Determination of the prerequisites for fetal macrosomia using biomarkers in the first trimester of pregnancy
Tysyachnyi O.V., Baev O.R., Zimina A.O., Krechetova L.V.
Low levels of maternal serum PAPP-A in the first trimester of pregnancy are associated with an increased risk of developing fetal growth restriction and/or low-weight fetuses. There is evidence of a correlation between the blood plasma concentration of PAPP-A in a pregnant woman and the weight of the fetus in the 1st, 2nd, and 3rd trimesters. However, when studying the relationship between PAPP-A and/or β-hCG and the birth of a large fetus, the data are contradictory, highlighting the need for further research to clarify the possibility of predicting fetal macrosomia.
Objective: To determine the relationship between prenatal screening indicators in the first trimester and the development of a large fetus.
Materials and methods: This retrospective cohort study included 408 healthy primiparas. The patients were divided into a study group (n=298), including newborns weighing up to 4000 g, and a control group (n=110), including newborns weighing 4000 g or more. In the first trimester, the levels of β-hCG, PAPP-A, and PIGF in the blood serum were assessed using an enzyme immunoassay.
Results: The data obtained showed that the levels of PAPP-A (MoM) were 1.08 versus 1.2 (p=0.04), PIGF (pg/ml) 21.18 versus 25 (p=0.008), and PIGF (MoM) 0.78 versus 0.85 (p=0.04), which was significantly higher in the group with large fetuses. Additionally, there was a direct correlation between the studied markers and birth weight.
Conclusion: The data obtained demonstrated the formation of associations with the development of macrosomia as early as the first wave of trophoblast invasion. However, its realization apparently requires a combination of predisposing factors.
Authors’ contributions: Tysyachnyi O.V. – statistical analysis, drafting of the manuscript; Baev O.R. – editing the manuscript; Zimina A.O. – data collection and processing; Krechetova L.V. – study design.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors’ Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Tysyachnyi O.V., Baev O.R., Zimina A.O., Krechetova L.V. Determination of the prerequisites for fetal macrosomia using biomarkers
in the first trimester of pregnancy.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (1): 68-73 (in Russian)
https://dx.doi.org/10.18565/aig.2023.236
Keywords
The introduction of combined biochemical screening in obstetric practice has made it possible to predict the development of various complications during pregnancy. Initially, serum markers were studied to screen for fetal chromosomal abnormalities, but they are now also used to diagnose placentation disorders such as preeclampsia and fetal growth restriction [1].
It is known that low maternal serum levels of pregnancy-associated plasma protein A (PAPP-A) in the first trimester of pregnancy are linked to an increased risk of fetal growth restriction or low birth weight [2–4]. Several studies have shown a direct correlation between high PAPP-A levels in the first trimester of pregnancy and fetal macrosomia [5–8]. For instance, Chełchowska et al. found a correlation between the concentration of PAPP-A in the blood plasma of a pregnant woman and fetal weight in the first, second, and third trimesters of pregnancy [9]. Schwartz et al. reported that the size of the placenta at birth correlates with birth weight, suggesting its role in the development of fetal macrosomia [10]. They also noted a reciprocal relationship between the concentration of this glycoprotein in umbilical cord blood and fetal weight [11].
However, some studies have failed to confirm the relationship between macrosomia and PAPP-A levels and/or the β-subunit of human chorionic gonadotropin (β-hCG) during the first trimester of pregnancy [12, 13]. According to Monari et al. (2021), the simultaneous consideration of factors such as birth parity and body mass index improves predictive accuracy [14].
Therefore, existing data on the relationship between biochemical markers in the first trimester of pregnancy and fetal macrosomia are contradictory, and further research is necessary to clarify the potential for predicting the formation of a large fetus using these markers.
This study aimed to determine the relationship between first-trimester prenatal screening indicators and the development of large-for-gestational-age fetuses.
Materials and methods
We conducted a retrospective cohort study at Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation analyzing 9672 births from an electronic database from 2022 to 2023.
