Pregestational and gestational diabetes mellitus as risk factors for predicting the development of congenital anomalies in newborns
Postoev V.A., Telkova A.A., Maevskaya P.S., Postoeva A.V., Usynina A.A., Grjibovski A.M.
Relevance: Epidemiological studies have shown an increased risk of congenital anomalies in children born to mothers with diabetes mellitus.
Objective: This study aimed to estimate the influence of maternal pregestational and gestational diabetes mellitus on the risk of congenital anomalies in newborns.
Materials and methods: We conducted a cohort study using data from the Murmansk and Arkhangelsk County birth registries, which contain information on the health of pregnant women and neonatal diaseses in newborns of 134,884 pregnancies.
Results: From 2006 to 2017, 4862 newborns with congenital anomalies were identified, resulting in a prevalence of 36.2 per 1,000 births. Pregnant women with pregestational diabetes mellitus had a higher risk of giving birth to babies with anomalies of the eye, ear, face, and neck, circulatory system, and other congenital anomalies (Q80-Q89), including multiple anomalies. Pregnant women with gestational diabetes mellitus had a higher risk of having children with respiratory system anomalies.
Conclusion: Diabetes mellitus in women increases the risk of congenital anomalies in their children. Prognostic models developed to determine the risk of congenital anomalies should include information regarding the presence of maternal diabetes.
Authors' contribution: Postoev V.A., Telkova A.A., Maevskaya P.S., Postoeva A.V., Usynina A.A., Grjibovski A.M. – conception and design of the study, data collection and analysis, drafting of the manuscript, editing of the manuscript, final approval of the version to be submitted.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: This study was supported by the Russian Research Fund (Project №22-15-20059).
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the Northern State Medical University.
Patient Consent for Publication: Implementation of birth registries were promuglated with Regional Ministries of Health orders. The registries contain anonymous information only and they are published as consolidated data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Postoev V.A., Telkova A.A., Maevskaya P.S., Postoeva A.V., Usynina A.A., Grjibovski A.M. Pregestational and gestational diabetes mellitus as risk factors for predicting the development of congenital anomalies in newborns. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (11): 78-86 (in Russian)
https://dx.doi.org/10.18565/aig.2023.125
Keywords
Congenital malformations (CM) are structural or functional abnormalities that occur during intrauterine development. CM is a leading cause of childhood morbidity, disability, and mortality [1]. These abnormalities can be detected before birth, immediately after birth, or at a later age [2]. During organogenesis, which spans the first 12 weeks of gestation, the embryo is most sensitive to unfavorable factors that can affect the formation of the central nervous system, cardiovascular system, skeletal system, and genitourinary system, leading to the development of CM [3]. In 2010, the World Health Assembly adopted a resolution that all member states of the World Health Organization should promote the primary prevention of CM and health promotion for children born with this condition. A key focus in this direction is the advancement of scientific research on the etiology, diagnosis, and prevention of CM [4].
Special attention is currently being paid to carbohydrate metabolism during pregnancy [5, 6]. Diabetes mellitus (DM) is a global epidemic of the 21st century. According to International Diabetes Federation 2018 data, 463 million people worldwide are diagnosed with DM, including approximately 60 million women of reproductive age [7]. In the Arkhangelsk County, 47 thousand patients diagnosed with DM were registered at the beginning of 2019 [8]. Epidemiological studies have demonstrated a significant increase in the risk of developing certain forms of congenital anomalies in newborns of mothers with diabetes mellitus [3]. The mechanism of CM formation in children born to mothers with DM is unclear. Significant factors include glycemic fluctuations [9, 10], accumulation of ketone bodies during decompensation of carbohydrate metabolism, increased peroxidation with accumulation of free radicals, increased production of insulin-like growth factors, and disorders of lipid and protein metabolism [9]. The role of impaired placental blood flow due to morphological changes in the walls of the capillaries and arteries cannot be excluded [11].
Previous epidemiological studies have shown that pregestational DM is a risk factor for non-chromosomal CM, such as malformations of the cardiovascular, genitourinary, digestive, and central nervous system [12]. According to the European Network for the Study of Congenital Anomalies (EUROCAT), maternal pregestational DM also increases the likelihood of developing multiple CM [13].
However, the association between gestational DM and CM development is poorly understood. However, previous studies have found an increased risk of having children with cleft lip and soft palate or cleft lip alone, as well as cleft hard palate [14], central nervous system CM [15], and genitalia [16] with maternal gestational DM.
