Natural killer cell immunoglobulin-like receptors and their ligands in spouses and pregnancy outcomes of recurrent miscarriage treated with immunocytic therapy
Krechetova L.V., Khoroshkeeva O.V., Tetruashvili N.K., Krechetov S.P., Jankevic T.E., Inviyaeva E.V., Trofimov D.Yu., Sukhikh G.T.
Relevance: Impaired fetoplacental blood flow is a primary cause of early pregnancy loss. The formation of the vascular bed during endometrial decidualization and control of trophoblast invasion involves natural killer (NK) cells, which express killer cell immunoglobulin-like receptors (KIR). The action of NK cells on target cells is determined by the interaction between the KIR and HLA class I molecules (HLA I). The characteristics of maternal and paternal KIR and HLA I molecules, which are KIR (KIR-L) ligands, as well as the NK cell-mediated allocompatibility reactions they induce have the potential to influence the efficacy of immunocytic therapy (ICT) during pregnancy.
Objective: To perform typing of classical HLA I and KIR genes in patients and their spouses among couples with idiopathic recurrent miscarriage (RM) to identify the characteristics of KIR genotypes and KIR-L representation in spouses with different pregnancy outcomes.
Materials and methods: This study included 39 married couples with RM. ICT was performed twice for each couple, both outside and during pregnancy. KIR and HLA I gene typing was conducted using maternal and paternal peripheral blood samples. Typing was performed using high-throughput NGS sequencing.
Results: Typing of KIR genes and classical HLA I genes in patients from married couples with idiopathic RM and treatment using ICT did not reveal any specific characteristics of KIR and KIR-L in spouses. Additionally, no association was found between the characteristics of KIR and KIR-L in spouses and pregnancy outcomes that occurred after ICT. These results do not provide sufficient evidence to suggest that KIR genotypes and classical HLA I in spouses are prerequisites for mother-fetus alloincompatibility in RM. Furthermore, the data did not reveal any combinations of KIR and KIR-L in spouses that affect the effectiveness of treatment involving ICT.
Conclusion: The lack of connection between the results of RM treatment involving ICT and the characteristics of the KIR genotypes and classical HLA I of spouses emphasizes the need for further investigation into the immunological processes involved in allogeneic mother-fetus interaction during pregnancy and maternal and paternal ICT. One promising area for further research is the study of KIR gene expression in NK cells of women with normal and pathological pregnancies.
Authors' contributions: Tetruashvili N.K., Trofimov D.Yu., Sukhikh G.T. – conception and design of the study; Khoroshkeeva O.V. – selection of clinical samples; Tetruashvili N.K., Khoroshkeeva O.V. – clinical examination of patients, obtaining biospecimens; Jankevic T.E. – KIR and HLA typing of biological samples; Krechetov S.P., Khoroshkeeva O.V., Krechetova L.V. – analysis of clinical and laboratory data, drafting of the manuscript; Inviyaeva E.V. – editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P (Ref. No: 35 of 09.04.2009).
Patient Consent for Publication: The couple signed a voluntary informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Krechetova L.V., Khoroshkeeva O.V., Tetruashvili N.K., Krechetov S.P., Jankevic T.E., Inviyaeva E.V., Trofimov D.Yu., Sukhikh G.T. Natural killer cell immunoglobulin-like receptors and their ligands in spouses and pregnancy outcomes of recurrent miscarriage treated with immunocytic therapy.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (11): 98-108 (in Russian)
https://dx.doi.org/10.18565/aig.2024.261
Keywords
The treatment of recurrent miscarriage (RM) has been a significant challenge for decades. The immunological aspects of RM have received particular attention and are largely influenced by the immunological principles of allocompatibility in transplantation. This focus has led to the concept of the fetus as a semi-allogeneic graft [1] and the association between immunological disorders and embryo rejection in early pregnancy [2].
According to the World Health Organization definition, RM is diagnosed when three or more consecutive spontaneous abortions occur at a gestational age of up to 22 weeks (ICD-10 code N96). However, it is generally accepted that examinations may be initiated after two consecutive losses, especially in women over 35 years of age [3]. Various authors have noted that in 5–20% of cases, the causes of abortions remain undetermined by modern diagnostic methods [4]. Accordingly, RM is often classified as idiopathic, and its pathogenesis is linked to immunological factors [5]. The validity of this assumption has been reinforced by the successful application of immunomodulatory therapies, primarily immunocytic therapy (ICT), which involves administering allogeneic leukocyte cells to women and has been practiced for over 40 years [6]. Nevertheless, there is currently no clear consensus on the necessity of ICT for treating RM [7], and some studies dispute its advantages over placebo [8]. However, it should be noted that the use of ICT in cases of idiopathic RM is associated with a significant increase in live birth rates [9]. Additionally, there are reports suggesting the benefits of immunization with partner cells compared to those with donor cells [10]. The lack of consensus regarding the effectiveness of ICT may be attributed to the variability in cell isolation procedures, quantity of cells administered, frequency and methods of administration, and grouping of patients without considering their individual history of pregnancy loss [11].
The determination of fetal allogeneity for the mother primarily involves Human Leukocyte Antigen (HLA) genes. This focus stems from long-term efforts to link the causes of pregnancy termination in the early gestational stages with the allelic characteristics of the parents according to the HLA system [12]. However, this hypothesis requires further clarification [13].
The main purpose of HLA is to identify cells carrying foreign genetic information by presenting epitopes of proteins synthesized within the cell as HLA class I molecules (HLA I), which are expressed on the surface of almost all nucleated cells and platelets. When HLA I molecules present epitopes that are absent in the body's own proteins, this leads to the activation of T killer clones within the adaptive immune system, which destroys cells synthesizing foreign proteins.
