Preimplantation genetic testing using haplotype-based single nucleotide polymorphisms analysis
Aim. To develop and apply haplotype-based single nucleotide polymorphisms (SNP) analysis for preimplantation genetic testing for monogenic diseases (PGT-M) using chips developed by Affymetrix, Inc., USA.Ekimov A.N., Karetnikova N.A., Shubina E.S., Gol'tsov A.Yu., Kuznetsova M.V., Mukosei I.S., Ritcher O.V., Trofimov D.Yu.
Materials and methods. Twenty-three families with monogenic pathology underwent testing with an integrated approach simultaneously using PGT-M (fragment analysis of STR markers, SNP haplotyping, Sanger sequencing, real-time PCR) and PGT for aneuploidy (PGT-A) (NGS and aCGH).
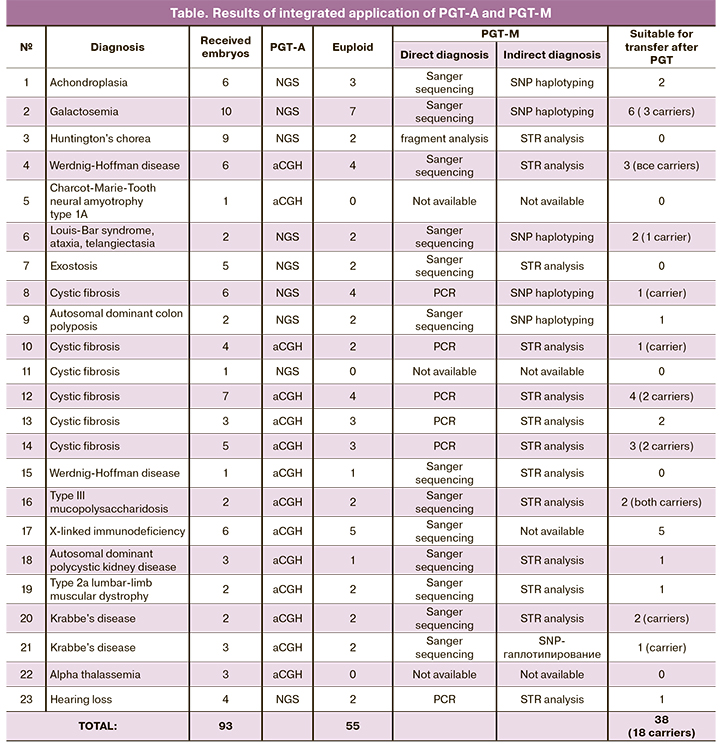
Results. Out of 93 embryos sent for testing, 55 were euploid. In three families, OGT-M was not performed due to the complete absence of euploid embryos after OGT-A. In the remaining 20 observations, 38 embryos (20 without mutations and 18 with heterozygous carriers) were transferred into the uterine cavity. Interpretation of SNP haplotyping results was more straightforward and reliable than the STR analysis. This technique is universal and does not require individual development for each case. The results of SNP haplotyping are described in detail for two cases, including PGT-M for achondroplasia (autosomal dominant inheritance) and galactosemia (recessive inheritance).
Conclusion. The versatility and reliability of the developed approach significantly accelerate obtaining the results, facilitating their interpretation, and reducing the likelihood of possible errors. Also, OGT-M should be preceded by OGT-A for the prevention of chromosomal pathology.
Keywords
Preimplantation genetic diagnosis (PGD) identifies genetic abnormalities in preimplantation embryos before embryo transfer in assisted reproductive technologies (ART) [1]. PGD is performed for couples with known genetic conditions to prevent transferring their genetic abnormality to their offspring. Currently, this method is termed as a preimplantation genetic diagnosis for monogenic disorders (PGT-M) [2]. PGT-M can be performed using direct and indirect diagnosis [3]. Direct diagnosis can detect a direct genetic disease (gene or chromosomal mutation that cause genetic abnormalities). However, using only direct methods may be insufficient due to the allele dropout [1].
Indirect diagnosis cannot detect a direct genetic disorder, but they can trace the inheritance of the corresponding parental chromosome regions. The classical method of indirect PGT-M is the fragment analysis of STR markers. It is based on the analysis of short tandem repeats (STR) [4]. This approach is also not without its drawbacks. First, successful testing requires the development of appropriate methods and time for their validation. Secondly, as noted above, for each testing, several such methods are needed simultaneously. In addition to the problem of allele dropout, some markers may not be informative due to the coincidence between the father and mother.