Because both DM and CM are relatively rare events from an epidemiological standpoint, the best way to study their association is through large population-based studies, including those based on population-based registries of pregnancy outcomes. Such registries, first organized in a number of Scandinavian countries in the 1960s, were introduced in the Murmansk and Arkhangelsk Counties of the Russian Federation and continue to be a valuable resource for studying rare pregnancy outcomes, including CM [17]. The study of the relationship between DM and CM will allow us to evaluate the importance of DM information for predicting CM when developing prediction models for clinical practice within the framework of personalized medicine using machine learning technologies.
This study aimed to estimate the influence of maternal pregestational and gestational diabetes mellitus on the risk of congenital anomalies in newborns.
Materials and methods
Study design and data sources
This historical cohort study was conducted using data from birth registries (BR) of the Arkhangelsk (BRAC) and Murmansk (BRMC) Counties. This study was based on BRMC data for 2006–2011 and BRAO data for 2012–2017. During the study period, 134884 pregnancy outcomes were recorded in two regional registries.
The BR is an electronic database containing detailed information on the health of all pregnant women and children born in a given territory, takes into account potential risk factors for adverse pregnancy outcomes, and helps to assess the effectiveness of the application of standards of care in obstetrics and perinatal medicine. The information collected on a regular basis is used to monitor the quality of work of the obstetrics and neonatology service, identify the causes of pathological conditions, and control the quality of implementation of management decisions. BRs are electronic databases containing information on all pregnancy outcomes in the above-mentioned regions with a gestational age of 22 weeks or more. Data for the registers were collected by obstetric medical organizations according to a developed questionnaire containing information on the parents' demographic data (age, education, and place of work), obstetric history, specific features of the course of the current pregnancy, and lifestyle factors. The BR registration card also contains a block of information about the newborn, including anthropometric data, Apgar score, birth status and diagnoses, including CM, made during the stay at the facility. The source of data for completing the BR registration card was primary medical documentation, namely antenatal care cards, delivery case records, and newborn developmental history [17].
In the BR, the presence of DM in pregnant women was confirmed using the appropriate code of the International Classification of Diseases 10th Revision (ICD-10). Taking into account the timing of glycemia onset in relation to conception and critical periods of ontogenesis, and to ensure comparability of results with previous large international studies [13, 14, 16], all pregnant women diagnosed with DM were divided into 2 groups: pregnant women with diagnosed pregestational DM (these included pregnant women with established diagnoses coded as E10-E11 according to ICD-10) and gestational DM (code O 24).
CM data were categorized according to ICD-10 malformations: congenital anomalies of the nervous system (Q00–Q07); congenital anomalies of the eye, ear, face, and neck (Q10–Q18); congenital anomalies of the circulatory system (Q20–Q28); congenital anomalies of the respiratory system (Q30–Q34); cleft lip and palate (Q35–Q37); other congenital anomalies of the digestive system (Q38–Q45); congenital anomalies of the genitals (Q50–Q56); congenital anomalies of the urinary system (Q60–Q64); congenital anomalies and deformities of the musculoskeletal system (Q65–Q79); congenital anomalies (Q80–Q89); and chromosomal abnormalities not classified in other headings (Q90–Q99).
Statistical analysis
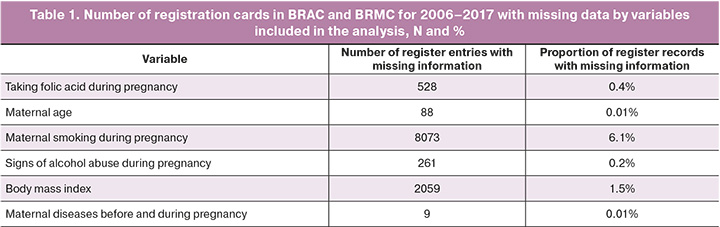
The study was conducted in 2 stages. In the first stage, the overall prevalence of CM was calculated using data on CM cases with available ICD-10 diagnoses in the registry, as well as the prevalence of CM by malformation group according to ICD-10, depending on the presence or absence of maternal DM. The CM rates in the groups of newborns of mothers without DM, with pregestational DM (pregnant women with type 1 or type 2 DM in the "Maternal Diseases Before Pregnancy" field were included), and gestational DM were compared using Pearson's chi-squared (χ2) test. At this stage, all pregnancy outcomes for which records were available in the BR, for which information on maternal diagnoses before and during pregnancy was present, and information on reported CM with diagnoses were included in the study. The number of observations at this stage was 134013 or 99.4% of all pregnancy outcomes in the registry.
