Chromosomal abnormalities in nonimmune hydrops fetalis: diagnosis and management of pregnancy
Lyushnina D.G., Tetruashvili N.K., Shubina Je., Rogacheva M.S., Zaretskaya N.V., Bolshakova A.S., Barkov I.Yu., Sadelov I.O., Pak V.S., Bokeriya E.L., Trofimov D.Yu.
Objective: To study the role of chromosomal abnormalities and optimal methods for their genetic testing in patients diagnosed with nonimmune hydrops fetalis (NIHF).
Materials and methods: After perinatal consultation and genetic counselling, 43 pregnant women with NIHF were selected; they underwent invasive prenatal diagnosis at 15 to 30 weeks’ gestation. The quantitative fluorescence polymerase chain reaction (QF-PCR) method was used in the first step of testing fetal material to detect abnormalities of chromosomes 13, 18, 21, X, Y. Molecular karyotyping using DNA microarrays was performed in the second step.
Results: The patients were divided into three groups according to the period of NIHF manifestation: group 1 included patients with manifestation of the disease up to 13+6 weeks, group 2 included patients with manifestation from 14 to 21+6 weeks, and group 3 included patients with manifestation from 22 weeks. Chromosomal abnormalities were found to be the most common cause of NIHF in group 1 (78.5%) and were associated with a higher risk of perinatal loss. Live births were noted only in 2 out of 14 cases with early manifestation of NIHF in patients with normal fetal karyotypes. A total of 11/43 (25.6%) patients had chromosomal abnormalities, including Turner syndrome (13.9%), Down syndrome (6.9%), Edwards syndrome (2.3%), and DiGeorge syndrome (2.3%). No chromosomal abnormalities were diagnosed in patients with manifestation of NIHF after 14 weeks, and it was associated with a higher live birth rate.
Conclusion: This study demonstrated the need for invasive prenatal diagnosis, the place and advantages of each of the methods for genetic testing performed prenatally in case of NIHF. The findings of genetic testing are relevant for counselling couples about the prognosis of current pregnancies and for assessing the risk of NIHF recurrence in subsequent pregnancies in a given family.
Authors’ contributions: Lyushnina D.G., Tetruashvili N.K., Shubina Je., Zaretskaya N.V., Bokeriya E.L., Trofimov D.Yu. – developing the concept and design of the study; Lyushnina D.G., Pak V.S., Bolshakova A.S., Sadelov I.O. – collecting and processing the material; Lyushnina D.G., Rogacheva M.S., Shubina Je. – statistical processing of the data; Lyushnina D.G., Tetruashvili N.K., Shubina Je. – writing the text; Tetruashvili N.K., Bolshakova A.S., Shubina Je., Bokeriya E.L.,
Trofimov D.Yu. – editing the article.
Conflicts of interest: Authors declare lack of the possible conflicts of interests.
Funding: The study was performed within the framework of the State Assignment on the subject: “Development of a test system for prenatal diagnosis of fetal cardiopathology”, 2-A21.
Ethical Approval: The study was approved by the Ethical Review Board of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
Patient Consent for Publication: The patients provided an informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Lyushnina D.G., Tetruashvili N.K., Shubina Je., Rogacheva M.S., Zaretskaya N.V., Bolshakova A.S.,
Barkov I.Yu., Sadelov I.O., Pak V.S., Bokeriya E.L., Trofimov D.Yu. Chromosomal abnormalities in
nonimmune hydrops fetalis: diagnosis and management of pregnancy.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (7): 66-73 (in Russian)
https://dx.doi.org/10.18565/aig.2024.75
Keywords
In recent years, there has been a significant progress in the field of molecular genetic diagnostics and fetal medicine. Therefore, understanding the causes of nonimmune hydrops fetalis (NIHF) is of crucial importance. The current literature characterizes NIHF as both a pathological accumulation of fluid in two or more serous cavities of the fetus and a lesion of one serous cavity combined with generalized skin edema (exceeding 5 mm) or cystic hygroma of the fetal neck. Polyhydramnios and placental thickening are common ultrasound findings that are associated with NIHF. The causes of NIHF are different; however, the data from numerous studies suggest that one of the most frequent causes is chromosomal abnormalities in the fetus and their rate ranges widely, from 8 to 80% of cases [6–9].