In 2010, karyomapping was proposed as an alternative to conventional PCR-based protocols. Essentially, karyomapping involves analyzing single nucleotide polymorphisms (SNP) in both parents [5]. Due to its technological features, this method allows PGT-M to be very rapidly performed, and the results are simply interpretable. It can be used to test any monogenic familial disease without the need to develop and validate a patient-specific test.
Karyomapping is a genome-wide linkage analysis in which several hundred thousand SNP scattered throughout the genome are genotyped in the two parents and their embryos. Each chromosomal region has a unique SNP fingerprint, allowing the inheritance of chromosomal segments (and the genes they contain) to be tracked from one generation to the next. By comparing SNP results obtained from the parents to those obtained from other family members of known genetic status (e.g., another relative carrying the same mutation as one of the parents), the combination of SNP alleles associated with a chromosome carrying a mutant gene can be identified. As a result, the transfer of embryos carrying this chromosome (or SNP pattern) can then be avoided.
Karyomapping is relatively expensive and can be performed using only one platform based on biological chips from Illumina, which limits its widespread use. However, there is currently a high clinical need for such methods, which are not tied to a limited number of technological platforms. This led to the development of several alternative approaches based on the same principle [6, 7].
The present study aimed to develop and apply haplotype-based SNP analysis for PGT-M using chips developed by Affymetrix, Inc., USA.
Materials and methods
Twenty-three families with monogenic diseases (patients who were referred, their children or relatives) were examined using PGT at the Molecular Genetics Laboratory of the Center. Characteristics of the pathology are presented in Table 1. Due to the small amount of material obtained for analysis (trophectoderm), the study began using genome-wide DNA amplification. The choice of the method depended on the subsequent testing approaches. For SNP haplotyping, MDA (Qiagen, USA) was used, in other cases, WGA-PCR (Picoplex, USA).
The testing was an integrated approach with the simultaneous use of PGT-M and PGT aneuploidy (PGT-A). PGT-M was performed using fragment analysis of STR markers, SNP haplotyping, Sanger sequencing; real-time polymerase chain reaction (PCR) was used to analyze the most frequent mutations (for example, F508del in cystic fibrosis, GJB2: 35delG in congenital sensorineural hearing loss). PGT-A was performed using next-generation sequencing (NGS) (ReproSeq ™ PGS Kit Thermo Fisher Scientific, USA) and array-based comparative genomic hybridization (aCGH) analysis (GenetiSure Agilent, USA).
An approach to SNP haplotyping was developed in the framework of this study. Sample preparation included restriction, ligation with adapters, amplification, purification of PCR products, fragmentation, fluorophore labeling, following the manufacturer's protocol (Affymetrix Inc., USA). After the samples passed all the quality controls required by the manufacturer, the obtained samples were applied to high-resolution CytoScan 750K microchips. Then, DNA hybridization was carried out for 18 hours at a temperature of 50°C. Subsequently, the microchips were washed and stained on a Fluidic Station 450 (ThermoFisher, USA) and scanned with a Scanner 3000 7G (ThermoFisher, USA). The results were analyzed using the Chromosome Analysis Suite (ChAS) software. Subsequently, lists of genotypes were compiled for SNP haplotypes presented on the microarrays.
Using genotyping data of both parents and sick child, the key positions were determined, by which it was possible to unambiguously determine which of the child’s chromosomes inherited from one of the parents. Comparing genotypes in blocks of key positions of a given size in a patient and embryos made it possible to determine whether the same or different fragments of chromosomes were inherited from the parents and to select embryos to prevent transferring parents' disease.
Since many points are examined simultaneously to determine inheritance, the allele drop-out phenomenon at individual points does not affect the result. The rest of the methods were carried out in a standard way.
Results
As shown in the table, there were significantly fewer euploid embryos (n=55) than all those sent for testing (n=93). According to the PGT-A data, in observations 5, 11, and 22, the PGT-M was inappropriate since all the embryos were aneuploid. Among the remaining 20 cases, where the analysis continued, only 38 embryos transfer were suitable for transfer. Of these, 20 had no mutations, and 18 were heterozygous carriers. In 14 out of 23 observations (table), PGT-A was performed using aCGH and 9 using NGS. The findings indicated that both methods were informative for detecting embryo aneuploidies. At the same time, NGS allows, in addition, for obtaining information about the level of chromosomal mosaicism.