In the second stage, to establish the strength of the effect of maternal DM on the risk of CM in newborns, regression analysis was performed using logistic regression. To assess the risk, unadjusted odds ratios (ORs) for the development of individual CM groups were calculated with 95% confidence intervals (CI) using binary logistic regression. The presence or absence of CM in the newborn was included in the model as a binary dependent variable, whereas the presence of maternal DM was included as an independent variable. Given that CM is a rare outcome, OR can be considered a proxy for relative risk (RR). Furthermore, to exclude the influence of potential confounders that could distort the strength and direction of the relationship, multivariate logistic regression analysis was performed both overall for all CMs and separately for each CM group. Potential confounders were selected based on a literature review [13, 14, 16, 18]. Independent variables were screened for multicollinearity, and the variance inflation factor (VIF) for all variables included in the model ranged from 1 to 5, indicating no pronounced multicollinearity requiring correction. Thus, the analysis included the fact of presence/absence of CM in the newborn as a binary dependent variable, and information about the presence of DM in the mother as independent variables (as a categorical variable with the following values: no diagnosis of diabetes, pregestational diabetes, gestational diabetes), data on maternal age (less than 20 years, 20–35 years, older than 35 years), body mass index (less than 18.5 kg/m2; 18.5–24.99 kg/m2; 25 or more kg/m2), folic acid intake during pregnancy, alcohol abuse during pregnancy, and information on smoking during pregnancy. As approaches to the diagnosis of gestational DM changed during the period under review, we also adjusted for the year of birth by including this variable in the regression model. At this stage, all pregnancy outcomes for which the registry did not have information on the variables included in the analysis were excluded. Thus, the number of observations included in each of the regression models was 123928 pregnancy outcomes, for which the registry database contained information on all variables used for correction. This amounted to 91.9% of all records available in the registries. Table 1 presents the number and proportion of missing data for each variable. Notably, the groups of neonates with and without CM did not differ significantly in the proportion of missing data (p for χ2 test=0.230).
Statistical analysis of the data was performed using STATA 17.0. (Stata Corp., TX, USA).
Results
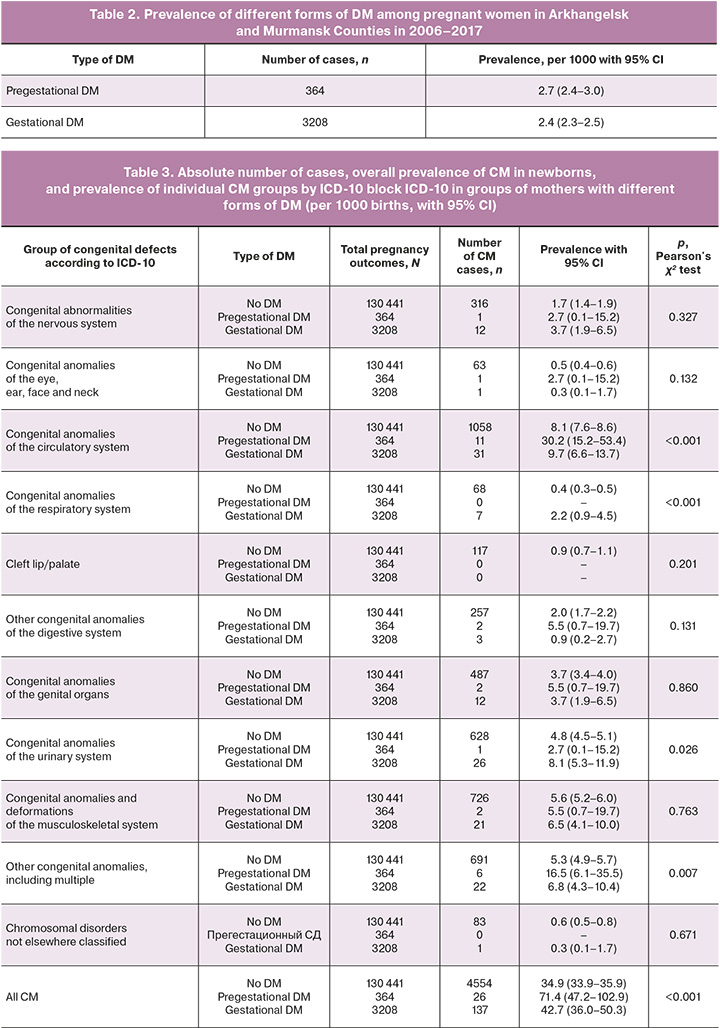
Among all pregnancy outcomes available in the combined BRMC and BRAC databases from 2006 to 2017, 4717 newborns with various forms of CM were recorded. Among pregnant women whose information was entered into these registries, 3592 cases of DM were identified, of which 364 cases were pregestational DM (Table 2). Among the pregnant women with pregestational DM, 232 (63.7%) had type 1 DM.
