Clinical characteristics of pregnancy in women with gestational diabetes mellitus
Objective. To study clinical, anamnestic and laboratory risk factors of gestational diabetes mellitus (GDM). Materials and methods. The study included 220 women aged from 18 to 43 years. The patients were divided into two groups and subgroups: group I consisted of 118 patients with GDM, group Ia - 94 patients with GDM on diet therapy, group Ib - 24 patients with GDM on insulin therapy; group II included 102 women with uncomplicated pregnancy. Age indicators, history of somatic and gynecological diseases, reproductive history, characteristics of the current pregnancy, indicators of oral glucose tolerance test (OGTT), ultrasound parameters of fetal growth have been studied in detail.Khodzhaeva Z.S., Snetkova N.V., Muminova K.T., Gorina K.A., Abramova M.E., Esayan R.M.
Results. GDM was significantly more common in multigravidas aged from 26 to 37 years. These patients were significantly more likely to have inherited type 2 diabetes mellitus, GDM in the previous pregnancy. They are characterized by an earlier age of menarche, uterine fibroids, infertility and a past history of artificial abortion. The course of pregnancy was complicated by the threatened miscarriage and dysbiotic disorders of the vaginal microbiota. The analysis of OGTT results showed the role of fasting hyperglycemia, as the most common reason for the insulin therapy. The tendency to higher fetal weight indicators in pregnant women with GDM starts at 18 weeks of gestation.
Conclusion. Тhe identified risk factors indicate the need for timely diagnosis of GDM to prevent pregnancy complications.
Keywords
Gestational diabetes mellitus (GDM) is associated with the presence of various pathological conditions that probably existed before pregnancy. Hyperglycemia appears to be their dominant manifestation, which affects not only the course and outcomes of pregnancy, but also the quality of the subsequent life of the woman and her child [1–3]. Therefore, the World Health Organization (WHO) and International Federation of Gynecology and Obstetrics (FIGO) identified hyperglycemia during pregnancy as a particularly important nosological condition with long-term adverse consequences for a woman posing an increased risk for the development of various diseases in her child.
According to the National Register of Diabetes, the prevalence of GDM in Russia is 8-9%, while, according to the international studies, about 17% of all pregnancies are complicated by GDM, and there is a tendency for an increase in the incidence rate [4–5].
The objective of the research is to study clinical and anamnestic risk factors for the development of GDM in pregnant women.
Materials and Methods
This was a cross-sectional comparative study of 220 women. The patients were divided into two groups and subgroups: group I consisted of 118 patients with GDM, group Ia included 94 patients with GDM on diet therapy, group Ib had 24 patients with GDM on insulin therapy; group II included 102 women with uncomplicated pregnancy.
All patients were evaluated for age parameters, history of somatic and gynecological diseases, surgical interventions in the past, obstetric history. Characteristics of the current pregnancy, indicators of oral glucose tolerance test (OGTT), ultrasound parameters of fetal growth dynamics were also analyzed.
The criteria for inclusion in group I were spontaneous singleton pregnancy and GDM confirmed by OGTT. The criteria for inclusion in group II was spontaneous singleton pregnancy with normal OGTT indicators. Informed consent to participate in the study was obtained from all patients.
The exclusion criteria were multiple pregnancy, pregnancy following the use of assisted reproductive technologies, structural and chromosomal abnormalities, severe extragenital pathology, types I and II diabetes mellitus, impaired carbohydrate and fat metabolism, autoimmune diseases, oncology, congenital malformations in the fetus.
Statistical analysis
Statistical processing of the obtained data was performed using the IBM SPSS Statistics program, version 22. Quantitative parameters were presented in the form of arithmetic mean and standard deviation M(SD). For the analysis of quantitative data, the methods of parametric statistics (Student’s t-test) were used with a normal distribution of data when assessing intergroup differences. When normal distribution of data was absent, the methods of nonparametric statistics were used, namely Mann-Whitney U-test was applied for two groups. The method χ2 was used for the comparison of the groups based on qualitative binary characteristics; if one of the values was less than 5, Fischer’s exact test was applied. Differences were considered statistically significant when the error rate was p<0.05.