The total incidence of NIHF is reported to vary from 1 in 1700 to 1 in 1300 pregnancies worldwide [10–12]. In general, most authors agree that NIHF significantly compromises the prognosis of pregnancy and leads to an increased incidence of complications such as antenatal fetal death, preterm labor, stillbirth, neonatal morbidity and mortality. The prognosis in each case depends largely on the term when NIHF manifested, timely diagnosis, presence of chromosomal abnormalities in the fetus, therapy, mode of delivery, and timely therapeutic or surgical care of the newborn [5, 13–16].
The combined prenatal screening during the first trimester most frequently shows cystic hygromas or generalized fetal edema [6, 17]. These patients are mainly referred for termination of pregnancy due to unfavorable fetal prognosis, and the fetal autopsy tissue samples are not subsequently examined. However, not all cases of these anomalies are caused by spontaneous disorder of chromosome number, which has a low probability of recurrence, and not all fetuses develop NIHF later in antenatal life. Thus, prenatal diagnosis of NIHF is important for choosing pregnancy management tactics, assessing the prognosis of both the fetus and the risk of recurrence of NIHF in subsequent pregnancies. In our country, invasive prenatal diagnosis (IPD) in case of NIHF is more commonly performed using cytogenetic testing. Quantitative fluorescence polymerase chain reaction (QF-PCR) and molecular karyotyping using DNA microarrays (CMA) are not currently included in the prenatal screening algorithm. The QF-PCR method is based on multiplex PCR with the use of fluorescently labeled primers that restrict polymorphic short tandem repeats. Its advantage is rapid detection (within 2–3 working days) of frequently occurring chromosomal aneuploidies and low cost of the study. Molecular karyotyping is a method of molecular genetic study of the karyotype which is based on DNA hybridization with microarrays. CMA provides information on microscopic and submicroscopic copy number variations (CNV) in the genome [18]. In addition to resolving power, the advantages of CMA include the requirements of the material (there is no need for dividing cells, examination of the amniotic fluid is performed much faster) and the less subjective nature of the study.
The introduction of these methods into the clinical practice will optimize the pregnancy management tactics and improve the understanding of the genetic causes of NIHF.
Therefore, the aim of the present study was to investigate the role of chromosomal abnormalities and optimal methods for their genetic testing in the diagnosis of NIHP.
Materials and methods
This was a prospective study that included the analysis of the data obtained from 43 pregnant women who sought perinatal care at the National Medical Research Centre for Obstetrics, Gynecology and Perinatology, Moscow. The patients were diagnosed with NIHF at 11 to 30 weeks’ gestation between September 2021 and November 2023. Ultrasound markers of NIHF included nuchal swelling, cystic hygromas of the neck, generalized edema, ascites, and hydrothorax. All patients underwent genetic counseling and were admitted to the Second Obstetric Department of Pregnancy Pathology for IPD. The prenatal stage included questionnaires, general clinical examination, instrumental examination, such as ultrasound assessment with measurement of maximal systolic blood flow velocity in the middle cerebral artery, infection screening and IPD which included transabdominal choriocentesis (TC) or transabdominal amniocentesis (TA).
After obtaining the informed consent from the patients, they were offered IPD. Samples of chorionic villi (CV) or amniotic fluid (AF) were collected at the hospital by TC or TA. TC and TA were performed at 11–14 and 15–30 weeks’ gestation, respectively. The obtained material was sent for infectious screening, which included PCR detection of parvovirus B19, Epstein-Barr virus, cytomegalovirus, herpes simplex virus types 1 and 2, and bacteriologic examination of AF. The first stage of genetic diagnosis consisted of detecting abnormalities of chromosomes 13, 18, 21, X, Y using QF-PCR. If there were no principal fetal aneuploidies, CMA was performed in the second stage. QF-PCR of maternal and fetal tissue samples was performed in all cases due to the risk of contamination with maternal material (Fig. 1).