The prevalence of CM among children born to women with pregestational DM was significantly higher than the prevalence of CM in other forms of maternal diabetes and women with normal glycemia (Table 2). When comparing the prevalence by individual CM groups, significant differences in prevalence were found for CM of the circulatory, respiratory, and urinary system anomalies, and a group of other congenital anomalies, including multiple congenital anomalies (Table 3).
In a univariate analysis of the relationship between the presence of DM and the risk of developing CM, expressed as OR, we found that the presence of pregestational DM (p<0.0001) and gestational DM (p=0.014) significantly increased the risk of developing CM in newborns. When analyzed by individual CM groups, the presence of pregestational DM increased the risk of congenital anomalies of the circulatory system (p<0.0001); the risk of congenital anomalies of the eye, ear, face, and neck (p=0.044); and the risk of CM from the other anomalies group (p=0.002). The presence of gestational DM was associated with an increased risk of developing CM of the nervous system (p=0.027), respiratory system (p<0.0001), and urinary system (p=0.014) (Table 4).
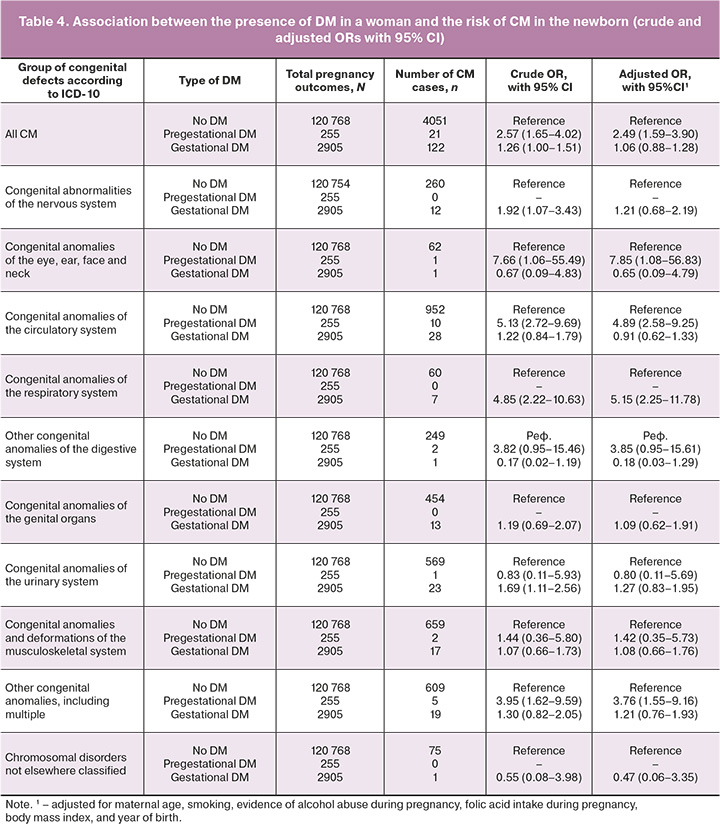
After adjusting for several potentially confounding factors (Table 4), a statistically significant association remained between pregestational DM and the risk of developing CM in general (p<0.0001), congenital anomalies of the eye, ear, face, and neck (p=0.042), congenital anomalies of circulation (p<0.0001), and other congenital anomalies (Q80–Q89) (p=0.004). However, for gestational DM, the association with an increased risk of developing respiratory CM remained statistically significant (p<0.0001).
Discussion
Analysis of the BRAC and BRMC data confirmed that pregestational and gestational DM were independent risk factors for CM in children. Moreover, children of women with type 1 DM were found to have the highest prevalence of CM. Type 1 DM in the pregnant woman was associated with the highest risk of congenital anomalies of the eye, ear, face and neck, as well as CM of the circulatory system and other CM from the same group (Q80–Q89). Gestational DM significantly increases the risk of congenital respiratory system anomalies.
According to previous studies, the risk of fetal CM in pregnant women with DM is 2–5 times higher than that in the general population [19]. In patients on insulin therapy, the risk increases to 10–12% [18], which explains the highest prevalence of CM in women with type 1 DM who receive insulin in 100% of cases [12]. Patients with type 1 DM are characterized by frequent alternation of hyper- and hypoglycemia. The use of insulin pump therapy to minimize glycemic fluctuations is now widespread; however, this device was available to a very limited number of patients during the study period and was not widely used during the time period we reviewed.