Results
The age of the patients in the study ranged from 20 to 43 years and averaged 31.8 (4.56) years: 33.15 (4.65) years in group I, 33.5 (4.5) years in group Ia, 32.8 (4.8) years in group Ib, 30.1 (4.4) years in the control group (group II). Patients aged 32-37 years were statistically significantly more frequent in the group with GDM (43.2%), compared to the control group (p=0.0001).
Body mass index (BMI) was 22.11 ± 0.27 in group I, 21.98 ± 0.32 in group Ia, 22.58 ± 0.54 in group Ib, and 20.89 ± 0.26 in group II. BMI of patients with GDM was higher in comparison with the control group (p=0.03); the highest BMI was observed in group Ib (p=0.01).
The comparative analysis of hereditary diseases showed that arterial hypertension in the first-degree relatives was statistically significantly more frequent in patients with GDM in comparison with the patients of the control group (p=0.0001). In the families of patients with GDM, myocardial infarction (p=0.007) and stroke (p=0.034) were significantly more common in comparison with the patients of the control group. The incidence of type II diabetes was higher in patients with GDM (p=0.004), especially in patients of group Ib (p=0.001). Type I diabetes was also statistically significantly more common in group Ib, 12.5% (p=0.016). These data are suggestive of the role of the genetic factor in the development of GDM.
The analysis of the patient histories showed that the pathology of the gastrointestinal tract was statistically significantly higher in patients of group Ib in comparison with patients of group Ia (p=0.03), and the frequency of cardiovascular diseases (mitral valve prolapse, arterial hypertension, varicose veins) was statistically significantly higher in pregnant women with GDM in comparison with the women of the control group (p=0.03).
The comparative analysis of the onset and characteristics of the menstrual cycle revealed a statistically significantly earlier age at menarche in pregnant women of group I (p=0.006).
The analysis of gynecological history showed that only uterine fibroids were statistically significantly more common in group with GDM (group I) (p=0.001), compared to the control group; the intragroup analysis detected that uterine fibroids were statistically significantly more common in group Ia (p=0.0001). The frequency of infertility was observed twice as often (12.5%) in group Ib (p=0.041) in comparison with group Ia. Polycystic ovarian syndrome (PCOS) was observed more often in group Ib (8.3%) compared to group Ia (1%) (p=0.034).
The analysis of the reproductive history of the women showed that the number of multigravidas was significantly higher in group I (73.7%), compared to group II (56.9%) (p=0.009). The number of primigravidas was significantly higher in group I (40.7%) in comparison with their number in group II (14.6%) (p=0.001); the intragroup analysis revealed that this indicator was higher in group Ia (45.7%), compared to group Ib (20.8%) (p=0.027).
The analysis of the course of previous pregnancy showed that the frequency of GDM was statistically significantly higher in patients of group I, compared to the control group (p=0.013); the intragroup analysis revealed that this indicator was higher in patients of group Ib (p=0.01). Thus, each subsequent pregnancy was complicated by the repeated development of GDM and required insulin therapy.
The above-mentioned data indicate that patients with GDM were significantly more likely to have diseases in the family and obstetric history. They had a higher BMI, and moreover, diabetes was noted in their nearest relatives. Pregnant women of group I also had a higher incidence of PCOS, infertility and GDM in their histories.
When analyzing the characteristics of the first trimester of pregnancy in the patients, the risk of miscarriage was found to be more common in the group of patients with GDM (20.3%) in comparison with the patients of the control group (4.9%) (p=0.001); the intragroup analysis showed that this indicator was higher in group Ib (25%) in comparison with the patients of groups Ia and II (p=0.003), which was statistically significant. In addition, hormonal therapy was more often prescribed in group I (18.6%), compared to group II (5.9%), which also turned out to be a statistically significant difference (p=0.005).
Pregnancy-related nausea and vomiting were slightly more frequent in patients of group Ib (41.7%), while the frequency of this complication in group Ia and in the control group was almost the same (33.0 and 36.3%, respectively), although no significant differences were obtained.
The analysis of the characteristics of the second trimester of pregnancy showed that threatened miscarriage (23.4%) (p=0.001), isthmic-cervical insufficiency (7.4%) (p=0.033) with its surgical correction (6.4%) (p=0.042) and/or the installation of an obstetric pessary (4.2%) (p=0.039) were significantly more often observed in patients with GDM in group Ia. There were no statistically significant differences in the frequency of anemia, edema of pregnant women, and dysbiotic disorders of the vaginal microbiota in patients of groups Ia and Ib.