It was found that the results of SNP haplotyping are influenced by the method of whole genome amplification. Valid results can only be obtained with MDA. In the course of the study, it was found that the interpretation of SNP haplotyping results was more straightforward and more reliable than the STR analysis. This technique does not require individual development for each case and is universal. Although in this study its results were confirmed by direct methods, SNP haplotyping can be used alone.
Here is a description of two clinical cases.
Case 1
Patient N., 22 years old, consulted the Center for ART due to her husband's achondroplasia. His earlier molecular genetic analysis showed the G1138A (Gly380Arg) mutation in the FGFR3 gene in a heterozygous state. The spouses were examined. The woman was phenotypically normal, somatically healthy, the menstrual cycle was normal. The man had teratozoospermia. There was an isolated case of achondroplasia in the family.
Given the high risk of achondroplasia in the offspring (50% due to the autosomal dominant type of inheritance) and the couple’s desire to undergo PGT-M, they were referred to reproductive specialists for molecular genetic examination.
Sequencing data confirmed that the man had heterozygous carriage of the mutant FGFR3 gene (Gly380Arg), while the woman had no mutations. Sequencing of the man's father and mother also did not reveal this mutation, which confirmed its de-novo occurrence.
After a clinical examination and an in vitro fertilization using the ICSI, 13 eggs were obtained, and 6 embryos were cryopreserved. Indirect DNA testing was performed using SNP haplotyping. It was found that embryos 1, 3, 4, 6 have one paternal allele of the FGFR3 gene, while embryos 2 and 5 have another. To validate the results obtained by direct sequencing according to Sanger, we searched for the Gly380Arg mutation in the FGFR3 gene in embryos 1, 2, 3, 4, 5, 6. In embryos 3 and 4, the Gly380Arg mutation was detected in the FGFR3 gene in a heterozygous state. In embryos 2 and 5, this mutation was not found, and valid data could not be obtained for embryos 1 and 6.
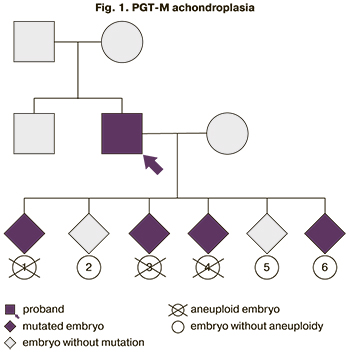 These findings suggested that embryos 1, 3, 4, 6 had the Gly380Arg mutation in the FGFR3 gene.
These findings suggested that embryos 1, 3, 4, 6 had the Gly380Arg mutation in the FGFR3 gene.
To exclude chromosomal pathology in the presented samples of trophectoderm, PGT-A was performed using the aCGH (Agilent, USA). A balanced genotype was found in the material of embryos 2, 5, and 6. As a result of the combined use of PGT-M and PGT-A in the framework of the IVF, no contraindications to the transfer of embryos 2 and 5 into the uterine cavity were revealed (Fig. 1). Patients were recommended invasive prenatal diagnostics at the onset of pregnancy to confirm the results of preimplantation testing.
One embryo was transferred into the uterine cavity, but pregnancy did not occur. The woman was offered contraception with the subsequent transfer of the second examined embryo. However, after four months, the woman had a spontaneous pregnancy. Considering the high risk of having a child with achondroplasia, an invasive prenatal diagnosis was proposed. At 11–12 weeks of gestation, the patient underwent transabdominal choriocentesis with no complications.
Chorionic tissue was examined by cytogenetic and molecular genetic methods. Cytogenetic analysis showed a normal male karyotype (46, XY). Sequencing showed a mutation in the FGFR3 gene (Gly380Arg) in a heterozygous state similar to the paternal one. Due to a fetal mutation associated with achondroplasia, the Center's council recommended the woman to undergo the pregnancy termination. The pregnancy was terminated at 12–13 weeks without complications. Contraception with subsequent transfer of the examined embryo into the uterine cavity was proposed.