CM development is also influenced by unsatisfactory glycemic control during the periconceptional period. According to the National Medical Research Center for Endocrinology of the Ministry of Health of the Russian Federation, only approximately 50% of patients with DM have an HbA1c level of less than 7%. Considering that for most women of reproductive age (< 40 years), the target HbA1c level is 6.5%, we can assume an even smaller number of women with satisfactory glycemic control [20].
Hyperglycemia before pregnancy and in the first trimester of pregnancy is more common in women with type 1 DM. The presence of hyperglycemia during the period corresponding to the formation of fetal organs and systems explains the maximum frequency of CM in this group [21]. Cardiovascular malformations are a common type of CM in the newborns of mothers with type 1 DM. According to our data, women with pregestational DM had a 4.81-fold higher risk of giving birth to a child with this group of malformations, which occur in the first 3–7 weeks of gestation. Other researchers have also noted a higher incidence of cardiovascular CM in children of women with type 1 DM [22, 23]. However, the mechanisms underlying these relationships are poorly understood. Animal studies have shown that hyperglycemia stimulates oxidative stress, in which the amount of oxidized oxygen and nitrogen radicals increases, contributing to genetic changes and impaired apoptosis in cardiac cells [24]. On the other hand, under conditions of hyperglycemia, there is an alteration in multiple signaling pathways in the myocardium, such as increased expression of transforming growth factor beta 1 (TGF-β1), which leads to excessive accumulation of extracellular matrix proteins in cardiac tissues. In addition, there was a decrease in the level of nitric oxide, which is necessary for the proper functioning of cardiac endothelial cells. Decreased NO, in turn, leads to the inhibition of other signaling pathways [25]. An association between high glucose levels and subsequent placental vascular dysfunction due to dysregulation of vascular endothelial growth factor with subsequent effects on cardiogenesis has also been described [24]. According to the literature, the most common variant of CM is cardiovascular anomaly, but 20–30% of this pathology is represented by transient hypertrophic cardiomyopathy due to unstable glycemia during pregnancy, which artificially increases the number of cases. Among the most frequent pathologies are mitral valve dysfunction and additional and ectopic chordae in the left ventricular cavity (70.1%); mitral valve prolapse without regurgitation was diagnosed in 23.6% of cases [12].
Gestational DM is most often diagnosed in the second trimester of pregnancy when the main stage of ontogenesis is completed. Therefore, the presence of gestational DM more often increases the risk of developing the so-called "minor" forms of CM. The probability of hyperglycemia increases with increasing gestational age and has a direct negative impact on the fetus, mainly in the second half of pregnancy [26]. In our study, women with gestational DM had a 4–5-fold increased risk of having a child with congenital anomalies of the bronchopulmonary system. According to the literature, these CMs are rare, accounting for 5–18% of all CMs. In the available literature, explanations for the possible mechanisms of the development of these malformations are limited, most likely due to an increase in the expression of fibroblast growth factor 10 (FGF10), leading to impaired bronchopulmonary system formation [9].
Another group of congenital anomalies associated with gestational DM is urinary system pathology. An earlier study based on BRMC data reported a 4.77-fold increased risk of genitourinary anomalies in newborns of mothers with pregestational and gestational DM, representing a single group [27]. Possible mechanisms include maternal hyperglycemia, compensatory hyperinsulinemia during pregnancy due to insulin resistance, and transient hypercriticism in the fetus [28].
It should be noted that the impact of DM during pregnancy on the fetus in general and on the development of its individual systems in particular is still not completely understood because of the complexity of the interpretation of the relationship and the large number of factors acting on the pregnant woman and the fetus.
This is the first population-based registry study in the Russian Federation to examine the association between DM in pregnant women and the risk of fetal CM using data from more than 134,000 observations, of which only 8% had missing data on the variables included in the analysis. However, the strength and significance of the associations found in this study demonstrate the importance of DM as a potential predictor of CM, which should be considered in the selection of prognostic factors for CM development when developing decision-making systems based on machine learning and intelligent data-processing techniques [29, 30].
The population-based registries used in this study covered more than 99% of births, with the proportion of missing data on individual variables not exceeding 6%. This suggests a low probability of selection errors, which is an advantage of the present study. At the same time, this study has a number of limitations related to the probability of information errors and historical changes in approaches to diagnosing the conditions under study. Changing diagnostic approaches to the definition of gestational DM may cause the registry based on primary medical records to not include all cases of this condition according to the current diagnostic criteria, which may affect the results of the relationship assessment, primarily in terms of its underestimation. Such misclassification, caused by the improvement in diagnostic quality in recent registries, may also be characteristic of CM. Owing to insufficient validity and a high proportion of missing data, the present study did not include information on treatment and, as a consequence, the degree of glycemic control, which did not allow for appropriate correction for these confounders. Simultaneously, we believe that in the absence of laboratory data on glycated hemoglobin, information on the treatment received (dietary therapy and insulin therapy) did not have a significant impact on the results obtained. In addition, it should be noted that both BRAC and BRMC registered cases of CM diagnosed in obstetric institutions, that is, those anomalies that manifested themselves in the first days of the child's life.