The results of OGTT showed that fasting glucose level in venous blood plasma ≥ 5.1 mmol/L was in 82 out of 220 (37.3%) pregnant women, which is consistent with the criteria of the diagnosis GDM; subsequently, 24 of them (29.3%) required insulin therapy. During the further loading test, 21 out of 138 (15.2%) pregnant women showed an increase in blood glucose at the second point (after one hour), and three of them required insulin therapy. There was an increase in blood glucose at the third point (after two hours) in 23 of 138 (16.7%) pregnant women, two of them were later prescribed insulin therapy. Hyperglycemia was observed in nine women at the second and third points, one of them was prescribed insulin therapy. Blood glucose levels differed significantly between groups Ia and Ib. Thus, patients in group Ib had a higher level of glycemia in all three points of OGTT, compared to patients of group Ia, where only the third indicator of blood glucose level was significantly higher, and it was 8.63 mmol/L (0.26) (p=0.001). Insulin therapy was prescribed by endocrinologists; the gestation period was 29.7 (0.6) weeks.
Thus, insulin therapy (group Ib) was prescribed to 24 out of 82 pregnant women with GDM (29.3%); most of them (n=20) had increased level of fasting glucose and ineffective diet therapy (83.3%), two women showed increased glucose level at the second point (8.3%), one patient had the increased glucose level at the third point (4.15%), and one patient had it at the second and third points (4.15%).
The results of OGTT are suggestive of the important role of fasting hyperglycemia in pregnant women, which is the most common reason for administering insulin therapy.
The data of OGTT results are presented in Table 1.
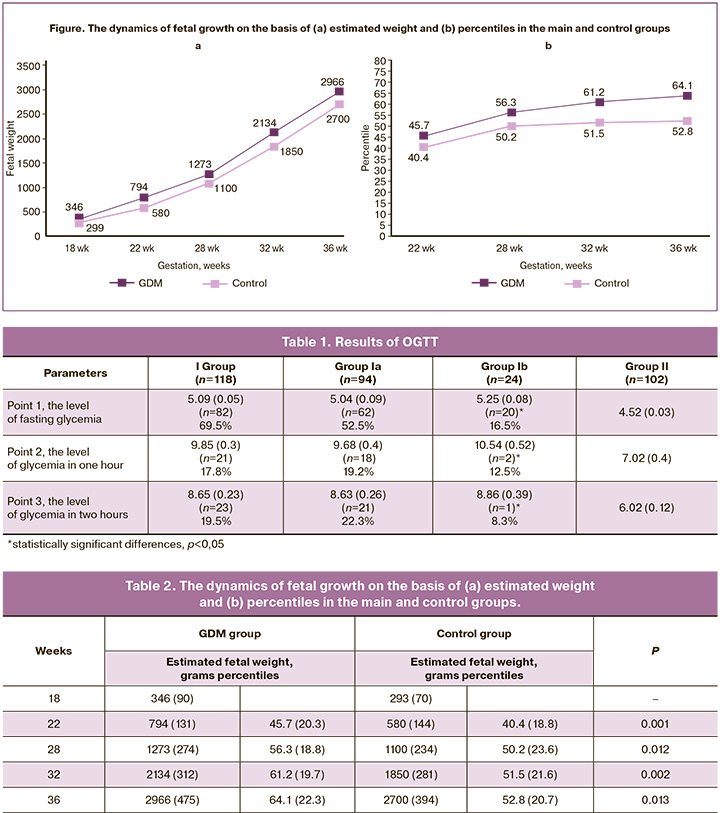
The comparative analysis of the third trimester of pregnancy revealed a similar frequency of anemia, asymptomatic bacteriuria, hydramnios, oligohydramnios, acute respiratory diseases in the groups. Threatened preterm labor was statistically significantly more frequent in group I (11.9%) (p=0.001); in group Ib, its rate was 16.7% (p=0.004), which was statistically significantly more frequent than in the control group. Vaginal dysbiosis was statistically significantly more frequent in the group with GDM (p=0.004), compared to the control group; this fact once again proves that patients with GDM are more susceptible to dysbiotic processes. Hypertensive disorders of pregnant women, namely gestational hypertension, were observed only in five pregnant women of group Ia (5.3%). Edematous syndrome was observed in 11 out of 94 pregnant women of group Ia and in 4 out of 24 pregnant women of group Ib; its frequency did not differ statistically between groups Ia and Ib (p=0.5). However, in comparison with the control group where 4 out of 102 pregnant women had edema, the difference was statistically significant (p=0.0286). Preeclampsia was not detected in the pregnant women from the study groups. Fetal macrosomia was observed in five pregnant women with GDM from group Ia and in one patient from group Ib (4.2%) (p=0.048). The estimated fetal weight varied from 4003.0 to 4614.0 g and corresponded to the 95th percentile as measured by ultrasound fetometry. Fetopathy was noted only in one patient with GDM, who received insulin therapy (4.2%); its manifestation was a double contour of the fetal head, thickening of the subcutaneous fat layer, and severe hydramnios. The analysis of the dynamics of fetal growth on the basis of ultrasound parameters is presented in Figure and Table 2. It can be seen that the tendency to higher fetal weight indicators and percentile values in pregnant women with GDM starts at 18 weeks of gestation.