Cells in the body that have a reduced number of HLA I molecules – due to viral infections or mutations—are characterized as “missing self” and become targets for natural killer (NK) cells [14], which are part of the innate immune system. Notably, NK cells make up to 70% of lymphoid cells in the placenta during the first trimester of pregnancy [15]. Additionally, altered expression of HLA I molecules has been observed in trophoblasts [16], allowing us to recognize NK cells as participants in alloimmune interactions.
Killer-cell immunoglobulin-like receptors (KIRs) play a crucial role in regulating the NK cell response to changes in epitopes of both classical HLA I (A/B/C) and non-classical (HLA-G/F) molecules, which are their ligands (KIR-L). KIRs modulate NK cell functions irrespective of the origin of the presented peptide epitopes, and are classified as either inhibitory (iKIR) or activating (aKIR). The interaction of iKIRs with their ligands (iKIR-L) is essential for "tuning" NK cells to respond to the "loss of self" [17]. KIR genes, such as HLA genes, exhibit high allelic polymorphisms, and not all known genes may be present in KIR haplotypes [18]. Existing A haplotypes are characterized by a high proportion of iKIRs, whereas B haplotypes contain a significant number of aKIRs. The KIR and HLA genes in the human genome are located on different chromosomes, resulting in a vast array of KIR and KIR-L combinations based on fetal genomes [19].
Compatibility testing of KIR and KIR-L sets in the fetus can occur not only after the emergence of NK cell sprouts from the 6th to the 15th week [20], but also after the acquisition of functional properties from the 15th to the 22nd week of gestation [21]. Therefore, in cases of recurrent miscarriage, the interaction of maternal NK cells (decidual NK cells, dNK) in the placenta with two groups of target cells carrying different KIR-L sets, maternal (decidual stromal cells, endothelial cells, and others) and fetal (trophoblasts), is significant.
Considering this, the characteristics of KIR and KIR-L in the mother, fetus, and father can lead to different pregnancy outcomes and varying responses of the maternal immune system to ICT, due to the involvement of NK cells in alloimmune interactions between the mother and fetus, as well as between the mother and father. In light of these considerations, the objective of the present study was to genotype the classical HLA I and KIR genes of patients and their spouses in couples experiencing idiopathic RM and to identify the characteristics of KIR genotypes and KIR-L representation in spouses with differing pregnancy outcomes. This may be viewed as either a contributing factor to the pathogenesis of RM or a prerequisite for the reduced efficacy of treatment involving ICT.
Materials and methods
Patients. The study included 39 married couples who had experienced RM (defined as two or more consecutive spontaneous miscarriages before 22 weeks) [22]. These couples were observed at V.I. Kulakov NMRC for OG&P from 2009 to 2017. The study was reviewed and approved by the Research Ethics Committee of V.I. Kulakov NMRC for OG&P (Ref. No: 35 dated 09.04.2009) as meeting the current guidelines of the Russian Federation and World Medical Association, as outlined in the Declaration of Helsinki. The study included women aged 20–45 years with a morphologically normal fetus based on ultrasound examination (US), and both spouses provided informed consent to participate in the study. Exclusion criteria included a pathological karyotype of the parents; anatomical causes of RM; developmental anomalies and/or congenital defects in the fetus as determined by ultrasound; maternal chronic infectious, oncological, or systemic autoimmune diseases; Rh sensitization during pregnancy; and maternal obesity (body mass index ≥30 kg/m²).
Treatment. All patients received standard treatment in accordance with the algorithm adopted by the Department of Prevention and Treatment of Miscarriage following the clinical guidelines for "Recurrent Miscarriage" [23]. After the administration of anti-inflammatory therapy for a period of two–three months during which normal laboratory parameters were observed, the couples were referred for the alloimmunization procedure. ICT was conducted using lymphocytes from the patient's spouse during both the pre-gestational preparation phase and during the initial trimester of pregnancy. For the procedure, the spouse's lymphocytes were tested to ensure compliance with the donation criteria [24]. A suspension of lymphocytes in 1 ml of saline solution containing between 30 and 50 million cells was used for ICT. The suspension was administered intradermally into the palmar surface of the forearm of female subjects on two occasions outside of pregnancy, specifically on days five–ten of the menstrual cycle, with an interval of one month between each administration. Additionally, the suspension was administered twice during pregnancy, at five–six weeks and eight–nine weeks of gestation. All patients conceived naturally. All couples in the study received the same treatment. The participants were divided into groups based on a single criterion, pregnancy outcome, which serves as an indicator of the effectiveness of ICT.
Biomaterial samples. Whole peripheral blood collected in EDTA test tubes was used as biological material from the spouses. Genomic DNA was isolated from the blood samples using the PROBA-MCh MAX kit (NPO DNA-Technology, Moscow).
Typing of HLA class I genes and determining the presence of KIR-L. For the typing of HLA Class I genes, DNA libraries were prepared using the "Reagent Kit for Preparation of DNA Fragment Libraries of HLA I and II Genes for Genotyping by High-Throughput Sequencing (NGS) HLA-Expert" (NPO DNA-Technology, Moscow, Russian Federation). Using the MiSeq Reagent Kit v3 (Illumina, Inc., San Diego, CA, USA), the resulting library was sequenced on a MiSeq device (Illumina, Inc., San Diego, CA, USA). Typing of HLA Class I genes based on the sequencing results was performed using HLA-Expert software (version 2.0; NPO DNA-Technology, Moscow, Russian Federation). The presence of the KIR-L epitopes A3/11, Bw4, B27, and C1/C2 was determined using HLA Class I alleles.
