of large fetal neck lymphangioma with OK-432. Fetal Diagn. Ther. 2009; 26(2): 102-6. https://dx.doi.org/10.1159/000238111.
- Jiao-Ling L., Hai-Ying W., Wei Z., Jin-Rong L., Kun-Shan C., Qian F. Treatment and prognosis of fetal lymphangioma. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018; 231: 274-9. https://dx.doi.org/10.1016/j.ejogrb.2018.10.031.
- Goldstein I., Leibovitz Z., Noi-Nizri M. Prenatal diagnosis of fetal chest lymphangioma. J. Ultrasound Med. 2006; 25(11): 1437-40. https://dx.doi.org/ 10.7863/jum.2006.25.11.1437.
- Reichler A., Bronshtein M. Early prenatal diagnosis of axillary cystic hygroma. J. Ultrasound Med. 1995; 14(8): 581-4. https://dx.doi.org/ 10.7863/jum.1995.14.8.581.
- Martinez R., Heredia F. Axillary hemangiolymphangioma. 2002. Available at: www.thefetus.net/
- Baytur Y.B., Ulkumen B.A., Pala H.G. Foetal axillary lymphangioma with ipsilateral pes equinovarus: pitfalls in sonographic differential diagnosis (axillary lymphangioma and pes equinovarus). J. Obstet. Gynaecol. 2015; 35(6): 647-9. https://dx.doi.org/10.3109/01443615.2014.992872.
- Niwa Y., Imai K., Kotani T., Nakano T., Ushida T., Moriyama Y. et al. A pitfall in diagnosing fetal abdominal lymphangioma: a report of two cases. J. Clin. Ultrasound. 2019; 47(8): 494-6. https://dx.doi.org/10.1002/jcu.22756.
- Deshpande P., Twining P., O’Neill D. Prenatal diagnosis of fetal abdominal lymphangioma by ultrasonography. Ultrasound Obstet. Gynecol. 2001; 17(5): 445-8. https://dx.doi.org/10.1046/j.1469-0705.2001.00367.x.
- Santo S.F., Marques J.P., Veca P., Melo A., Graça L.M. Prenatal ultrasonographic diagnosis of abdominal cystic lymphangioma: a case report. J. Matern. Fetal Neonatal Med. 2008; 21(8): 565-6. https://dx.doi.org/10.1080/14767050802165927.
- York D.G., Wolfe H., von Allmen D. Fetal abdomino-perineal lymphangioma: differential diagnosis and management. Prenat. Diagn. 2006; 26(8): 692-5. https://dx.doi.org/10.1002/pd.1481.
- Медведев М.В., Юдина Е.В., Сыпченко Е.В. Исчезающие аномалии у плода при динамическом эхографическом наблюдении. Ультразвуковая диагностика. 1997; 1: 71-4. [Medvedev M.V., Yudina E.V., Sypchenko E.V. Disappearing fetal anomalies with dynamic echographic observation. Ultrasound. Diagn. 1997; 1: 71-4. (in Russian)].
- Стыгар А.М., Демидов В.Н. Ультразвуковая оценка состояния органов пищеварительной системы плода. В кн.: Митьков В.В., Медведев М.В., ред. Клиническое руководство по ультразвуковой диагностике. т. 2. М.: Издательский дом Видар-М; 1996: 181-204. [Stygar A.M., Demidov V.N. Ultrasound assessment of the state of the fetal digestive system. Clinical guidelines for ultrasound diagnostics. Vol. 2. Ed. by V.V. Mitkov, M.V. Medvedeva. M.: Vidar; 1996: 181-204. (in Russian)].
- Malpas T.J., MacLachlan N., Dyres E., Kiely E.M. Prenatal intestinal perforation and intra-abdominal lymphangioma. Prenat. Diagn. 2007; 27(9): 882-3. https://dx.doi.org/10.1002/pd.1794.
- Sung E.R., Jae Y.B., Kim H.H., Shin J.C., Ahn H.Y., Kim D.C. et al. Prenatal sonographic and MRI imaging findings of extensive fetal lymphangioma: a case report. Korean J. Radiol. 2003; 4(4): 260-3. https://dx.doi.org/10.3348/kjr.2003.4.4.260.
- Dionisio A.C., Gomes R., Cernadas E., Caballero I., Semião M., Branco V. et al. Giant cystic mediastinal lymphangioma. Eur. J. Case Rep. Intern. Med. 2019; 7(1): 001323. https://dx.doi.org/ 10.12890/2019_001323.
- Adaletli I., Towbin A.J., Ozbayrak M., Madazli R. Anterior mediastinal limphangioma: pre- and postnatal sonographic findings. J. Clin. Ultrasound. 2013; 41(6): 383-5. https://dx.doi.org/10.1002/jcu.21960.