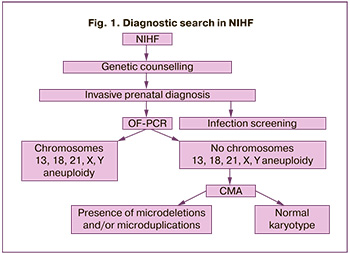
The women with multiple pregnancies, NIHF due to fetal heart rhythm abnormalities, sacrococcygeal teratoma did not meet the criteria for inclusion in the study.
The course and outcomes of pregnancies in three groups were analyzed, depending on the gestational age of NIHF manifestation: group 1 – from 11 weeks to 13+6 weeks (n=14); group 2 – from 14 weeks to 21+6 weeks (n=13); group 3 – from 22 to 30 weeks (n=16). Pregnancies in the above groups resulted in medical termination, antenatal fetal death, live births with signs of NIHF, and live births without signs of NIHF. The patients were followed up by studying their medical records and by telephone interviews; the information was then confirmed by official documents from medical institutions.
Genomic DNA was extracted from CV and AF using the IGENatal kit according to the manufacturer’s instructions. The study was performed using QF-PCR kits at the Institute of Reproductive Genetics, V.I. Kulakov National Medical Research Centre for Obstetrics, Gynecology and Perinatology, Moscow.
CMA was carried out using the GenoScan3000 system on CytoScan Optima microarrays (Thermo Fisher Scientific, USA) according to the manufacturer’s protocol. The obtained data were analyzed using the ChAS software (“Chromosome Analysis Suite”). The obtained CNVs were interpreted into five categories based on the ACMG criteria. The size, location of gene copy number variations, and gene composition were assessed when interpreted according to the guidelines. The clinical significance of CNV was analyzed using the available databases including the Database of Genomic Variants (DGV), Online Mendelian Inheritance in Man (OMIM), ISCA, DECIPHER, and data from the world literature. Pathogenic and probably pathogenic gene copy number variations were reported; the variants of unclear clinical significance were included only if they were highly consistent with the clinical picture. Parental samples (peripheral blood) were also examined to determine the inheritance of CNV.
Statistical analysis
The data were analyzed using Microsoft Excel software (USA). Qualitative parameters were expressed in absolute and relative values (%); quantitative parameters were expressed as median (Me), interquartile range (Q1; Q3), arithmetic mean (M), standard deviation (SD). Fisher’s exact test was used to check statistical significance. At a significance level of p<0.05 the results were considered statistically significant. However, the Bonferroni correction was used because three groups were compared. In pairwise multiple comparisons between groups, p was considered significant at a value of <0.017.
Sidak correction was used for pairwise comparisons.
Results
The clinical and anamnestic data of 43 pregnant women included in the study were analyzed. The age of the patients in three groups was comparable (Table 1). The p value in all comparison cases was > 0.017.
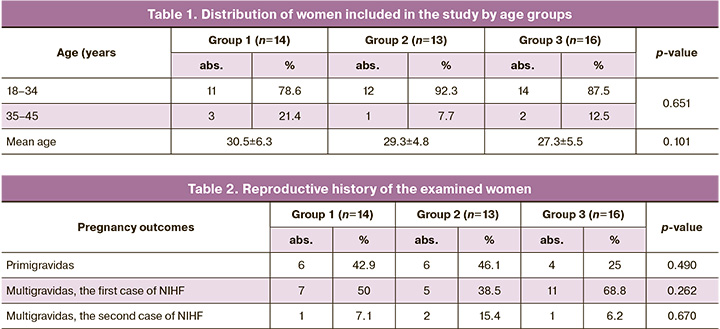
The data of reproductive history presented in Table 2 were comparable among the patients of the study groups. The p value in all comparison cases was >0.017.