Typing of KIR genes and determination of KIR genotype. Amplification of DNA regions containing exons of KIR genes and pseudogenes was performed using primers of our own design using the polymerase chain reaction method. Library preparation and sequencing were conducted as described above for the typing of HLA Class I genes. Subsequent typing of KIR genes was carried out using software that is a modification of "HLA-Expert" version 2.0. To determine the presence of KIR genes, the sequences obtained from the sequencing were compared with the reference sequences of exons in the IPD-KIR version 2.8 database. Consequently, the KIR genotype was determined for each study participant, indicating the presence or absence of the following genes in the studied genome: KIR2DL1, KIR2DL2, KIR2DL3, KIR2DL4, KIR2DL5A, KIR2DL5B, KIR2DS1, KIR2DS2, KIR2DS3, KIR2DS4, KIR2DS5, KIR3DL1, KIR3DL2, KIR3DL3, KIR3DS1, KIR2DP1, and KIR3DP. The KIR genotype type was determined based on the presence of typical centromeric and telomeric KIR A and B half-haplotypes using Microsoft Office Excel 2007 software (Microsoft, Redmond, WA, USA).
Description of NK cell alloreactivity in ICT. The participation of maternal NK cells in the alloimmune response during ICT was characterized by a set of states, each with different directions of cytolytic activity that NK cells can acquire as a result of their "tuning" (training), based on the interaction of individual iKIRs with affinity iKIR-L [26]. This approach was developed based on the assessment of NK cell alloreactivity during donor selection for bone marrow transplantation [27], in which the presence of iKIR genes was considered. In our case, the tuning of maternal and paternal NK cells occurred through the interaction of their iKIRs with iKIR-L on both their own and maternal target cells, suggesting the existence of different functional states of NK cells due to the stochastic expression of KIR genes [28].
Maternal NK cells (host) can tune to the cytolytic activity of the "host-versus-graft" (HvG) type, perceiving paternal cells as having "lost the characteristics of their own" when the maternal genome contains both the iKIR and HLA I genes that express affinity iKIR-L, while the paternal genome (graft) lacks the HLA I genes that express affinity iKIR-L. Maternal NK cells can also tune to the cytolytic activity of the "host-versus-host" (HvH) type, perceiving the body's own cells as having "lost the characteristics of their own" if the maternal genome contains the iKIR gene and lacks the HLA I genes that express affinity iKIR-L, while paternal cells do contain such HLA I genes.
In cases where the maternal genome has the iKIR gene and both spouses possess HLA I genes expressing affinity iKIR-L, the mother's NK cells can acquire cytolytic activity against the cells of either spouse when they "lose their own characteristics," resulting in a bidirectional (BD) cytolyticity type. The absence of the iKIR gene in the mother and/or the absence of HLA I genes expressing affinity iKIR-L in both spouses creates a combination in which the mother's NK cells cannot acquire cytolytic activity and must consequently become hyporeactive (HYPO).
Paternal NK cell tuning can be described in a similar manner: cytolytic activity that perceives maternal cells as having “lost their own characteristics” results in a “graft-versus-host” (GvH) reaction; cytolytic activity that perceives paternal cells as having “lost their own characteristics” leads to a “graft-versus-graft” (GvG) reaction; BD protective cytotoxicity occurs when cells of either spouse experience the “loss of their own characteristics,” while the lack of tuning capability corresponds to the HYPO state. Microsoft Office Excel 2007 (Microsoft, Redmond, WA, USA) was used to identify the types of NK cell tuning, according to the described algorithm.
Statistical analysis
Statistical analysis was performed using Microsoft Office Excel 2007 (Microsoft, Redmond, WA, USA) and MedCalc statistical software package version 14.8.1 (MedCalc, Ostend, Belgium). Continuous variables are presented as M (SD), where M is the mean and SD is the standard deviation. The non-parametric Mann-Whitney U test was used to assess differences between clinical groups in continuous variables (Fig. 5). Fisher's exact test was used to compare the frequencies between groups (Figs. 1–4). Differences were considered statistically significant at p<0.05.
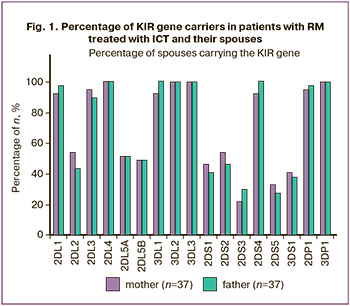
Results
The mean age of the women included in the study was 31.8 (4.7) years. Somatic comorbidities among women included vegetative-vascular dystonia of the hypertensive type (1/39, 2.6%), autoimmune thyroiditis (1/39, 2.6%), hypothyroidism (1/39, 2.6%), diabetes mellitus (1/39, 2.6%), chronic gastritis (1/39, 2.6%), chronic cystitis (2/39, 5.1%), urolithiasis (1/39, 2.6%), and chronic pyelonephritis (1/39, 2.6%). Gynecological comorbidities included chronic endometritis (13/39, 33.3%), chronic salpingo-oophoritis (7/39, 17.9%), uterine myoma (6/39, 15.4%), endometriosis (1/39, 2.6%), endometrial polyps (3/39, 7.7%), intrauterine adhesions (3/39, 7.7%), and cervical ectopy (12/39, 30.8%). Among the reported sexually transmitted infections were chlamydia (4/39, 10.3%), mycoplasma (1/39, 2.6%), and ureaplasma (6/39, 15.4%) infections. The course of pregnancy during the study was uncomplicated in the first trimester for one patient (1/39, 2.6%), in the second trimester for three patients (3/39, 7.7%), and in the third trimester for ten patients (10/39, 25.6%).
