The effectiveness of targeted interferon replacement therapy in patients with extragenital endometriosis
Aim. To compare the effectiveness of extragenital endometriosis (EGE) treatment regimens with and without interferon-alpha-2b (IFN-α2b).Yarmolinskaya M.I., Durneva E.I., Sel'kov S.A., Chepanov S.V., Selyutin A.V., Sokolov D.I.
Materials and methods. The study comprised 46 patients with stage I and II EGE. Twenty-one patients in the study group received standard six months therapy with gonadotropin-releasing hormone agonists (GnRHa) concurrently with IFN-α2b, while 25 patients in the control group were administered GnRHa monotherapy. Before surgery and six months after the treatment initiation, the composition of the lymphocyte subpopulations in the peripheral blood was determined by flow cytofluorometry.
Results. Compared to the control group, combination therapy of patients with EGE using IFN-α2b was associated with an increase in peripheral blood T and B lymphocyte counts, preservation of NK cell activity with a decrease in their number.
Conclusion. The effect of IFN-α2b as part of combination therapy on the composition of the lymphocyte subpopulations is associated with better immune response toward heterotopic endometrial cells and protective effect against recurrent endometriosis, compared with monotherapy.
Keywords
Endometriosis is one of the most common gynecological diseases affecting approximately 10% of women of reproductive age. Histologically, endometriosis is characterized by the presence of endometrium-like epithelium and stroma outside the endometrium and myometrium [1].
Commonly associated with symptoms of chronic pelvic pain, dysmenorrhea, dyspareunia, infertility, and pelvic organ dysfunction, endometriosis can significantly impact the health and quality of life, including social and family life of individuals afflicted by this disease [2]. Despite numerous studies, the etiology and pathogenesis of this disease are not fully understood. Factors accounting for survival, implantation, and proliferation of endometrial cells include hormones, growth factors, cytokines and prostaglandins, cells of the immune system, stromal cells, and vascular endothelial cells [3]. A decrease in cytotoxic NK and T cell activity, changes in cytokine secretion by T-helpers, and production of autoantibodies by B-lymphocytes play a significant role in the development and progression of the disease [4]. Clarification of roles for each immune cell type in endometriosis pathogenesis may offer the possibility of developing targeted interventions aimed to modulate the specific functions of immune cells.
Intercellular molecules that play an essential role in the pathogenesis of this disease include interferons (IFN) [5]. There are two major classes of interferons: type 1 interferons (IFN-α, IFN-β, and IFN-ω) and type 2 interferon (IFN-γ). The main functions of IFN include modulation of immune cell function, regulation of apoptosis, and angiogenesis [6]. The described biological effects of interferon are promising in the search for new approaches in the treatment of endometriosis since IFN exerts an immunomodulating effect on various components of the immune system that are involved in the pathogenesis of this disease [7].
The efficacy of human IFN-α in the treatment of experimental endometriosis in rats has been previously established. Also, there have been studies investigating the effect of various concentrations of human IFN-α on the growth of endometrioma cell lineage in vitro [8–11]. However, only a few clinical studies have investigated the potential role of IFN-α2b in the management of women with endometriosis, and the results were contradictory [12, 13]. After administration of IFNα2b into the abdominal cavity during laparoscopic surgery, patients with endometriosis showed a decrease in symptoms and the spread of the disease, as well as an increased pregnancy rate [12]. In another study, intraperitoneal use of IFN-α2b was associated with the recurrence of endometriosis [13].
Thus, the use of interferon in the management of endometriosis seems relevant and requires further investigation.
This study aimed to investigate changes in the cells of the lymphoid lineage in patients with extragenital endometriosis (EGE) and compare the effectiveness of EGE treatment regimens with and without IFN-α2b.
Materials and methods
The study comprised 46 patients with a laparoscopically and histologically confirmed diagnosis of stage I-II EGE. The extent of endometriosis was assessed according to the revised American Fertility Society (rAFS) classification. After surgery, all patients were administered gonadotropin-releasing hormone agonists (GnRHa) (Buserelin acetate, Pharm-synthesis JSC, Russia) for six months (1 injection every 28 days). Subsequently, patients were divided into two groups. The study group included 21 patients with EGE, who concurrently received rectal suppositories of recombinant α2b-interferon (Viferon, Feron LLC, Russia) 3 million units twice daily for 10 days; then, after a 10-day break, the second course of immunomodulating therapy was given. The second group (comparison group) consisted of 25 EGE patients who received only GnRHa therapy after surgery. A control group (n=20) was also created, including women who underwent diagnostic laparoscopy for infertility investigation before IVF. In this group of patients, neither EGE, nor other benign neoplasms, nor acute or chronic pelvic inflammatory diseases were found intraoperatively.