Early stage of NIHF manifestation was diagnosed in 14 out of 43 pregnant women (32.5%). These changes were diagnosed during the prenatal screening of the first trimester at 11 weeks to 13 weeks 6 days (group 1). In all cases, the patients underwent genetic counseling and IPD was recommended. On average, the period of diagnosing early manifestation of NIHF was 12+5 weeks. Samples examined in the first trimester included CV samples in 14/14 (100%) cases. QF-PCR was performed in all cases. Chromosomal aneuploidies were revealed in 10/14 (71.4%) cases based on fetal tissue samples analysis, namely 45.X0 fetal karyotype in 6/14 (42.9%) cases, trisomy 21 in 3/14 (21.4%) cases, trisomy 18 in 1/14 (7.1%) case. No chromosomal aneuploidy was found in 4/14 (28.6%) cases; therefore, CMA of fetal tissue samples was performed and Di Georgi syndrome was diagnosed in 1/4 cases.
NIHF manifested at more than 14 weeks in 29/43 (67.4%) cases, from 14 weeks to 21 weeks 6 days (group 2) in 13/43 (30.2%) cases, and from 22 to 30 weeks (group 3) in 16/43 (37.2%) cases (Table 2). QF-PCR and CMA were performed in all cases; no chromosomal aneuploidies, microdeletions and microduplications were found in all 29 cases (Fig. 2).
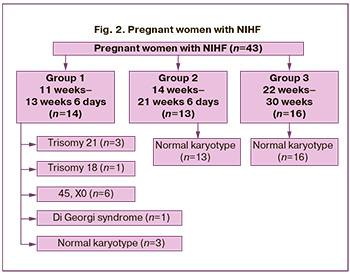
According to the presented data, chromosomal abnormalities were detected only in 11/14 (78.6%) patients of group 1; the differences with groups 2 and 3 were statistically significant, p<0.001 (Table 3).
The data on the period of manifestation and pregnancy outcomes in NIHF are presented in Table 4.
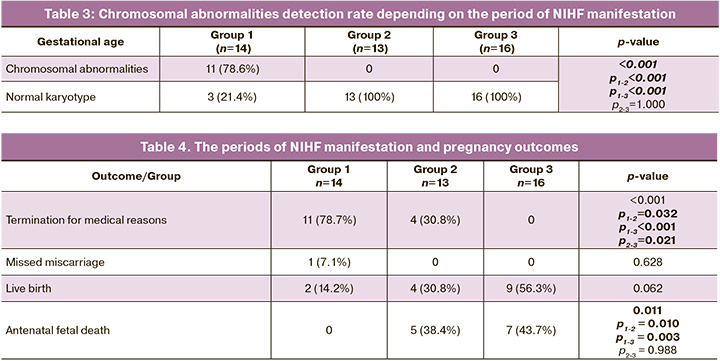
There was a statistically significant difference in the rate of termination for medical reasons among the comparison groups, and it was highest in group 1 and lowest in group 3. The rate of missed miscarriages was comparable among the comparison groups. The rate of live births was highest in group 3, but the differences did not reach the level of statistical significance. The rate of antenatal fetal death in groups 2 and 3 was statistically significantly higher than in group 1.
Thus, chromosomal abnormalities were diagnosed in 11/14 (78.6%) cases in group 1; all of these pregnancies were terminated for medical reasons. The genetic factor was not found in 3/14 (21.4%) cases, and pregnancies were prolonged.
The course of pregnancy was normal in 2 of the 3 cases, the signs of NIHF regressed, and there was a livebirth at 35–36 weeks’ gestation, the newborns were born without signs of hydrops. Antenatal fetal death at 21 weeks’ gestation occurred in 1 of 3 cases.