In the first trimester, the most prevalent complication was threatened miscarriage (37/39; 94.9%). In the second trimester, threatened miscarriage remained the most common complication (25/39, 64.1%), followed by isthmic cervical insufficiency (14/39, 35.9%). Third trimester complications included threatened preterm birth at 28–31+6 weeks (12/39, 30.7%), 32–34+6 weeks (8/39, 20.5%), and 35–37+6 weeks (9/39, 23.1%). Pregnancy outcomes during the study are presented in table.
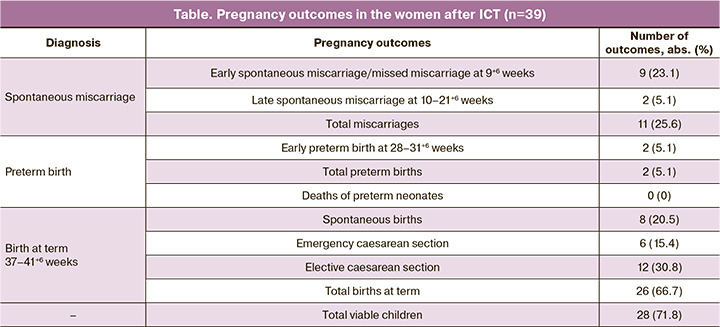
The data indicate that 71.8% of women who underwent treatment gave birth to a viable child. To evaluate the influence of maternal and paternal genetic profiles on ICT efficacy, married couples were classified into two groups based on their pregnancy outcomes: those with full-term pregnancies (n=26) and those with miscarriages during treatment (n=11). Couples with preterm delivery (n=2) were excluded from genotyping because of ambiguity in classifying such outcomes.
Figure 1 shows the results of KIR gene typing in mothers and fathers of married couples with RMs without subdivision into groups. The data in Figure 1 indicate that the proportions of carriers of the studied KIR genes among mothers and fathers in the married couples included in the study were similar and did not differ significantly.
Analysis of the proportion of KIR gene carriers among mothers with RM (Fig. 2A) and their spouses (Fig. 2B) across groups with different pregnancy outcomes during the study did not reveal statistically significant differences in the frequency of KIR gene carriage. No specific combinations of KIR genes were identified in the genotypes of either spouse (Fig. 2С, D), which statistically differentiated the groups with varied outcomes following ICT.
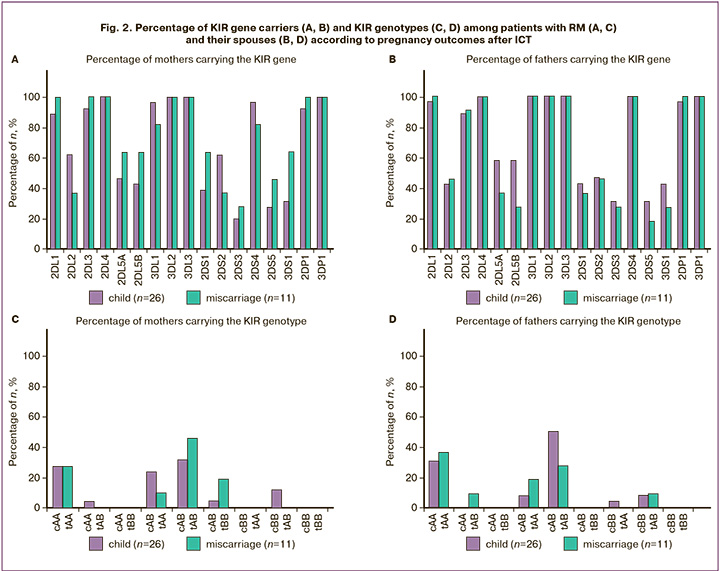
The analysis of the proportion of KIR-L epitope carriers in mothers with RM (Fig. 3A) and their spouses (Fig. 3B), based on HLA I gene typing results, also did not reveal statistically significant differences between the groups with different pregnancy outcomes following treatment with ICT in this study.
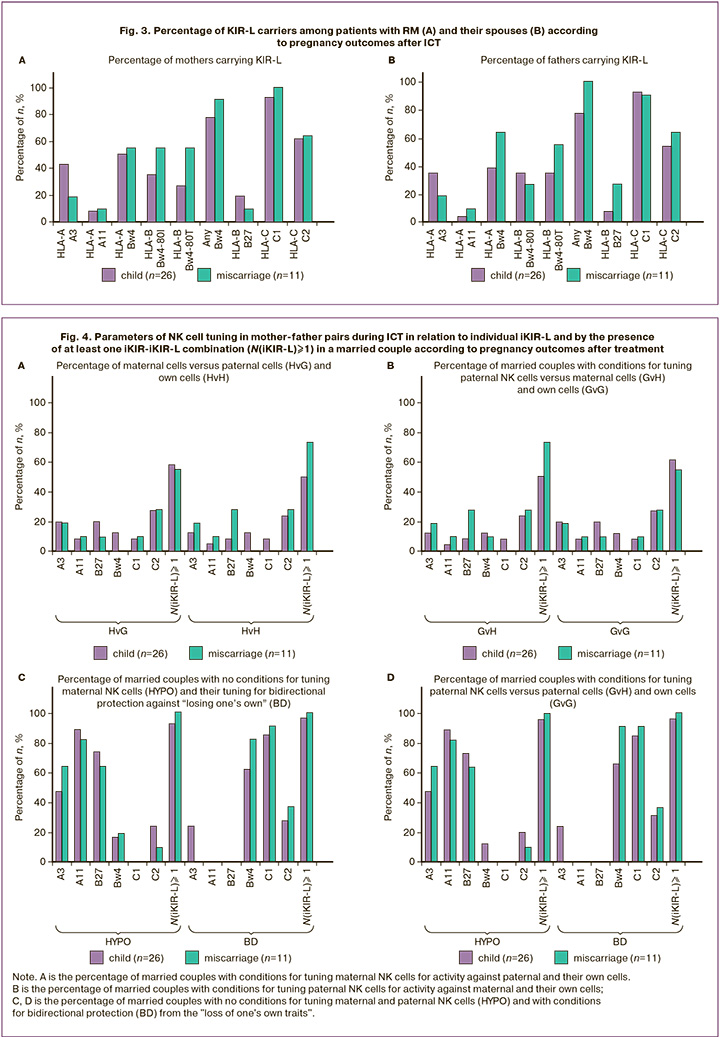
Using the obtained data on the presence of KIR and KIR-L in spouses, an assessment was made (Fig. 4) regarding the possible participation of NK cells in the alloimmune response during ICT, based on the direction of cytolytic activity (see Materials and Methods). Considering the stochastic expression of KIR genes, which often feature the predominance of one or several genes [29], the presence of NK cell populations with one, two, or three iKIR on the surface during ICT should lead to populations of paternal and maternal NK cells in the maternal body exhibiting different directions of cytolytic activity. As shown in the results presented in Figure 4, statistically significant features of NK cell tuning during the interaction of an individual iKIR with affinity iKIR-L in the selected clinical groups, defined by different pregnancy outcomes, were not detected for any of the possible variants of the direction of functional tuning of killers.