Discussion
GDM is an important medical and social global problem in obstetric practice, which can have short-term and long-term consequences for the health of mothers and children [6-10]. This study allowed us to establish the multifactorial nature of the pregnancy complication. Most women with GDM were aged 26-37 years (79.6%). In our study, the analysis of heredity revealed that patients with GDM were more likely to have type II diabetes and cardiovascular diseases; these findings are consistent with ones in foreign publications that type II diabetes increases twice the risk for developing GDM [2, 5].
More severe susceptibility to vaginal dysbiotic processes (p=0.004) in patients with GDM has also been found out in our research.
The results of OGTT showed the important role of fasting hyperglycemia in pregnant women, which is the most common cause of insulin therapy; therefore, the appropriate examination should be started before pregnancy and/or in the early stages of pregnancy.
According to the results of ultrasound fetometry, the tendency to higher fetal weight indicators and percentile values in pregnant women with GDM starts at 18 weeks’ gestation.
The obtained data on statistically higher body mass index in the group of patients with GDM in comparison with the control group are consistent with the data on the role of overweight in other studies. Overweight can be a risk factor of GDM [11–12], pregnancy-related nausea and vomiting, accompanied by metabolic disorders [1], which can also be often observed in pregnant women with GDM.
Conclusion
Thus, GDM is a clinically significant multifactorial disease, the course of which depends on the timely detection of this pathology during pregnancy and timely administration of complex therapy. The analysis of clinical and anamnestic characteristics of patients showed that in some cases the disease can be prevented. However, a comprehensive study of this problem will make it possible to develop approaches to the prevention of the disease before pregnancy.
References
- Metzger B.E., Lowe L.P., Dyer A.R., Trimble E.R., Chaovarindr U., Coustan D.R. et al.; HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008; 358(19): 1991- 2002. https://dx.doi.org/10.1056/NEJMoa0707943.
- Kharroubi A.T., Darwish H.M. Diabetes mellitus: The epidemic of the century. World J. Diabetes. 2015; 6(6): 850-67. https://dx.doi.org/10.4239/wjd. v6.i6.850.
- McIntyre H.D., Colagiuri S., Roglic G., Hod M. Diagnosis of GDM: a suggested consensus. Best Pract. Res. Clin. Obstet. Gynaecol. 2015; 29(2): 194-205. https://dx.doi.org/10.1016/j. bpobgyn.2014.04.022.
- Huhn E.A., Massaro N., Streckeisen S., Manegold-Brauer G., Schoetzau A., Schulzke S.M. et al. Fourfold increase in prevalence of gestational diabetes mellitus after adoption of the new International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria. J. Perinat. Med. 2016; 45(3): 359- 66. https://dx.doi.org/10.1515/jpm-2016-0099.
- Chan J.C., Malik V., Jia W., Kadowaki T., Yajnik C.S., Yoon K.-H. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009; 301(20): 2129-40. https://dx.doi.org/10.1001/jama.2009.726.
- Ornoy A., Reece E.A., Pavlinkova G., Kappen C., Miller R.K. Effect of maternal diabetes on the embryo, fetus, and children: congenital anomalies, genetic and epigenetic changes and developmental outcomes. Birth Defects Res. C Embryo Today. 2015; 105(1): 53-72. https://dx.doi.org/10.1002/ bdrc.21090.