- Varlet F., Guye E., Varlet M.N., Tronchet M., Mariat G., Chene G. Isolated mediastinal lymphangioma: prenatal diagnosis and thoracoscopic treatment. Surg. Sci. 2010; 1: 20-3. https://dx.doi.org/10.4236/ss.2010.11004.
- Демидов В.Н., Машинец Н.В. Ультразвуковая диагностика больших размеров нетипичной локализации лимфангиом плода. Пренатальная диагностика. 2007: 6(3): 193-7. [Demidov V.N., Mashinets N.V. Ultrasound diagnostics of large sizes of atypical localization of fetal lymphangiomas. Prenatal diagnostics. 2007; 6 (3):193-7. (in Russian)].
- Демидов В.Н., Машинец Н.В. Изолированная лимфангиома средостения больших размеров у плода с летальным исходом: обзор литературы и собственное наблюдение. Пренатальная диагностика. 2016; 15(2): 155-9. [Demidov V.N., Mashinets N.V. Isolated lymphangioma of the mediastinum of large sizes in a fetus with a fatal outcome: a review of the literature and our own observation. Prenatal diagnostics. 2016; 15(2):155-9. (in Russian)].
- Машинец Н.В., Демидов В.Н., Подуровская Ю.Л., Дорофеева Е.И. Абдоминальная лимфангиома плода: обзор литературы и собственные наблюдения. Пренатальная диагностика. 2017; 16(4): 334-9. [Mashinets N.V., Demidov V.N., Podurovskaya Yu.L., Dorofeeva E.I. Fetal abdominal lymphangioma: a review of the literature and our own observations. Prenatal diagnostics. 2017; 16 (4): 334-9. (in Russian)].
- Демидов В.Н., Машинец Н.В., Подуровская Ю.Л., Дорофеева Е.И., Панин А.П. Лимфангиома подмышечной области гигантских размеров: обзор литературы и собственные наблюдения. Пренатальная диагностика. 2017; 16(4): 319-24. [Demidov V.N., Mashinets N.V.,Podurovskaya Yu.L., Dorofeeva E.I., Panin A.P. Giant axillary lymphangioma: a review of the literature and our own observations. Prenatal diagnostics. 2017; 16 (4): 319-24. (in Russian)].
Received 28.05.2020
Accepted 14.09.2020
About the Authors
Natalya V. Mashinets, Ph.D., Senior Researcher at the Department of Functional Diagnostics, Department of Diagnostic Imaging, V.I. Kulakov NMRC for OG&P,Ministry of Health of Russia. Tel.: +7(906)795-66-47. E-mail: natashamashinets@yandex.ru. 117997, Russia, Moscow, Ac. Oparin str., 4.
Vladimir N. Demidov, Dr. Med. Sci., Professor at the Department of Functional Diagnostics, Department of Diagnostic Imaging, V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia. Tel.: +7(910)451-25-68. E-mail: demydow@yandex.ru. 117997, Russia, Moscow, Ac. Oparin str., 4.
Elena I. Dorofeeva, Ph.D., Pediatric Surgeon, Clinical Care Supervisor at the Department of Neonatal Surgery, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Tel.: +7(916)114-21-18. E-mail: dorofey_i@mail.ru. 117997, Russia, Moscow, Ac. Oparin str., 4.
Yulia L. Podurovskaya, Ph.D., Department of Neonatal Surgery, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Tel.: +7(916)107-13-88. E-mail: podurovskaya@yandex.ru. 117997, Russia, Moscow, Ac. Oparin str., 4.
Andrey P. Panin, Ph.D., Pediatric Surgeon at the Department of Neonatal Surgery, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Tel.: +7(495)531-44-44. E-mail: an_panin@oparina4.ru. 117997, Russia, Moscow, Ac. Oparin str., 4.
Denis V. Nikiforov, Anesthesiologist at the Department of Neonatal Surgery, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Tel.: +7(495)531-44-44. E-mail: dennik2009@mail.ru. 117997, Russia, Moscow, Ac. Oparin str., 4.
Elena A. Filippova, Ph.D., Head of the Department of Ultrasound Diagnostics in Neonatology and Pediatrics, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Tel.: +7(495)531-44-44. E-mail: fla77@mail.ru. 117997, Russia, Moscow, Ac. Oparin str., 4.
Elena A. Kulabukhova, Ph.D., Physician at the Department of Diagnostic Imaging, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Tel.: +7(495)531-44-44. E-mail: e_kulabuhova@oparina4.ru. 117997, Russia, Moscow, Ac. Oparin str., 4.
For citation: Mashinets N.V., Demidov V.N., Dorofeeva E.I., Podurovskaya Yu.L., Panin A.P., Nikiforov D.V., Filippova E.A., Kulabukhova E.A. Fetal lymphangiomas of various locations. The Center’s 14-year experience.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2020; 10: 83-93 (in Russian)
https://dx.doi.org/10.18565/aig.2020.10.83-93