Conclusion
Pregestational and gestational DM increase the risk of congenital abnormalities, and newborns of women with pregestational DM have a significantly higher risk of congenital malformations. Among the individual CM groups, a notable increase in risk was observed for congenital anomalies of the eye, ear, face, and neck, congenital anomalies of the circulatory system, and a group of other congenital anomalies, including multiple anomalies. Gestational DM is associated with a five-fold increase in the risk of having a child with respiratory CM.
The results of this study support the importance of adequate glycemic control during pregnancy planning and medical surveillance during pregnancy to reduce the risk of adverse fetal and infant outcomes. Data on pregestational and gestational DM should be included in prediction models developed to determine the risk of congenital anomalies.
References
- WHO. Congenital amonalies. Fact sheet № 370. http://www.who.int/mediacentre/factsheets/fs370/en/ (assesed: 09.04.2023).
- WHO, CDC, ICBDSR. Birth defects surveillance: A manual for programme managers. 2014. 115 p.
- Kirby R.S., Browne M.L. Birth defects surveillance: epidemiology, health services research, public health, and prevention. Birth Defects Res. A Clin. Mol. Teratol. 2013; 97(10):617-8. https://dx.doi.org/10.1002/bdra.23192.
- Всемирная организация здравоохранения. Пороки развития: основные факты. https://www.who.int/ru/news-room/fact-sheets/detail/congenital-anomalies (дата обращения: 14.10.2022). [WHO. Congenital disorders: Key facts. https://www.who.int/ru/news-room/fact-sheets/detail/congenital-anomalies (assesed: 14.10.2022)].
- Зенкова Е.В., Бондарь И.А. Различия в течении беременности у пациенток с сахарным диабетом 2 типа и гестационным сахарным диабетом. Сахарный диабет-2017: от мониторинга к управлению. Материалы II Российской мультидисциплинарной конференции с международным участием, Новосибирск, 19–20 апреля 2017 г. Общество с ограниченной ответственностью «Манускрипт»; 2017: 43-5. [Zenkova E.V., Bondar I.A. Differences in the course of pregnancy in patients with type 2 diabetes mellitus and gestational diabetes mellitus. Diabetes mellitus-2017: from monitoring to management. Materials of the II Russian Multidisciplinary Conference with International Participation, Novosibirsk, April 19-20, 2017. LLC "Manuscript"; 2017: 43-5. (in Russian)].
- Волкова Н.И., Давиденко И.Ю., Дегтярева Ю.С. Гестационный сахарный диабет. Акушерство и гинекология. 2021; 9: 174-9. [Volkova N.I., Davidenko I.Yu., Degtyareva Yu.S. Gestational diabetes mellitus. Obstetrics and Gynecology. 2021; (9): 174-9 (in Russian)]. https://dx.doi.org/10.18565/aig.2021.9.174-179.
- International Diabetes Federation. IDF Diabetes Atlas 9th edition; 2019.
- Статистика и показатели. https://rosinfostat.ru (дата обращения: 25.12.2022). [Statistics and indicators. https://rosinfostat.ru (accessed: 25.12.2022). (in Russian)].
- Goto M.P., Goldman A.S. Diabetic embryopathy. Curr. Opin. Pediatr. 1994; 6(4):486-91. https://dx.doi.org/10.1097/00008480-199408000-00023.
- Lucas M.J., Leveno K.J., Williams M.L., Raskin P., Whalley P.J. Early pregnancy glycosylated hemoglobin, severity of diabetes, and fetal malformations. Am. J. Obstet. Gynecol. 1989; 161(2):426-31. https://dx.doi.org/10.1016/0002-9378(89)90536-x.
- Wender-Ozegowska E., Wróblewska K., Zawiejska A., Pietryga M., Szczapa J., Biczysko R. Threshold values of maternal blood glucose in early diabetic pregnancy--prediction of fetal malformations. Acta Obstet. Gynecol. Scand. 2005; 84(1):17-25. https://dx.doi.org/10.1111/j.0001-6349.2005.00606.x.