- Rayanagoudar G., Hashi A.A., Zamora J., Khan K.S., Hitman G.A., ThangaratinamNS. Quantification of the type 2 diabetes risk in women with gestational diabetes: a systematic review and meta-analysis of 95,750 women. Diabetologia 2016; 59(7): 1403-11. https://dx.doi.org/10.1007/s00125-016-3927-2.
- Gupta Y., Gupta A. Post-partum screening after gestational diabetes. Lancet Diabetes Endocrinol. 2013; 1(2): 90-1. https://dx.doi.org/10.1016/S2213- 8587(13)70066-4.
- Eades С.Е., Styles M., Leese G.P., Cheyne H., Evans J.M. Progression from gestational diabetes to type 2 diabetes in one region of Scotland: an observational follow-up study. BMC Pregnancy Childbirth. 2015; 15(1): 11. https://dx.doi.org/10.1186/s12884-015-0457-8.
- Webber J., Charlton M., Johns N. Diabetes in pregnancy: management of diabetes and its complications from preconception to the postnatal period (NG3). Br. J. Diabetes. 2015; 15(3): 107-11.
- Cheng Y.W., Chung J.H., Kurbisch-Block I., Inturrisi M., Shafer S., Caughey A.B. Gestational weight gain and gestational diabetes mellitus: perinatal out- comes. Obstet. Gynecol. 2008; 112(5): 1015-22. https://dx.doi.org/10.1097/ AOG.0b013e31818b5dd9.
- Olmos P.R., Borzone G.R., Olmos R.I., Valencia C.N., Bravo F.A., Hodgson M.I. et al. Gestational diabetes and pre-pregnancy overweight: possible factors involved in newborn macrosomia. J. Obstet. Gynaecol. Res. 2012; 38(1): 208-14. https:// dx.doi.org/10.1111/j.1447-0756.2011.01681.x.
Received 07.02.2020
Accepted 23.06.2020
About the Authors
Zulfiya S. Khodzhaeva, M.D., Professor, Deputy Director of Obstetrics Institute, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Healthcare of Russian Federation. Tel.: +7(916)407-75-67. E-mail: zkhodjaeva@mail.ru.117997, Russia, Moscow, Akademika Oparina str., 4.
Nina V. Snetkova, graduate student in the Department of Pregnancy Pathology, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Healthcare of Russian Federation. Tel.: +7(915)113-31-98. E-mail: ninasnetkova@mail.ru.
117997, Russia, Moscow, Akademika Oparina str., 4.
Kamilla T. Muminova, Ph.D., Junior researcher the Department of Pregnancy Pathology, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Healthcare of Russian Federation. Tel.: +7(916)373-77-07. E-mail: k_muminova@oparina4.ru.
117997, Russia, Moscow, Akademika Oparina str., 4.
Ksenia A. Gorina, Junior researcher the Department of Pregnancy Pathology, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Healthcare of Russian Federation. Tel.: +7(926) 649-77-32. E-mail: k_gorina@oparina4.ru.
117997, Russia, Moscow, Akademika Oparina str., 4.
Maria Ye. Abramova, graduate student in the Department of Pregnancy Pathology, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Healthcare of Russian Federation. Tel.: +7(917)577-12-77. E-mail:
m_abramova@oparina4.ru. 117997, Russia, Moscow, Akademika Oparina str., 4.
Roza M. Yesayan, Ph.D., head of the therapeutic department, National Medical Research Center for Obstetrics, Gynecology and Perinatology named
after Academician V.I. Kulakov Ministry of Healthcare of Russian Federation. Tel.: +7(926)395-81-06. E-mail: rozaes@mail.ru.
117997, Russia, Moscow, Akademika Oparina str., 4.
For citation: Khodzhaeva Z.S., Snetkova N.V., Muminova K.T., Gorina K.A., Abramova M.E., Esayan R.M. Clinical characteristics of pregnancy in women with gestational diabetes mellitus.
Akusherstvo i Ginekologiya / Obstetrics and gynecology. 2020; 7: 47-52 (in Russian)
https://dx.doi.org/10.18565/aig.2020.7.47-52