For a generalized assessment of alloreactivity in ICT involving NK cells of different origins in the maternal body, we calculated the sums of maternal and paternal iKIR, as well as the sums of maternal and paternal iKIR-L, which led to a specific direction of cytolytic activity, for each married couple. The sums of iKIR and iKIR-L of both spouses were calculated for the following cases: XvH-=(HvH+GvH) – tuning of NK cells of both spouses against maternal cells; XvG-=(HvG+GvG) – against paternal cells; HvX-=(HvG+HvH) – tuning of maternal NK cells against the cells of both spouses; GvX-=(GvH+GvG) – tuning of paternal NK cells against the cells of both spouses. As shown in Figure 5, no differences were found in the proposed estimates of the total alloreactivity of maternal and paternal NK cells in ICT among the clinical groups with different pregnancy outcomes after treatment.
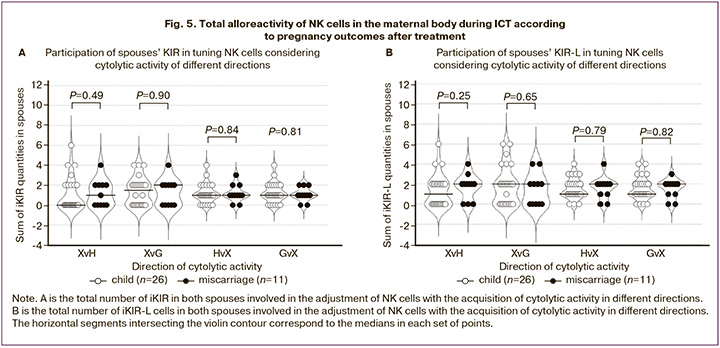
Discussion
This study did not identify any specific characteristics of KIR and classical HLA I genotypes that would determine differential NK cell involvement in the response to ICT in couples experiencing RM. It was observed that various KIR genes and genotypes, as well as HLA-I genes with KIR-L epitopes, were present at the same frequency in both mothers and fathers in couples with differing responses to RM treatment (Fig. 1–3). The lack of linkage between KIR and HLA genotypes due to their location on different chromosomes (19 and 6) and their incompatible inheritance precludes the emergence of specific combinations of iKIR and iKIR-L. The obtained data did not reveal any discernible differences in the indices of mutual alloreactivity of NK cells between spouses in couples with varying pregnancy outcomes during RM treatment involving ICT (Fig. 4, 5). The results, coupled with the absence of compelling evidence linking pregnancy complications to the specific compatibility of spouses based on the alleles of HLA I and II [30], suggest that the allocompatibility parameters commonly employed in transplantation are not critical determinants in the maternal gestation of an allogeneic fetus due to paternal antigens. Limited expression of HLA I on trophoblasts, which occurs in the absence of highly polymorphic HLA A and HLA B on their surface while maintaining the expression of HLA C [31], along with the expression of low polymorphic HLA G [32], suggests a low cytolytic response to trophoblasts from maternal T-cell adaptive immunity [33]. However, the fact that more than half of the decidual lymphoid cells at the beginning of pregnancy are maternal NK cells [15] indicates the important role of innate immunity mechanisms in the allogeneic mother-fetus interaction. The involvement of polymorphic HLA I molecules in the mechanism controlling the “loss of self traits” via KIR-L also suggests the polymorphism of another participant in this interaction – KIR molecules – as a condition for maintaining the high affinity of KIR–KIR-L in the population; therefore, the allelic polymorphism of KIR can be viewed as a phenomenon associated with HLA I polymorphism. However, the existence of more than a dozen different KIR genes in the genotypes [17], along with the optional presence of genes in KIR haplotypes [18], and the presence of KIR with inhibitory and activating activity warrants further consideration. In this regard, the presence of several iKIR genes combined with their stochastic expression [28, 29] indicates the possibility of influencing the overall ability of the entire NK cell population to control the “loss of self-traits among individual groups of NK cells with varying numbers of iKIR. The randomness of gene expression may lead to a situation in which NK cells with none, one, or two iKIRs, or a large number of iKIRs, predominate [34]. In the first scenario, because of the minimal number of iKIRs and the absence of iKIR-L in some iKIRs, NK cell populations in a hyporeactive state (HYPO) should predominate, as their tuning will not occur. In the second scenario, groups of NK cells in the HYPO state will be represented in limited numbers, but the proportion of NK cells tuned for cytolytic action will increase when target cells appear at the site of contact, having “lost the signs of their own.”