After obtaining the results of normal molecular karyotype in group 2, the couples decided to terminate pregnancy in 4/13 (30.8%) cases of repeated prenatal counseling due to the increasing severity of NIHF which had a very unfavorable prognosis for the life of the fetus. The pregnancy was prolonged in 9/13 (69.2%) cases, but antenatal fetal death was diagnosed in 5/13 (38.4%) cases (NIHF was probably caused by the presence of cytomegalovirus and parvovirus infections in 2 out of 5 cases). Antenatal death occurred at a median gestational age of 24 weeks (23; 27).
The course of pregnancy was without complications in 4/13 (30.8%) cases. Hydrops fetalis regressed in 2 of the 4 cases and there was a preterm delivery at 35 weeks, the infants are demonstrating growth and development in accordance with their age. There was a timely operative delivery at 38 weeks’ gestation in 1 of 4 cases. A live premature boy was born without any signs of hydrops. Due to the worsening of NIHF and unfavorable prognosis for fetal life after diagnosis of normal karyotype, termination of pregnancy was suggested in 1 of 4 cases; however, the family refused to terminate the pregnancy, and preterm spontaneous delivery occurred at 26 weeks because of premature rupture of membranes. The baby could live for seven days.
After the results of the absence of chromosomal abnormalities were obtained in group 3, all pregnancies were prolonged as there were no gross fetal malformations. Antenatal fetal death occurred in 7/16 (43.7%) cases; the median gestational age of antenatal death was 26 weeks (24; 27). There was a live birth in 9/16 (56.3%) cases; the median gestational age at delivery was 38 weeks (34; 39); the Apgar scores ranged from 1 to 8 points in the first minute and from 3 to 8 points in the fifth minute; the newborns were observed and treated in intensive care units. Infant deaths occurred within the period from 3 hours of life to 9 months in 5 of the 9 cases; of these cases, 4 were classified as early neonatal deaths, and 1 was an infant death. One newborn was diagnosed with cystic adenomatous malformation of the upper lobe of the left lung, but due to complications of the current disease and characteristics of the postoperative period, late neonatal death occurred on the 15th day of life. Hydrops fetalis regressed in 4 out of 9 cases by the time of birth; healthy babies were born and had a score of 6 to 8 on the Apgar scale in the first minute and 7 to 8 in the fifth minute; they are demonstrating growth and development in accordance with their age. In these observations, no genetic cause of NIHF was diagnosed at this stage.
Discussion
This study found that chromosomal abnormalities represent the most common cause of NIHF in the first trimester and are associated with a higher risk of perinatal loss; the findings of the study are consistent with those of other authors [5, 12, 16, 19]. Shereshevsky–Turner syndrome (13.9%), Down syndrome (6.9%), and Edwards syndrome (2.3%) were the most common chromosomal aneuploidies; these results are also consistent with the findings of multicenter studies [6, 9]. According to the world literature, chromosomal abnormalities in NIHF occur in 4–20% of cases at gestational age of more than 14 weeks [7, 19]. In our study, no chromosomal abnormality was diagnosed in patients with gestational age more than 14 weeks. The live birth rate was found to be higher in those cases of NIHF where clinical manifestations occurred at a later gestational age, which is also consistent with the findings of other researchers [6, 9, 16, 17, 19].
This study demonstrated the need for IPD, the place and advantages of each method for genetic testing performed prenatally in NIHF. The choice of testing method depends directly on the time of manifestation, the presence of additional ultrasound markers of chromosomal disease or fetal malformations, the time of performing IPD, and further severity of the course of NIHF. The introduction of the CMA method increases the number of chromosomal abnormalities detected. Therefore, it is possible to confirm or exclude chromosomal abnormalities in the shortest possible time and optimize the management of the pregnancy in each individual case. However, the pathology caused by a single gene dysfunction cannot be detected by these methods, which means that monogenic diseases such as autosomal dominant (in particular, RASopathies) or autosomal recessive diseases cannot be diagnosed but they may cause NIHF [6, 17]. Thus, when a normal molecular karyotype is verified, it is necessary to continue the diagnostic search in the direction of full exome sequencing, which will improve the detectability of the underlying causes of NIHF [4, 20–22].