The inclusion criteria were primiparous women, aged 18 to 40 years, spontaneous singleton pregnancy, cephalic presentation of the fetus, full-term pregnancy, normal body mass index, weight gain during pregnancy of less than 12 kg, and delivery that occurred at the V.I. Kulakov NMRC for OG&P.
The criteria for non-inclusion were obesity, weight gain of more than 12 kg during pregnancy, severe somatic comorbidities, complicated pregnancy (preeclampsia, gestational hypertension, gestational diabetes mellitus), developmental anomalies of the female genital organs, fetal malformations, and multiple pregnancies.
Clinical and anamnestic data, the nature of the pregnancy of women, and the condition of children at birth were obtained from archival delivery case notes and neonatal case notes.
All women underwent combined prenatal screening in the first trimester at 11+1–13+6 weeks of pregnancy. We assessed the levels of β-hCG, PAPP-A, and placental growth factor (PlGF). After collecting maternal blood samples from the antecubital, blood serum levels of β-hCG, PAPP-A, and PlGF were determined using an enzyme-linked immunosorbent assay (ELISA; DELFIA Xpress, USA) and the corresponding DELFIA Xpress kit reagents (Wallac Oy, Turku, Finland). Since the concentration of placental hormones is influenced by gestational age, determined by the crown-rump length of the fetus, age and maternal anthropometric data, and medical history, using the Astraia Software Version 2.8 program (Germany), the absolute values were converted into multiples of the medians. The indicators were considered reference values ranging from 0.5 to 2.0 MoM.
In total, 408 women met the inclusion criteria. After passing the selection criteria, all patients were divided into two groups. Group 1, study group (n=298), included newborns weighing up to 4000 g; Group 2, control group (n=110), included newborns weighing 4000 g or more.
The primary outcome was the differences in the studied markers between groups; the secondary outcome was the relationship between the levels of screening markers in the first trimester and the size of the fetus.
The study was reviewed and approved by the Research Ethics Committee of V.I. Kulakov NMRC for OG&P.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 22 for Windows” and MedCalc version 22.007 for Windows. The normality of the distribution was tested using the Shapiro–Wilk test. Quantitative variables showing normal distribution were expressed as mean (M) and standard deviation (SD) and presented as M (SD); otherwise, the median (Me) with interquartile range (Q1; Q3) was reported. Equality of variance was assessed using Fisher's test. Categorical variables were described using frequency and percentage (%) and compared using Pearson’s chi-square test (χ2). Differences were considered statistically significant at p<0.05. Correlation analysis was performed using Spearman's rank coefficient, and differences were considered significant at p˂0.05.
Results
We found no differences between the study groups in terms of age, average body mass index, and total body weight gain during pregnancy, frequency and structure of somatic comorbidities, gynecological history and the course of pregnancy, which is presented in Table 1.
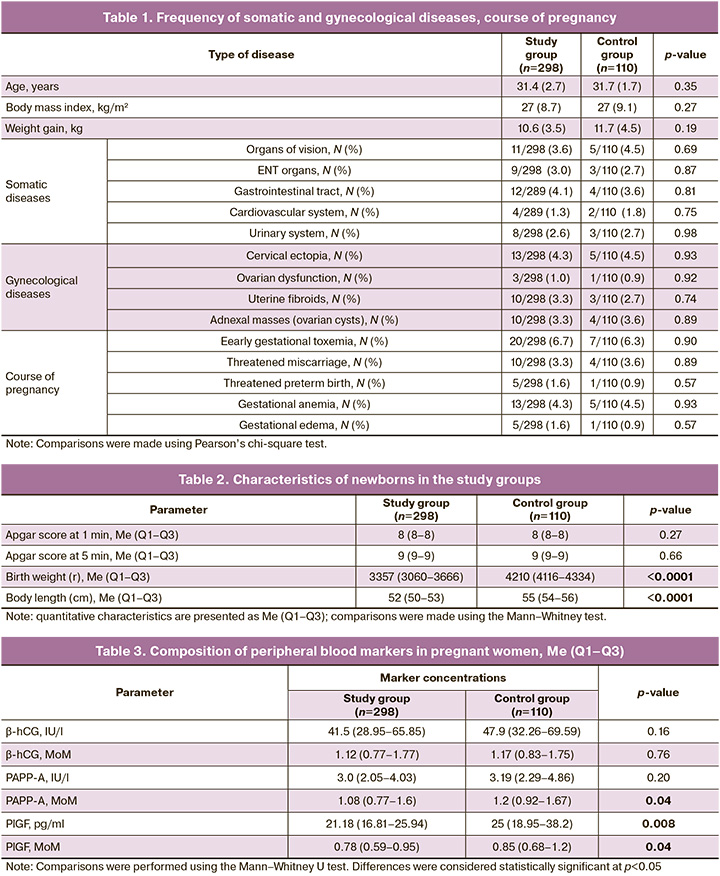
The gestational age at the time of delivery did not differ between the groups, and in the study group it was 278.3 (2.7) days – 39 weeks 5 days versus 278.8 (1.9) days, or 39 weeks 5 days in the control group (p=0.32).