- Логинова Е.В., Аракелян Г.А., Савенкова И.В., Гагаев Д.Ч., Оразмурадов А.А., Гагаев Ч.Г. Современные представления о здоровье детей, рожденных матерями с сахарным диабетом. Акушерство и гинекология: новости, мнения, обучение. 2019; 7(3) Приложение: 56-62. [Loginova E.V., Arakelyan G.A., Savenkova I.V., Gagaev D.Ch., Orazmuradov A.A., Gagaev Ch.G. Modern view of the health of infants born to mothers with diabetes mellitus. Obstetrics and Gyneology: News, Opinions, Training. 2019; 7(3) Supplement: 56-62. (in Russian)]. https://dx.doi.org/10.24411/2303-9698-2019-13907.
- Garne E., Loane M., Dolk H., Barisic I., Addor M.-C., Arriola L. et al. Spectrum of congenital anomalies in pregnancies with pregestational diabetes. Birth Defects Res. A Clin. Mol. Teratol. 2012; 94(3):134-40. DOI: 10.1002/bdra.22886.
- Correa A., Gilboa S.M., Besser L.M., Botto L.D., Moore C.A., Hobbs C.A. et al. Diabetes mellitus and birth defects. Am. J. Obstet. Gynecol. 2008;199(3):237.e1-9. https://dx.doi.org/10.1016/j.ajog.2008.06.028.
- Anderson J.L., Waller D.K., Canfield M.A., Shaw G.M., Watkins M.L., Werler M.M. Maternal obesity, gestational diabetes, and central nervous system birth defects. Epidemiology. 2005;16(1):87-92. https://dx.doi.org/10.1097/01.ede.0000147122.97061.bb.
- Arendt L.H., Lindhard M.S., Henriksen T.B., Olsen J., Cnattingius S., Petersson G. et al. Maternal diabetes mellitus and genital anomalies in male offspring: a nationwide cohort study in 2 Nordic countries. Epidemiology. 2018;29(2):280-9. https://dx.doi.org/10.1097/EDE.0000000000000781.
- Усынина А.А., Одланд Йон Ойвинд, Пылаева Ж.А., Пастбина И.М., Гржибовский А.М. Регистр родов Архангельской области как важный информационный ресурс для науки и практического здравоохранения. Экология человека. 2017; 2: 58-64. [Usynina A.A., Odland Jon Øyvind, Pylaeva Zh.A., Pastbina I.M., Grjibovski A.M. Arkhangelsk county birth registry as an important source of information for research and healthcare. Human Ecology. 2017; 2: 58-64. (in Russian)]. https://dx.doi.org/10.33396/1728-0869-2017-2-58-64.
- Arendt L.H., Pedersen L.H., Pedersen L., Ovesen P.G., Henriksen T.B., Lindhard M.S. et al. Glycemic control in pregnancies complicated by pre-existing diabetes mellitus and congenital malformations: a Danish population-based study. Clin. Epidemiol. 2021;13:615-26. https://dx.doi.org/10.2147/CLEP.S298748.
- Riskin A., Garcia-Prats J.A. Infants of women with diabetes (IMD). Literature review. Jun 2019. https://www.uptodate.com/contents/infants-of-women-with-diabetes
- Sirico A., Sarno L., Zullo F., Martinelli P., Maruotti G.M. Pregestational diabetes and fetal heart rate in the first trimester of pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019; 232:30-2. https://dx.doi.org/10.1016/j.ejogrb.2018.11.003.
- Basu M., Zhu J.Y., LaHaye S., Majumdar U., Jiao K., Han Z., Garg V. Epigenetic mechanisms underlying maternal diabetes-associated risk of congenital heart disease. JCI Insight. 2017;2(20):e95085. https://dx.doi.org/10.1172/jci.insight.95085.
- Chen L., Yang T., Chen L., Wang L., Wang T., Zhao L. et al. Risk of congenital heart defects in offspring exposed to maternal diabetes mellitus: an updated systematic review and meta-analysis. Arch. Gynecol. Obstet. 2019; 300(6):1491-506. https://dx.doi.org/10.1007/s00404-019-05376-6.
- Maduro C., de Castro L.F., Moleiro M.L., Guedes-Martins L. Pregestational Diabetes and Congenital Heart Defects. Rev. Bras. Ginecol. Obstet. 2022;44(10):953-61. https://dx.doi.org/10.1055/s-0042-1755458.
- Basu M., Garg V. Maternal hyperglycemia and fetal cardiac development: Clinical impact and underlying mechanisms. Birth Defects Res. 2018;110(20):1504-16. https://dx.doi.org/10.1002/bdr2.1435.