Conclusion
Due to the different time and variety of clinical manifestations as well as possible outcomes in NIHF, genetic counseling of couples in each case should be based not only on the obtained data on normal karyotype, but also on the data on the presence of fetal anomalies, their compatibility with the life of the child, the severity of hydrops fetalis at the antenatal stage. The above information helps to determine the fetal prognosis, therapeutic options, and the necessity of prolonging the current pregnancy. The findings of genetic testing are relevant for counselling couples about the prognosis of current pregnancies and for assessing the risk of NIHF recurrence in subsequent pregnancies in a given family.
References
- Guo D., He S., Lin N., Dai Y., Li Y., Xu L. et al. Genetic disorders and pregnancy outcomes of non-immune hydrops fetalis in a tertiary referral center. BMC Med. Genomics. 2023; 16(1): 83. https://dx.doi.org/10.1186/s12920-023-01505-y.
- Norton M.E., Chauhan S.P., Dashe J.S.; Society for Maternal-Fetal Medicine (SMFM). Society for maternal-fetal medicine (SMFM) clinical guideline #7: nonimmune hydrops fetalis. Am. J. Obstet. Gynecol. 2015; 212(2): 127-39. https://dx.doi.org/10.1016/j.ajog.2014.12.018.
- Iskaros J., Jauniaux E., Rodeck C. Outcome of nonimmune hydrops fetalis diagnosed during the first half of pregnancy. Obstet. Gynecol. 1997; 90(3):321-5. https://dx.doi.org/10.1016/s0029-7844(97)00290-1.
- Sparks T.N., Lianoglou B.R., Adami R.R., Pluym I.D., Holliman K., Duffy J. et al.; University of California Fetal-Maternal Consortium; University of California, San Francisco Center for Maternal-Fetal Precision Medicine. Exome sequencing for prenatal diagnosis in nonimmune hydrops fetalis. N. Engl. J. Med. 2020; 383(18):1746-56. https://dx.doi.org/10.1056/NEJMoa2023643.
- Кадырбердиева Ф.З., Шмаков Р.Г., Бокерия Е.Л., Тетруашвили Н.К., Костюков К.В., Донников А.Е., Белоусов Д.М. Неиммунная водянка плода: основные причины. Акушерство и гинекология. 2019; 11: 186-91. [Kadyrberdieva F.Z., Shmakov R.G., Bokeriya E.L., Tetruashvili N.K., Kostyukov K.V., Donnikov A.E., Belousov D.M. Nonimmune hydrops fetalis: main causes. Obstetrics and Gynecology. 2019; (11): 186-91. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.11.186-191.
- Cherian A.G., Kamath V., Srivastava V., Danda S., Sebastian T., Beck M.M.Spectrum of chromosomal abnormalities detected by conventional cytogenetic analysis following invasive prenatal testing of fetuses with abnormal ultrasound scans. J. Obstet. Gynaecol. India. 2022; 72(Suppl. 1): 209-16.https://dx.doi.org/10.1007/s13224-022-01626-x.
- Beke A., Joó J.G., Csaba A., Lázár L., Bán Z., Papp C. et al. Incidence of chromosomal abnormalities in the presence of fetal subcutaneous oedema, such as nuchal oedema, cystic hygroma and non-immune hydrops. Fetal Diagn. Ther. 2009; 25(1): 83-92. https://dx.doi.org/10.1159/000201946.
- Laterre M., Bernard P., Vikkula M., Sznajer Y. Improved diagnosis in nonimmune hydrops fetalis using a standardized algorithm. Prenat. Diagn. 2018; 38(5): 337-43. https://dx.doi.org/10.1002/pd.5243.
- He S., Wang L., Pan P., Wei H., Meng D., Du J. et al. Etiology and perinatal outcome of nonimmune hydrops fetalis in Southern China. AJP Rep. 2017; 7(2): e111-e115. https://dx.doi.org/10.1055/s-0037-1603890.