In all the cases, the children were born alive. The Apgar scores at 1 min were not significantly different (p=0.27). At the 5th minute, no significant differences were found between the groups (p=0.66). An assessment of the anthropometric data of newborns revealed significantly greater birth weight and body length in the group of large fetuses (p<0.0001). The characteristics of the newborn groups are presented in Table 2.
In the first stage, we compared the levels of β-hCG, PAPP-A, and PlGF between the study and control groups. Our data showed that the levels of PAPP-A (MoM), PlGF (pg/ml), and PIGF (MoM) were significantly higher in the group with large fetuses (Table 3).
To assess the relationship between biochemical parameters and birth weight, correlation analysis was performed (Table 4).
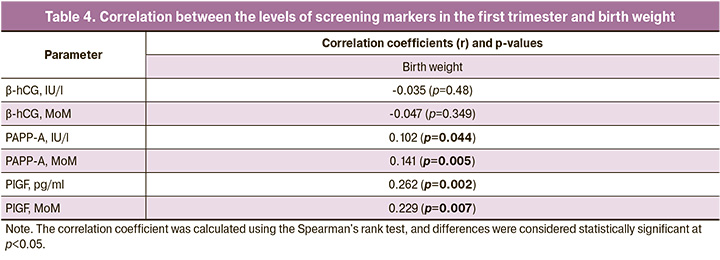
The analysis found a very weak association between large fetal size and PAPP-A (MoM), PlGF (MoM) and a weak association with PlGF (pg/ml). An assessment of the relationship between biochemical parameters and fetal weight showed a very weak relationship with PAPP-A (IU/l) and PAPP-A (MoM), and a weak relationship with PlGF (pg/ml) and PlGF (MoM).
Discussion
In our study, we investigated the relationship between prenatal screening indicators in the first trimester and the risk of developing a large fetus. It is established in the literature that advanced maternal age (> 35 years), body mass index (BMI) exceeding 29 kg/m2, weight gain of over 10 kg during pregnancy, and parity of more than two births are all risk factors for the development of a large fetus [15–17]. Consequently, in our study, we specifically selected primiparous women with a normal BMI and weight gain during pregnancy, thereby excluding the influence of these factors and focusing solely on placental risk factors.
The literature indicates that PAPP-A levels <0.4 mmol and β-hCG <0.3 MoM are correlated with a high risk of low gestational weight in fetuses [18]. In our study, we did not observe any differences in β-hCG levels between the study groups. However, we found variations in the levels of PAPP-A (MoM), Placental Growth Factor (PlGF) (pg/ml), and PlGF (MoM).
PAPP-A is a zinc-containing metalloproteinase that belongs to the metzincin superfamily. The concentration of PAPP-A increase during pregnancy and delivery. Low protease levels during the first trimester of pregnancy in women without chromosomal abnormalities are associated with adverse perinatal outcomes, including intrauterine growth restriction, miscarriage, and low birth weight [19]. Ozdemir S. et al., in their study, demonstrated a positive correlation between PAPP-A (MoM) levels and fetal birth weight (p=0.04) [20]. Our data revealed that PAPP-A (MoM) in women with large fetuses was 1.2, which was significantly higher than that in women with normal fetuses 1.08 (p=0.04). Similar findings were reported by Tul N. et al., who showed that PAPP-A content (MoM) of 0.76 MoM is typical for low-birth-weight newborns (p=0.002), and 1.12 MoM for a large fetus (p=0.03) [21].