The assessment of maternal-paternal alloreactivity parameters (Fig. 4, 5) can be considered as a description of the population composition of paternal and maternal NK cells, reflecting their tuning and the direction of cytolytic activity when interacting with only two types of target cells under conditions of minimal KIR gene expression.
As previously noted, the data indicated an absence of genetic features of KIR and KIR-L (HLA I) in both mothers and fathers in couples with varying pregnancy outcomes when treated with ICT. This result suggests that evolution has preserved only functionally compatible sets of KIR and HLA I genes, where any combination implies not only mutual tuning through iKIR and iKIR-L in any organism but also a lack of cytolytic effects from alloimmune interactions during ICT.
However, it is a characteristic of stochastic expression of these genes that may underlie both the differing maternal responses to allostimulation with paternal lymphocytes during ICT and contribute to impaired placentation during RM and other pregnancy complications. Specifically, with minimal expression of KIR genes, the discussed duality of tuning indicated the presence of dNK populations with opposing cytolytic effects in the placenta. On one hand, dNK populations tuned solely by maternal decidual cells should adopt the HvG state and limit excessive trophoblast invasion; on the other hand, dNK populations influenced only by trophoblasts should adopt the HvH state and disrupt the existing endometrial structure. Together, these processes facilitate placentation while considering the needs of the growing fetus [35].
It is important to note that the expression of HLA-G, which carries the iKIR-L epitope for KIR2DL4 – a "framework" gene inherent in trophoblasts – suggests the obligatory presence of a dNK population with HvH activity in every pregnancy. Increased expression of KIR genes, coupled with a decrease in the proportion of dNKs with single iKIRs, could lead to a reduction in the number of dNKs in the HvG and HvH states, potentially disrupting normal placental formation. Simultaneously, an increase in the proportion of dNKs with a large number of iKIRs will result in an increased number of dNKs in the BD state, which can respond to the "loss of signs of their own" in both maternal and fetal target cells. Notably, the emergence of dNKs in the BD state enhances protection, particularly against viral infections in the placenta.
Notably, even the expression of an iKIR for the KIR and HLA I genotypes present in the human population (using the example of the spouses' genotypes that we studied) suggests, in almost 100% of cases, the presence of at least one combination for tuning both maternal (Fig. 4B), and paternal (Fig. 4D) NK cells to the BD state.
Conclusion
The results of this study indicate that there is no relationship between the outcomes of treatment for RMs using ICT and the KIR and classical HLA I genotypes in spouses. The findings, along with the inconclusiveness of the data regarding the relationship between RM and the characteristics of the spouses' matches in HLA alleles, highlight the need for new research into the immunological mechanisms of allogeneic mother-fetus interaction during pregnancy and mother-father interaction during ICT. These results suggest that the expression of a large number of KIR genes in dNK cells may contribute to the disruption of placentation, particularly the restructuring of endometrial vessels. This disruption may result from a decrease in the representation of dNK cells with HvH activity directed against maternal decidual cells. In this context, a promising area of study is the analysis of KIR gene expression in NK cells in women with normal and pathological pregnancies.
References
- Beer A.E., Billingham R.E. Immunobiology of mammalian reproduction. Adv. Immunol. 1971; 14: 1-84. https://dx.doi.org/10.1016/s0065-2776(08)60283-7.
- Сидельникова В.М., Сухих Г.Т. Невынашивание беременности: руководство для практикующих врачей. М.: ООО «Медицинское информационное агенство»; 2011. 516 с. [Sidelnikova V.M., Sukhikh G.T. Miscarriage: a guide for practitioners. Moscow: Medical Information Agenсy; 2011. 516p. (in Russian)].
- El Hachem H., Crepaux V., May-Panloup P., Descamps P., Legendre G., Bouet P.E. Recurrent pregnancy loss: current perspectives. Int. J. Womens Health. 2017; 9: 331-45. https://dx.doi.org/10.2147/IJWH.S100817.
- Carp H.J.A., ed. Recurrent pregnancy loss: causes, controversies, and treatment. 2nd ed. London: CRC Press; 2014. 456 p.
- Сарибегова В.А., Тетруашвили Н.К., Кречетова Л.В., Агаджанова А.А., Вторушина В.В. Течение и исходы беременностей у женщин с идиопатическим привычным выкидышем при использовании иммуноцитотерапии. Акушерство и гинекология. 2017; 8: 68-73. [Saribegova V.A., Tetruashvili N.K., Krechetova L.V., Agadzhanova A.A., Vtorushina V.V. The course and outcomes of pregnancy in women with idiopathic recurrent miscarriage during immunocytic therapy. Obstetrics and Gynegology. 2017; (8): 68-73. (in Russian)]. https://dx.doi.org/10.18565/aig.2017.8.68-73.
- Говалло В.И., Быкова Е.Я., Кальке И.К. Сравнительный анализ методов иммунотерапии самопроизвольных выкидышей. Акушер-гинеколог (Москва). 1985; 3: 41-3. [Govallo V.I., Bykova E.Ya., Kalke I.K. Immunotherapy for spontaneous abortion: a comparative study of different methods. Obstetrician-Gynecologist (Moscow). 1985; (3): 41-3. (in Russian)].