Case 2
Patient Z., 30 years old, consulted the Center for ART due to the absence of pregnancy despite having regular unprotected sex for four years. She had a history of spontaneous pregnancy and uneventful delivery of a full-term boy, who died on the 17th day in the hospital's intensive care unit. According to the autopsy, the child has a brain tumor, acute liver failure, agalactosemia. His blood analysis revealed the mutation c.A563G (p.Q188R) in a homozygous state in the GALT gene. A similar disorder in the heterozygous state was found in the child's father. The mother was suspected of carrying an extended deletion in the GALT gene. She was found to have a heterozygous microdeletion of the segment of the short arm of chromosome 9 from position 34646154 to position 34651207, capturing the 9p13.3 region with the involvement of the GALT gene (arr [hg19] 9p13.3 (34646154_34651207) x1).
The couple was examined at the Center. The woman had no abnormalities, while the man had teratozoospermia. There were no contraindications to ART. IVF/ICSI protocol was carried out, yielding 19 oocytes and ten embryos. The obtained trophectoderm material underwent PGT-A using NGS. Various chromosomal abnormalities were found in embryos 6, 3, and 9. Later, these embryos were not used for indirect DNA testing.
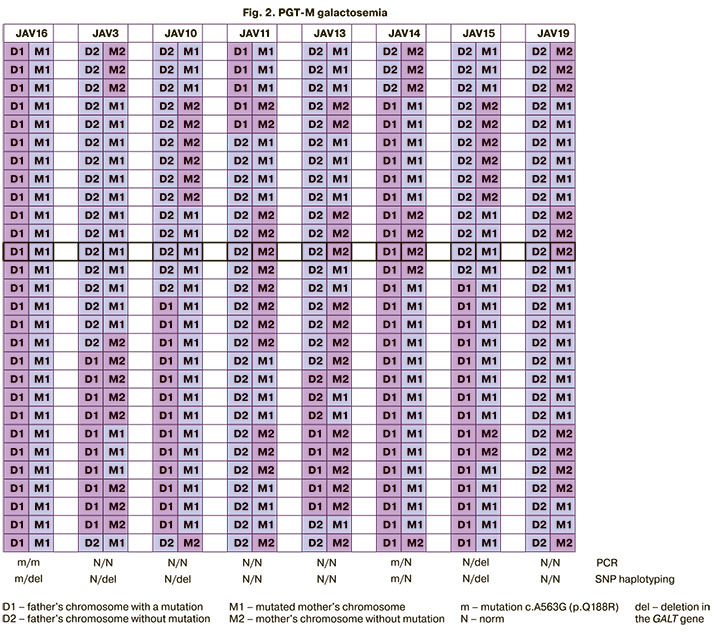
Indirect DNA testing using SNP haplotyping showed that embryos 11, 13, and 19 had no mutations. Embryo 14 has a heterozygous Q188R mutation and no deletion. Embryos 10 and 15 had a heterozygous deletion and no Q188R mutation, and embryo 16 had both mutations. To validate these results, real-time PCR was used to detect the Q188R mutation, which was confirmed in the man in a heterozygous state and two embryos in a homozygous (embryo 16) and heterozygous (embryo 14) state.
Combined results of PGT-M and PGT-A suggested that there were no contraindications to the transfer of embryos 11, 13, and 19 in the IVF program (no mutations), as well as embryos 10, 14, and 15 (only one of the mutations in a heterozygous state, that is, they are carriers of an autosomal recessive pathology, Fig. 2). Patients were recommended to undergo invasive prenatal diagnostics in case of pregnancy to confirm PGT results.
Discussion
As noted above, direct genetic testing alone may not be enough for PGT. A small amount of biological material (single blastomeres, polar bodies, trophectoderm samples) warrants their amplification to use amplification products for further analysis. The amplification from each of the parental chromosomes can proceed with different efficiency, leading to allele drop-out. When analyzing such an unbalanced amplification, both false-positive results (selective amplification of a pathogenic allele in a heterozygous state can lead to an erroneous interpretation that it is in a homozygous state) and false-negative ones are possible. This means that detecting a homozygous state of a normal allele can also happen when a mutant allele drops out. Still, in the case of testing autosomal recessive diseases, the transfer of such an embryo is in any case possible since the child will be healthy. If a homozygous state of a mutant allele is found, such an embryo will be recognized as unsuitable for transfer. However, in the event of a loss of a normal allele, such an embryo would be recognized as a carrier and could be recommended for transfer. Thus, we see that in the case of PGT-M autosomal recessive diseases, such a phenomenon as allele drop-out cannot lead to the transfer of embryos with two mutations and the birth of a sick offspring, but in some cases, heterozygous embryos suitable for transfer can be discarded [1].