Research on PlGF has garnered considerable interest as the placenta is its primary source [22]. Circulating levels of PlGF significantly increase during pregnancy and stimulate the proliferation, migration, and activation of endothelial cells, playing a central role in the development and maturation of the placental vascular system and circulation [23]. Additionally, PlGF has been shown to play a role in the development and function of the placental vasculature [24]. PlGF levels increase from early pregnancy to 29–32 weeks and then decrease, reflecting a decline in production as the placenta ages and diminishes in functional capacity [25]. Previous studies have indicated that very low levels of PlGF in maternal serum (111.5 µmol/l) in the first trimester contribute to the development of decompensated forms of placental insufficiency and premature termination of pregnancy (r=0.89, p<0.01) [26]. Our findings indicate that when the fetus is large, the PlGF content is significantly higher than when it is normal (25 versus 21.18 pg/ml, p=0.008 or 0.85 versus 0.78 MoM, p=0.04). Similar results were reported by Zbucka-Kretowska et al., who showed that large fetuses had higher PlGF levels (p=0.04) [27].
Furthermore, we identified a direct correlation between the markers studied and birth weight.
Conclusion
The data obtained demonstrated the formation of associations for the development of macrosomia as early as the first wave of trophoblast invasion. However, it appears that a combination of other predisposing factors (BMI >29 kg/m2, weight gain >10 kg during pregnancy, gestational diabetes mellitus, and parity of more than two births) is necessary for its occurrence.
The strength of this study lies in being the first domestic study to establish a association between these markers and fetal macrosomia. One of the main limitations of the present study was the relatively small sample size.
Therefore, further investigation of prenatal screening indicators in the first trimester will help to identify a risk group for the development of interventions to prevent fetal macrosomia in primiparous women.
References
- Ижойкина Е.В., Трифонова Е.А., Куценко И.Г., Степанов И.А., Гавриленко М.М., Степанов В.А. Возможность прогнозирования задержки роста плода на основе определения биомаркеров в плазме крови. Акушерство и гинекология. 2023;2:18-24. [Izhoykina E.V., Trifonova E.A., Kutsenko I.G., Stepanov I.A., Gavrilenko M.M., Stepanov V.A. Feasibility of predicting fetal growth restriction, by identifying plasma biomarkers. Obstetrics and Gynecology. 2023;(2):18-24. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.269.
- Honarjoo M., Zarean E., Tarrahi M., Kohan S. Role of pregnancy-associated plasma protein A (PAPP-A) and human-derived chorionic gonadotrophic hormone (free β-hCG) serum levels as a marker in predicting of small for gestational age (SGA): A cohort study. J. Res. Med. Sci. 2021;26(1):104. https://dx.doi.org/10.4103/jrms.JRMS_560_20.
- Mohamad Jafari R., Masihi S., Barati M., Maraghi E., Sheibani S., Sheikhvatan M. Value of pregnancy-associated plasma protein-A for predicting adverse pregnancy outcome. Arch. Iran Med. 2019; 22(10):584-7.
- Poon L.C.Y., Karagiannis G., Stratieva V., Syngelaki A., Nicolaides K.H. First-trimester prediction of macrosomia. Fetal Diagn. Ther. 2011; 29(2):139-47. https://dx.doi.org/10.1159/000318565.
- Gabbay-Benziv R., Esin S., Baschat A.A. Incorporating first trimester analytes to predict delivery of a large for gestational infant in women with impaired glucose tolerance. J. Perinat. Med. 2015;43(3):299-303. https://dx.doi.org/10.1515/jpm-2014-0041.
- Baer R.J., Lyell D.J., Norton M.E., Currier R.J., Jelliffe-Pawlowski L.L. First trimester pregnancy-associated plasma protein-A and birth weight. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016;198:1-6. https://dx.doi.org/10.1016/j.ejogrb.2015.12.019.