- Hajipour H., Nejabati H.R., Latifi Z., Hamdi K., Bahrami-Asl Z., Fattahi A. et al. Lymphocytes immunotherapy for preserving pregnancy: Mechanisms and Challenges. Am. J. Reprod. Immunol. 2018; 80(3): e12853. https://dx.doi.org/10.1111/aji.12853.
- Wong L.F., Porter T.F., Scott J.R. Immunotherapy for recurrent miscarriage. Cochrane Database Syst. Rev. 2014; 2014(10): CD000112. https://dx.doi.org/10.1002/14651858.CD000112.pub3.
- Francisco P.D., Tan-Lim C.S.C., Agcaoili-De Jesus M.S.L. Efficacy of lymphocyte immunotherapy in the treatment of recurrent pregnancy loss from alloimmunity: A systematic review and meta-analysis. Am. J. Reprod. Immunol. 2022; 88(4): e13605. https://dx.doi.org/10.1111/aji.13605.
- Pandey M.K., Agrawal S. Induction of MLR-Bf and protection of fetal loss: a current double blind randomized trial of paternal lymphocyte immunization for women with recurrent spontaneous abortion. Int. Immunopharmacol. 2004; 4(2): 289-98. https://dx.doi.org/10.1016/j.intimp.2004.01.001.
- Ali S., Majid S., Niamat Ali M., Taing S., El-Serehy H.A., Al-Misned F.A. Evaluation of etiology and pregnancy outcome in recurrent miscarriage patients. Saudi J. Biol. Sci. 2020; 27(10): 2809-17. https://dx.doi.org/10.1016/j.sjbs.2020.06.049.
- Faulk W.P., McIntyre J.A. Immunological studies of human trophoblast: markers, subsets and functions. Immunol. Rev. 1983; 75: 139-75. https://dx.doi.org/10.1111/j.1600-065x.1983.tb01094.x.
- Хорошкеева О.В., Тетруашвили Н.К., Бурменская О.В., Агаджанова А.А., Трофимов Д.Ю. Роль антигенов главного комплекса гистосовместимости в реализации привычного выкидыша. Акушерство и гинекология. 2016; 3: 5-10. [Khoroshkeeva O.V., Tetruashvili N.K., Burmenskaya O.V., Agadzhanova A.A., Trofimov D.Yu. Role of major histocompatibility complex antigens in recurrent miscarriage. Obstetrics and Gynegology. 2016; (3): 5-10(in Russian)]. https://dx.doi.org/10.18565/aig.2016.3.5-10.
- Quatrini L., Della Chiesa M., Sivori S., Mingari M.C., Pende D., Moretta L. Human NK cells, their receptors and function. Eur. J. Immunol. 2021; 51(7): 1566-79. https://dx.doi.org/10.1002/eji.202049028.
- Moffett-King A. Natural killer cells and pregnancy. Nat. Rev. Immunol. 2002; 2(9): 656-63. https://dx.doi.org/10.1038/nri886.
- Hackmon R., Pinnaduwage L., Zhang J., Lye S.J., Geraghty D.E., Dunk C.E. Definitive class I human leukocyte antigen expression in gestational placentation: HLA-F, HLA-E, HLA-C, and HLA-G in extravillous trophoblast invasion on placentation, pregnancy, and parturition. Am. J. Reprod. Immunol. 2017; 77(6): e12643. https://dx.doi.org/10.1111/aji.12643.
- Roe D., Vierra-Green C., Pyo C.W., Geraghty D.E., Spellman S.R., Maiers M. et al. A detailed view of KIR haplotype structures and gene families as provided by a new motif-based multiple sequence alignment. Front. Immunol. 2020; 11: 585731. https://dx.doi.org/10.3389/fimmu.2020.585731.
- Hsu K.C., Chida S., Geraghty D.E., Dupont B. The killer cell immunoglobulin-like receptor (KIR) genomic region: gene-order, haplotypes and allelic polymorphism. Immunol. Rev. 2002; 190: 40-52. https://dx.doi.org/10.1034/j.1600-065x.2002.19004.x.
- Trowsdale J. Genetic and functional relationships between MHC and NK receptor genes. Immunity. 2001; 15(3): 363-74. https://dx.doi.org/10.1016/s1074-7613(01)00197-2.
- Phillips J.H., Hori T., Nagler A., Bhat N., Spits H., Lanier L.L. Ontogeny of human natural killer (NK) cells: fetal NK cells mediate cytolytic function and express cytoplasmic CD3 epsilon, delta proteins. J. Exp. Med. 1992; 175(4): 1055-66. https://dx.doi.org/10.1084/jem.175.4.1055.
- Ivarsson M.A., Loh L., Marquardt N., Kekäläinen E., Berglin L.,Björkström N.K. et al. Differentiation and functional regulation of human fetal NK cells. J. Clin. Invest. 2013; 123(9): 3889-901. https://dx.doi.org/10.1172/JCI68989.
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Привычный выкидыш. 2022. [Ministry of Health of the Russian Federation. Clinical guidelines. Habitual miscarriage. 2022.(in Russian)].
- Приказ Министерства здравоохранения Российской Федерации от 28.10.2020 № 1166н «Об утверждении порядка прохождения донорами медицинского обследования и перечня медицинских противопоказаний (временных и постоянных) для сдачи крови и (или) ее компонентов и сроков отвода, которому подлежит лицо при наличии временных медицинских показаний, от донорства крови и (или) ее компонентов». Доступно по: https://publication.pravo.gov.ru/Document/View/0001202011260032. [Order of the Ministry of Health of the Russian Federation dated 28.10.2020 No. 1166n "On approval of the procedure for donors to undergo a medical examination and a list of medical contraindications (temporary and permanent) for donating blood and (or) its components and the terms of exemption to which a person is subject in the presence of temporary medical indications from donating blood and (or) its components." Available at: http://publication.pravo.gov.ru/Document/ View /0001202011260032.(in Russian)].