The situation with autosomal dominant diseases is much more challenging. On the one hand, detecting the homozygous state of the mutant allele is unambiguously interpreted as a contraindication for embryo transfer. On the other hand, detecting the homozygous state of the normal allele is not sufficient for considering an embryo unsuitable for transfer. Indeed, in case of a loss of a mutant allele, the child will develop a related disease. Therefore, the effect of allele dropout when only direct genetic testing is used can lead to both the transfer of embryos with pathogenic alleles and the decision to forgo the transfer of healthy embryos that are carriers of the mutant allele [3].
For indirect PGT-M, at present, as a rule, fragment analysis of short tandem repeats (STR, short tandem repeats) is used [4]. It is based on the fact that the human genome contains the so-called STRs, repeating sequences of two, three, four, or more nitrogenous bases. Such repeats are located practically throughout the entire human genome, and there is a theoretical possibility to select the appropriate markers for almost every studied region. Since these markers differ in different people, it is possible, by assessing their presence in embryonic DNA, to determine which parental chromosome (containing or not containing the pathogenic allele) entered the embryonic genome. Thus, even if it is impossible to detect a mutation in the genome of an embryo by a direct diagnosis , its genetic status can be predicted. The problem of allele dropout is also present in indirect testing. However, it is possible to select several markers at once and test several of them at once. All in all, these findings suggest the possibility to conclude about the genetic status of the embryo under study [6].
The main advantage of SNP haplotyping for PGT-M is the versatility of this approach. As seen from the examples above, this approach can be applied to various genetic diseases. There is no need to select and test specific STR markers depending on the studied pathology, which complicates and slows down the analysis. Interpretation of PGT results is unified, which reduces the likelihood of erroneous results. The main disadvantage of the method is its high cost due to the need for chromosomal microarray analysis for each sample, including samples of embryos, parents, and affected relatives. At the same time, given the rapid advances of modern technologies, reducing the cost of this technique will allow it to cover a significant number of patients requiring PGT-M.
Even with SNP haplotyping for PGT-M, direct testing cannot be abandoned entirely, since in some cases (including autosomal dominant inheritance), the genetic status of embryos cannot be determined only by indirect testing. However, according to our data, direct methods did not always provide the expected results due to the allele dropout effect, which was not observed when using SNP haplotyping.
Aneuploidy testing in female embryos in ART (with the further use of only normal ones), as shown in many studies, leads to better chances of a progressive pregnancy and reduces the risks of recurrent pregnancy loss and birth of children with aneuploidies. Therefore, combining testing for monogenic genetic diseases with screening for aneuploidy improves the clinical performance of PGT.
Conclusion
The present study's findings suggest that patients requiring OGT-M should also be advised to undergo OGT-A since there is a risk of aneuploidies in their embryos in any age group of patients. OGT-M alone does not guarantee embryo transfer without chromosomal pathology.
The following universal scheme for PGT seems to be optimal.
1. Whole-genome amplification by MDA on the obtained samples.
2. NGS‑based PGT-A to identify embryos' chromosomal status.
3. NGS‑based SNP haplotyping in the presence of euploid embryos.
4. Validation of results by direct mutation testing (optional).
5. Analysis of the findings and reporting on the possibility of tested embryos' transfer.
The introduction into clinical practice of the approach developed in this study will increase the availability of OGT for patients due to its reliability and less time consuming procedure.
References
- Delhanty J.D.A., Harper J.C. Pre-implantation genetic diagnosis. Baillieres Best Pract. Res. Clin. Obstet. Gynaecol. 2000; 14(4): 691-708. https://dx.doi.org/10.1053/beog.2000.0105.
- Zegers-Hochschild F., Adamson G.D., Dyer S., Racowsky C., de Mouzon J., Sokol R. et al. The international glossary on infertility and fertility care. Hum. Reprod. 2017; 108(3): 393-406. https://dx.doi.org/10.1016/j.fertnstert.2017.06.005.