- Rossi A., Vogrig E., Ganzitti L., Forzano L., Simoncini G., Romanello I. et al. Prediction of large-for-gestation neonates with first-trimester maternal serum PAPP-A. Minerva Ginecol. 2014;66(5):443-7.
- Turrado Sánchez E.M., De Miguel Sánchez V., Macía Cortiñas M. Correlation between PAPP-A levels determined during the first trimester and birth weight at full-term. Reprod. Sci. 2023;30(11):3235-42. https://dx.doi.org/10.1007/s43032-023-01270-4.
- Chełchowska M., Gajewska J., Mazur J., Ambroszkiewicz J., Maciejewski T.M., Leibschang J. Serum pregnancy-associated plasma protein A levels in the first, second and third trimester of pregnancy: relation to newborn anthropometric parameters and maternal tobacco smoking. Arch. Med. Sci. 2016;6:1256-62. https://dx.doi.org/10.5114/aoms.2016.62908.
- Schwartz N., Quant H.S., Sammel M.D., Parry S. Macrosomia has its roots in early placental development. Placenta. 2014;35(9):684-90.https://dx.doi.org/10.1016/j.placenta.2014.06.373.
- DiPrisco B., Kumar A., Kalra B., Savjani G.V., Michael Z., Farr O. et al. Placental proteases PAPP-A and PAPP-A2, the binding proteins they cleave (IGFBP-4 and -5), and IGF-I and IGF-II: Levels in umbilical cord blood and associations with birth weight and length. Metabolism. 2019;100:153959.https://dx.doi.org/10.1016/j.metabol.2019.153959.
- Dugoff L., Hobbins J.C., Malone F.D., Porter T.F., Luthy D., Comstock C.H. et al. First-trimester maternal serum PAPP-A and free-beta subunit human chorionic gonadotropin concentrations and nuchal translucency are associated with obstetric complications: A population-based screening study (The FASTER Trial). Am. J. Obstet. Gynecol. 2004;191(4):1446-51.https://dx.doi.org/10.1016/j.ajog.2004.06.052.
- Goetzinger K.R., Singla A., Gerkowicz S., Dicke J.M., Gray D.L., Odibo A.O. The efficiency of first-trimester serum analytes and maternal characteristics in predicting fetal growth disorders. Am. J. Obstet. Gynecol. 2009; 201(4):412.e1-412.e6. https://dx.doi.org/10.1016/j.ajog.2009.07.016.
- Monari F., Menichini D., Spano’ Bascio L., Grandi G., Banchelli F., Neri I. et al. A first trimester prediction model for large for gestational age infants: a preliminary study. BMC Pregnancy Childbirth. 2021;21(1):654. https://dx.doi.org/10.1186/s12884-021-04127-3.
- Одинокова В.А., Шмаков Р.Г., Чаговец В.В. Прогнозирование, профилактика и тактика ведения беременности и родоразрешения при фетальной макросомии. Акушерство и гинекология. 2018;1:14-20. [Odinokova V.A., Shmakov R.G., Chagovets V.V. Fetal macrosomia: prediction, prevention, and tactics for management of pregnancy and delivery. Obstetrics and Gynecology. 2018;(1):14-20. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.1.14-20.
- Yadav H., Lee N. Factors influencing macrosomia in pregnant women in a tertiary care hospital in Malaysia. J. Obstet. Gynaecol. Res. 2014;40(2):439-44. https://dx.doi.org/10.1111/jog.12209.
- Nkwabong E., Nzalli Tangho G.R. Risk factors for macrosomia. J. Obstet. Gynecol. India. 2015;65(4):226-9. https://dx.doi.org/10.1007/s13224-014-0586-4.
- Kirkegaard I., Henriksen T.B., Uldbjerg N. Early fetal growth, PAPP-A and free β-hCG in relation to risk of delivering a small-for-gestational age infant. Ultrasound Obstet. Gynecol. 2011;37(3):341-7. https://dx.doi.org/10.1002/uog.8808.
- Costa M.A. The endocrine function of human placenta: an overview. Reprod. Biomed. Online. 2016;32(1):14-43. https://dx.doi.org/10.1016/j.rbmo.2015.10.005.