- Кречетова Л.В., Ванько Л.В., Вторушина В.В., Николаева М.А., Инвияева Е.В., Тетруашвили Н.К. Активация лимфоцитов в формировании иммунной толерантности у женщин с привычным выкидышем. Биохимия. 2020; 85(5): 682-94. [Krechetova L.V., Vanko L.V., Vtorushina V.V., Nikolaeva M.A., Inviyaeva E.V., Tetruashvili N.K. Lymphocyte activation in immune tolerance development in women with recurrent pregnancy loss. Biochemistry (Mosc.). 2020; 85(5): 682-94. (in Russian)]. https://dx.doi.org/10.31857/S0320972520050073.
- Boudreau J.E., Hsu K.C. Natural killer cell education in human health and disease. Curr. Opin. Immunol. 2018; 50: 102-11. https://dx.doi.org/10.1016/j.coi.2017.11.003.
- https://www.ebi.ac.uk/ipd/kir/matching/ligand/
- Raulet D.H., Vance R.E., McMahon C.W. Regulation of the natural killer cell receptor repertoire. Annu. Rev. Immunol. 2001; 19: 291-330. https://dx.doi.org/10.1146/annurev.immunol.19.1.291.
- Horowitz A., Strauss-Albee D.M., Leipold M., Kubo J., Nemat-Gorgani N., Dogan O.C. et al. Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Sci. Transl. Med. 2013; 5(208): 208ra145. https://dx.doi.org/10.1126/scitranslmed.3006702.
- Beydoun H., Saftlas A.F. Association of human leucocyte antigen sharing with recurrent spontaneous abortions. Tissue Antigens. 2005; 65(2): 123-35. https://dx.doi.org/10.1111/j.1399-0039.2005.00367.x.
- Juch H., Blaschitz A., Dohr G., Hutter H. HLA class I expression in the human placenta. Wien Med. Wochenschr. 2012; 162(9-10): 196-200. https://dx.doi.org/10.1007/s10354-012-0070-7.
- Dunk C.E., Bucher M., Zhang J., Hayder H., Geraghty D.E., Lye S.J. et al. Human leukocyte antigen HLA-C, HLA-G, HLA-F, and HLA-E placental profiles are altered in early severe preeclampsia and preterm birth with chorioamnionitis. Am. J. Obstet. Gynecol. 2022; 227(4): 641.e1-641.e13. https://dx.doi.org/10.1016/j.ajog.2022.07.021.
- Ander S.E., Diamond M.S., Coyne C.B. Immune responses at the maternal-fetal interface. Sci. Immunol. 2019; 4(31): eaat6114. https://dx.doi.org/10.1126/sciimmunol.aat6114.
- Andersson S., Fauriat C., Malmberg J.A., Ljunggren H.G., Malmberg K.J. KIR acquisition probabilities are independent of self-HLA class I ligands and increase with cellular KIR expression. Blood. 2009; 114(1): 95-104. https://dx.doi.org/10.1182/blood-2008-10-184549.
- Strunz B., Bister J., Jönsson H., Filipovic I., Crona-Guterstam Y., Kvedaraite E. et al. Continuous human uterine NK cell differentiation in response to endometrial regeneration and pregnancy. Sci. Immunol. 2021; 6(56): eabb7800. https://dx.doi.org/10.1126/sciimmunol.abb7800.
Received 21.10.2024
Accepted 12.11.2024
About the Authors
Lyubov V. Krechetova, Dr. Med. Sci., Head of the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Oparin str., 4, l_krechetova@oparina4.ru, https://orcid.org/0000-0001-5023-3476Olga V. Khoroshkeeva, Obstetrician-Gynecologist at the Department of Pregnancy Disorders (Miscarriage Treatment and Prevention Unit), V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Oparin str., 4, o_khoroshkeeva@oparina4.ru, https://orcid.org/0000-0002-5153-5422
Nana K. Tetruashvili, Dr. Med. Sci., Head of the Department of Pregnancy Disorders (Miscarriage Treatment and Prevention Unit), V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia, 117997, Russia, Moscow, Oparin str., 4, n_tetruashvili@oparina4.ru, https://orcid.org/0000-0002-9201-2281
Sergey P. Krechetov, PhD, Leading Researcher at the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Oparin str., 4, s_krechetov@oparina4.ru, https://orcid.org/0000-0003-2861-6010
Tatjana E. Jankevic, PhD, Junior Researcher at the Human Histocompatibility Genetics Laboratory, NRC Institute of Immunology, FMBA of Russia, 115522, Russia, Moscow, Kashirskoye Shosse, 24, tat-shapovalov@yandex.ru, https://orcid.org/0000-0002-0105-3812
Evgenya V. Inviyaeva, PhD, Leading Researcher at the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
117997, Russia, Moscow, Oparin str., 4, e_inviyaeva@oparina4.ru, https://orcid.org/0000-0001-9878-3637
Dmitry Yu. Trofimov, Corresponding Member of the RAS, Dr. Bio. Sci., Director of the Institute of the Reproductive Genetics, V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia, 117997, Russia, Moscow, Oparin str., 4, d_trofimov@oparina4.ru, https://orcid.org/0000-0002-1569-8486
Gennady T. Sukhikh, Academician of the RAS, Dr. Med. Sci., Professor, Director, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow,
Oparin str., 4, g_sukhikh@oparina4.ru, https://orcid.org/0000-0002-7712-1260