- Gigarel N., Frydman N., Burlet P., Kerbrat V., Steffann J., Frydman R. et al. Single cell co-amplification of polymorphic markers for the indirect preimplantation genetic diagnosis of hemophilia A, X-linked adrenoleukodystrophy, X-linked hydrocephalus and incontinentia pigmenti loci on Xq28. Hum. Genet. 2004; 114(3): 298-305. https://dx.doi.org/10.1007/s00439-003-1063-9.
- Traversa M.V., Carey L.,Leigh D. A molecular strategy for routine preimplantation genetic diagnosis in both reciprocal and Robertsonian translocation carriers. Mol. Hum. Reprod. 2010; 16(5): 329-37. https://dx.doi.org/10.1093/molehr/gaq013.
- Handyside A.H., Harton G.L., Mariani B., Thornhill A.R., Affara N., Shaw M.A., Griffin D.K. Karyomapping: a universal method for genome wide analysis of genetic disease based on mapping crossovers between parental haplotypes. J. Med. Genet. 2010; 47(10): 651-8. https://dx.doi.org/10.1136/jmg.2009.069971.
- Masset H., Zamani Esteki M., Dimitriadou E., Dreesen J., Debrock S., Derhaag J. et al. Multi-centre evaluation of a comprehensive preimplantation genetic test through haplotyping-by-sequencing. Hum. Reprod. 2019; 34(8): 1608-19. https://dx.doi.org/10.1093/humrep/dez106.
- Zamani Esteki M., Dimitriadou E., Mateiu L., Melotte C., Van der Aa N., Kumar P. et al. Concurrent whole-genome haplotyping and copy-number profiling of single cells. Am. J. Hum. Genet. 2015; 96(6): 894-912. https://dx.doi.org/10.1016/j.ajhg.2015.04.011.
Received 24.12.2020
Accepted 24.02.2021
About the Authors
Alexey N. Ekimov, Clinical Geneticist at the Molecular Genetics Laboratory, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.Tel.: +7(495)531-44-44. Е-mail: a_ekimov@oparina4.ru. ORCID: 0000-0001-5029-0462. 4, Ac. Oparin str., Moscow, Russian Federation, 117997.
Natalia A. Karetnikova, Clinical Geneticist at the Molecular Genetics Laboratory, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Tel.: +7(495)531-44-44. Е-mail: n_karetnikova@oparina4.ru. 4, Ac. Oparin str., Moscow, Russian Federation, 117997.
Ekaterina S. Shubina, Head of Genomic Data Analysis Laboratory, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Tel.: +7(495)531-44-44. Е-mail: e_shubina@oparina4.ru. 4, Ac. Oparin str., Moscow, Russian Federation, 117997.
Andrey Yu. Gol’tsov, Researcher at the Molecular Genetics Laboratory, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Tel.: +7(495)531-44-44. Е-mail: andrey.goltsov@gmail.com. 4, Ac. Oparin str., Moscow, Russian Federation, 117997.
Maria V. Kuznetsova, Ph.D. (biol.sci.), Senior Researcher at the Molecular Genetics Laboratory, V.I. Kulakov NMRC, Ministry of Health of Russia.
Tel.: +7(495)438-13-41. Е-mail: mkarja@mail.ru. 4, Ac. Oparin str., Moscow, Russian Federation, 117997.
Irina S. Mukosey, Researcher at the Molecular Genetics Laboratory, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Tel.: +7(495)531-44-44. Е-mail: irina.mukosey@yandex.ru. 4, Ac. Oparin str., Moscow, Russian Federation, 117997.
Olga V. Ritcher, Clinical Laboratory Pathologist at the Molecular Genetics Laboratory, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Tel.: +7(495)531-44-44. Е-mail: o_ritcher@oparina.ru. 4, Ac. Oparin str., Moscow, Russian Federation, 117997.
Dmitry Yu. Trofimov, Professor, Head of the Institute of Reproductive Genetics, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Tel.: +7(495)531-44-44. Е-mail: d_trofimov@oparina4.ru. 4, Ac. Oparin str., Moscow, Russian Federation, 117997.
For citation: Ekimov A.N., Karetnikova N.A., Shubina E.S., Gol'tsov A.Yu., Kuznetsova M.V., Mukosei I.S., Ritcher O.V., Trofimov D.Yu. Preimplantation genetic testing using haplotype-based single nucleotide polymorphisms analysis.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 5: 100-107 (in Russian)
https://dx.doi.org/10.18565/aig.2021.5.100-107