- Ozdemir S., Sahin O., Acar Z., Demir G.Z., Ermin E., Aydin A. Prediction of pregnancy complications with maternal biochemical markers used in down syndrome screening. Cureus. 2022;14(3):e23115. https://dx.doi.org/10.7759/cureus.23115.
- Tul N., Pusenjak S., Osredkar J., Spencer K., Novak-Antolic Z. Predicting complications of pregnancy with first-trimester maternal serum free-betahCG, PAPP-A and inhibin-A. Prenat. Diagn. 2003;23(12):990-6.https://dx.doi.org/10.1002/pd.735.
- Павлов К.А., Дубова Е.А. Щеголев А.И. Фетоплацентарный ангиогенез при нормальной беременности: роль плацентарного фактора роста и ангиопоэтинов. Акушерство и гинекология. 2010;6:10-5. [Pavlov K.A., Dubova Ye.A., Shchegolev A.I. Fetoplacental angiogenesis during normal pregnancy: a role of placental growth factor and angiopoietins. Obstetrics and Gynecology. 2010;(6):10-5 (in Russian)].
- Yanachkova V., Staynova R., Stankova T., Kamenov Z. Placental growth factor and pregnancy-associated plasma protein-A as potential early predictors of gestational diabetes mellitus. Medicina (Kaunas). 2023;59(2):398.https://dx.doi.org/10.3390/medicina59020398.
- Ahmed A., Dunk C., Ahmad S., Khaliq A. Regulation of placental vascular endothelial growth factor (VEGF) and placenta growth factor (PlGF) and soluble Flt-1 by oxygen – a review. Placenta. 2000;21:S16-24.https://dx.doi.org/10.1053/plac.1999.0524.
- Levine R.J., Maynard S.E., Qian C., Lim K.-H., England L.J., Yu K.F. et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 2004;350(7):672-83. https://dx.doi.org/10.1056/NEJMoa031884.
- Баринов С.В., Рогова Е.В., Кадцына Т.В. Шамина И.В. Прогнозирование плацентарной недостаточности при многоплодной беременности на основании определения фактора роста плаценты. Акушерство и гинекология. 2015;7:43-7. [Barinov S.V., Rogova E.V., Kadtsyna T.V., Shamina I.V. Prediction of placental insufficiency in multiple pregnancy, by identifying placental growth factor. Obstetrics and Gynecology. 2015;(7):43-7 (in Russian)].
- Zbucka-Kretowska M., Kuzmicki M., Telejko B., Goscik J., Ciborowski M., Lipinska D. et al. First-trimester irisin and fetuin-A concentration in predicting macrosomia. J. Matern. Neonatal Med. 2019;32(17):2868-73.https://dx.doi.org/10.1080/14767058.2018.1450859.
Received 11.10.2023
Accepted 04.12.2023
About the Authors
Oleg V. Tysyachnyi, Ph.D., Researcher at the 1st Maternity Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, o_tysyachny@oparina4.ru, 117997, Russia, Moscow, Oparina str., 4, https://orcid.org/0000-0001-9282-9817Oleg R. Baev, Dr. Med. Sci., Professor, Head of the Maternity Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4; Professor of the Department of Obstetrics, Gynecology, Perinatology, and Reproductology, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia, 119991, Russia, Moscow, Trubetskaya str., 8-2, +7(495)438-11-88,
o_baev@oparina4.ru, https://orcid.org/0000-0001-8572-1971
Anna O. Zimina, PhD, Teaching Assistant at the Department of Obstetrics and Gynecology of the Department of Professional Education, obstetrician-gynecologist of the 1st Maternity Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia,
+7(905)706-84-81, anne-89@mail.ru, 117997, Russia, Moscow, Ac. Oparina str., 4, https://orcid.org/0000-0001-8555-144X
Lubov V. Krechetova, Dr. Med. Sci., Head of the Laboratory of Clinical Immunology, Academician V.I. Kulakov National Medical Research Center for Obstetrics,
Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-11-83, 117997, Russia, Moscow, Ac. Oparina str., 4, https://orcid.org/0000-0001-5023-3476