- Heinonen S., Ryynänen M., Kirkinen P. Etiology and outcome of second trimester non-immunologic fetal hydrops. Acta Obstet. Gynecol. Scand. 2000; 79(1): 15-8.
- Hutchison A.A., Drew J.H., Yu V.Y., Williams M.L., Fortune D.W., Beischer N.A. Nonimmunologic hydrops fetalis: a review of 61 cases. Obstet. Gynecol. 1982; 59(3): 347-52.
- Meng D., Li Q., Hu X., Wang L., Tan S., Su J. et al. Etiology and outcome of non-immune hydrops fetalis in Southern China: report of 1004 cases. Sci. Rep. 2019; 9(1): 10726. https://dx.doi.org/10.1038/s41598-019-47050-6.
- Derderian S.C., Jeanty C., Fleck S.R., Cheng L.S., Peyvandi S., Moon-Grady A.J. et al. The many faces of hydrops. J. Pediatr. Surg. 2015; 50(1): 50-4; discussion 54. https://dx.doi.org/10.1016/j.jpedsurg.2014.10.027.
- Berger V.K., Sparks T.N., Jelin A.C., Derderian C., Jeanty C., Gosnell K. et al. Non-immune hydrops fetalis: do placentomegaly and polyhydramnios matter? J. Ultrasound Med. 2018; 37(5):1185-91. https://dx.doi.org/10.1002/jum.14462.
- Gedikbasi A., Oztarhan K., Gunenc Z., Yildirim G., Arslan O., Yildirim D. et al. Preeclampsia due to fetal non-immune hydrops: mirror syndrome and review of literature. Hypertens. Pregnancy. 2011; 30(3): 322-30. https://dx.doi.org/10.3109/10641950903323244.
- Кадырбердиева Ф.З., Шмаков Р.Г., Бокерия Е.Л., Костюков К.В. Тетруашвили Н.К. Эффективность применения алгоритма обследования на антенатальном этапе при неиммунной водянке плода. Акушерство и гинекология. 2020; 7: 71-8. [Kadyrberdieva F.Z., Shmakov R.G., Bokeriya E.L., Kostyukov K.V., Tetruashvili N.K. The effectiveness of the antenatal examination algorithm for nonimmune hydrops fetalis. Obstetrics and Gynecology. 2020; (7): 71-8. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.7.71-78.
- Chainarong N., Muangpaisarn W., Suwanrath C. Etiology and outcome of non-immune hydrops fetalis in relation to gestational age at diagnosis and intrauterine treatment. J. Perinatol. 2021; 41(10): 2544-8. https://dx.doi.org/10.1038/s41372-021-01202-7.
- Faucett W.A., Savage M. Chromosomal microarray testing. JAAPA. 2012; 25(1): 65-6. https://dx.doi.org/10.1097/01720610-201201000-00016.
- Sileo F.G., Kulkarni A., Branescu I., Homfray T., Dempsey E., Mansour S.et al. Non-immune fetal hydrops: etiology and outcome according to gestational age at diagnosis. Ultrasound Obstet. Gynecol. 2020; 56(3): 416-21.https://dx.doi.org/10.1002/uog.22019.
- Al-Kouatly H.B., Shivashankar K., Mossayebi M.H., Makhamreh M., Critchlow E., Gao Z. et al. Diagnostic yield from prenatal exome sequencing for non-immune hydrops fetalis: a systematic review and meta-analysis. Clin. Genet. 2023; 103(5): 503-12. https://dx.doi.org/10.1111/cge.14309.
- Zhou X., Zhou J., Wei X., Yao R., Yang Y., Deng L. et al. Value of exome sequencing in diagnosis and management of recurrent non-immune hydrops fetalis: a retrospective analysis. Front. Genet. 2021; 12: 616392. https://dx.doi.org/10.3389/fgene.2021.616392.