- Дедов И.И., Шестакова М.В., Викулова О.К. Эпидемиология сахарного диабета в Российской Федерации: клинико-статистический анализ по данным Федерального регистра сахарного диабета. Сахарный диабет. 2017; 20(1): 13-41. [Dedov I.I., Shestakova M.V., Vikulova O.K. Epidemiology of diabetes mellitus in Russian Federation: clinical and statistical report according to the federal diabetes registry. Diabetes mellitus. 2017;20(1):13-41. (in Russian)]. https://dx.doi.org/10.14341/DM8664.
- Annunziata F., Bush A., Borgia F., Raimondi F., Montella S., Poeta M. et al. Congenital lung malformations: unresolved issues and unanswered questions. Front. Pediatr. 2019;7:239. https://dx.doi.org/10.3389/fped.2019.00239.
- Postoev V.A., Grjibovski A.M., Kovalenko A.A., Anda E.E., Nieboer E., Odland J.Ø. Congenital anomalies of the kidney and the urinary tract: A Murmansk county birth registry study. Birth Defects Res. A Clin. Mol. Teratol. 2016;106(3):185-93. https://dx.doi.org/10.1002/bdra.23475.
- Волкова Н.И., Паненко С.О. Гестационный сахарный диабет: проблемы современного скрининга. Сахарный диабет. 2022;25(1):72-80. [Volkova N.I., Panenko S.O. Gestational diabetes mellitus: current screening problems. Diabetes mellitus. 2022; 25(1):72-80. (in Russian)]. https://dx.doi.org/10.14341/DM12727.
- Наркевич А.Н., Виноградов К.А., Гржибовский А.М. Интеллектуальные методы анализа данных в биомедицинских исследованиях: деревья классификации. Экология человека. 2021;3:54-64. [Narkevich A.N., Vinogradov K.A., Grjibovski A.M. Intelligent data analysis in biomedical research: classification trees. Human Ecology. 2021; 3: 54-64. (in Russian)]. https://dx.doi.org/10.33396/1728-0869-2021-3-54-64.
- Наркевич А.Н., Виноградов К.А., Параскевопуло К.М., Гржибовский А.М. Интеллектуальные методы анализа данных в биомедицинских исследованиях: нейронные сети. Экология человека. 2021; 4: 55-64. [Narkevich A.N., Vinogradov K.A., Paraskevopulo K.M., Grjibovski A.M. Intelligent data analysis in biomedical research: artificial neural networks. Human Ecology. 2021;4:55-64. (in Russian)]. https://dx.doi.org/10.33396/1728-0869-2021-4-55-64.
Received 17.05.2023
Accepted 05.10.2023
About the Authors
Vitaly A. Postoev, Cand. Sci. (Med), Ph.D., Acting Head of the Department of Methodology of Scientific Research, Head of the Arkhangelsk International School of Public Health, Northern State Medical University, Ministry of Health of the Russian Federation, ispha@nsmu.ru, https://orcid.org0000-0003-4982-4169,163069, Russia, Arkhangelsk, Troitsky Ave., 51.
Alena A. Telkova, Northern State Medical University, Ministry of Health of the Russian Federation, https://orcid.org/0009-0002-1472-0546, 163069, Russia, Arkhangelsk, Troitsky Ave., 51.
Polina S. Maevskaya, Northern State Medical University, Ministry of Health of the Russian Federation, https://orcid.org/0009-0004-4486-6225,
163069, Russia, Arkhangelsk, Troitsky Ave., 51.
Anna V. Postoeva, Cand. Sci. (Med), Associate Professor at the Department of Hospital Therapy and Endocrinology, Northern State Medical University, Ministry of Health of the Russian Federation, https://orcid.org0000-0003-3749-0173, 163069, Russia, Arkhangelsk, pr. Troitsky, 51.
Anna A. Usynina, Dr. Med. Sci., Ph.D., Head of the Department of Neonatology and Perinatology, Northern State Medical University, Ministry of Health of the Russian Federation, perinat@mail.ru, https://orcid.org0000-0002-5346-3047, 163000, Russia, Arkhangelsk, pr. Troitsky, 51.
Andrej M. Grjibovski, MD, Head of the Division for Research and Innovations, Northern State Medical University, Ministry of Health of the Russian Federation,
163069, Russia, Arkhangelsk, 51 Troitsky Ave.; Chief researcher at the Arctic biomonitoring laboratory, Northern (Arctic) Federal University,
163001, Arkhangelsk, 17 Severnaya Dvina Emb., a.grjibovski@yandex.ru, https://orcid.org0000-0002-5464-0498
Corresponding author: Vitaly A. Postoev, ispha@nsmu.ru