- Люшнина Д.Г., Тетруашвили Н.К., Шубина Е., Зарецкая Н.В., Толмачева Е.Р., Свирепова К.А., Большакова А.С., Пак В.С., Бокерия Е.Л., Трофимов Д.Ю. Лизосомные болезни накопления как одна из причин неиммунной водянки плода. Акушерство и гинекология. 2023; 12: 78-86. [Lyushnina D.G., Tetruashvili N.K., Shubina E., Zaretskaya N.V., Tolmacheva E.R., Svirepova K.A., Bol'shakova A.S., Pak V.S., Bokeriya E.L., Trofimov D.Yu. Lysosomal storage diseases as a cause of non-immune hydrops fetalis. Obstetrics and Gynecology. 2023; (12): 78-86. (in Russian)]. https://dx.doi.org/10.18565/aig.2023.221.
Received 01.04.2024
Accepted 28.06.2024
About the Authors
Daria G. Lyushnina, PhD student, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia,4, Acad. Oparin str., Moscow, Russia, 117997, +7(906)308-60-78, d_lyushnina@oparina4.ru, https://orcid.org/0009-0004-3160-8737
Nana K. Tetruashvili, PhD, Нead of the Obstetric Department of Pregnancy Pathology No. 2, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology
and Perinatology, Ministry of Health of Russia, 4, Acad. Oparin str., Moscow, Russia, 117997, +7(495)438-14-77, n_tetruashvili@oparina4.ru,
https://orcid.org/0000-0002-9201-2281
Jekaterina Shubina, PhD (Bio), Head of Laboratory of Genomic Data Analysis, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 4, Acad. Oparin str., Moscow, Russia, 117997, +7(495)531-44-44, e_shubina@oparina4.ru, https://orcid.org/0000-0003-4383-7428
Margarita S. Rogacheva, Researcher at the Laboratory of Genomic Data Analysis, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 4, Acad. Oparin str., Moscow, Russia, 117997, +7(495)531-44-44, m_rogacheva@oparina4.ru, https://orcid.org/0000-0002-2495-2554
Nadezhda V. Zaretskaya, PhD, Head of the Laboratory of Clinical Genetics of the Department of Clinical Genetics, V.I. Kulakov National Medical Research Center
for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 4, Acad. Oparin str., Moscow, Russia, 117997, +7(495)438-24-11, znadezda@yandex.ru,
https://orcid.org/0000-0001-6754-3833
Anna S. Bolshakova, MD, geneticist at the Department of Clinical Genetics, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 4, Acad. Oparin str., Moscow, Russia, 117997, +7(495 438-24-11, a_bolshakova@oparina4.ru, https://orcid.org/0000-0002-7508-0899
Ilya Yu. Barkov, PhD, Head of the Laboratory of Prenatal DNA Screening, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 4, Acad. Oparin str., Moscow, Russia, 117997, +7(495)438-24-10, i_barkov@oparina4.ru, https://orcid.org/0000-0001-6297-2073
Igor O. Sadelov, MD, geneticist at the Laboratory of Genomic Data Analysis, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 4, Acad. Oparin str., Moscow, Russia, 117997, +7(495)438-24-10, a_sadelov@oparina4.ru, https://orcid.org/0000-0002-5144-6307
Viktoriia S. Pak, PhD student, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia,
4, Acad. Oparin str., Moscow, Russia, 117997, +7(913)897-28-49, v_pak@oparina4.ru, https://orcid.org/0009-0002-1444-9071
Ekaterina L. Bokeriya, PhD, Researcher at the Department of Pathology of Newborn and Prematurely-born Children No. 2, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 4, Acad. Oparin str., Moscow, Russia, 117997, +7(495)438-27-05, e_bokeriya@oparina4.ru,
https://orcid.org/0000-0002-8898-9612
Dmitrii Yu. Trofimov, PhD (Bio), Head of the Department of Clinical Genetics, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 4, Acad. Oparin str., Moscow, Russia, 117997, +7(495)438-49-51, d_trofimov@oparina4.ru, https://orcid.org/0000-0002-1569-8486
Corresponding author: Daria G. Lyushnina, d_lyushnina@oparina4.ru