Before surgery and six months after the treatment initiation, the composition of lymphocyte subpopulations, the count, and activity of NK cells was examined. These parameters were determined by a FacsCanto II flow cytometer (Beckton Dickenson (BD), USA). Flow-through fluorometry was performed by the standard method in the total fraction of mononuclear cells. The cell surface phenotype was determined using monoclonal antibodies (MCA) conjugated with the following fluorochromes: anti-CD3FITC, anti-CD16-PE, anti-CD56-PE, anti-CD45PerCP-Cy5.5, anti-CD4-PE-Cy7, anti-CD19-APC, anti-CD8-APC-Cy7, anti-CD16+ 56-PE, anti-CD3PE-Cy7, anti-CD107a – Alexa Fluor®700 (BD, USA). Using standard kits (BD, USA), Absolute and relative counts of lymphocytes with the phenotype CD3+ (T-lymphocytes), CD3+CD4+ (T-helpers), CD3+CD8+ (cytotoxic T-lymphocytes), CD19+ (B-lymphocytes), CD16+CD56+ (NK cells) were determined. NK cells were isolated by the phenotype CD3-CD16+CD56+, NK cells by the phenotype CD3+CD16+CD56+.
To evaluate NK cell activity, peripheral blood mononuclear cells were isolated by ficoll-verographin density-gradient centrifugation (Sigma, USA). Then the mononuclear cells were incubated in vitro for 3 hours in RPMI-1640 culture medium (Sigma, USA), 10% ETS (Sigma, USA), 1% streptomycin and penicillin (Sigma, USA), 1% glutamine (Sigma, USA) in a humid atmosphere at 37 ºС and 5% CO2 at a concentration of 1million cells/ml. Cell degranulation was evaluated using a method proposed by G. Alter was used. et al. [13]. A part of the cells from each patient was incubated in the presence of antibodies to CD 107a (BD, USA) and 8 μg/ml monensin (BD, USA). The second part of the cells from the same patients was incubated in the presence of antibodies to CD 107a, 8 μg/ml monensin, and a standard reagent for activating white blood cells. Then, the count of peripheral blood NK cells expressing CD107a– glycoprotein associated with lysosomal membranes was estimated, which reflects the degree of NK cell activation if they are expressed on the surface of the cell membrane. Activated NK cells were divided into spontaneously activated (NKA-), that is, which expressed CD107a without exposure to an activation reagent, and activated (NKA+) were induced, which respectively expressed this antigen after addition of a standard activator. Expression of CD107a characterizes the activation of NK cells, accompanied by degranulation of lysosomes, followed by exposure of the LAMP (Lysosomal associated membrane protein) to the cell surface.
Statistical analysis was performed using nonparametric tests. Microsoft Office Excel 2016 spreadsheets were used to enter, calculate, analyze, and visually represent numerical data. Statistical analysis was performed using the STATISTICA 13.3 software (StatSoft, Russia). The critical level of significance when testing statistical hypotheses was considered at p <0.05 (5%). The normality of the distribution was tested by the Shapiro-Wilk test (with the number of subjects less than 50). Quantitative variables showing normal distribution were expressed as the mean and CI 95%; otherwise, the median (Me) and the quartiles Q1 and Q3 in the Me (Q1; Q3) format were reported. The differences between independent groups were assessed by Mann-Whitney U-test. The Wilcoxon W test was used to compare baseline and follow-up data.
Results
Clinical and demographic characteristics of participants
The mean age of patients in the control group was 25.5±1.1 years (minimum – 21.2 years, maximum – 33 years). A regular menstrual cycle was observed in 98% of patients. None of the patients complained of pain throughout the menstrual cycle. The main reason for seeking medical care was primary or secondary infertility. All couples had male factor infertility.
The group of patients with EGE included 46 women aged 26 to 43 years (mean 31.3±1.4 years). Based on the revised R-AFS classification, 29 (63%) and 17 (37%) patients were diagnosed with stage I EGE and stage II EGE, respectively. A regular menstrual cycle was observed in the majority (83%) of patients. The main reason to visit a gynecologist was abdominal cramping, which was reported by all patients. The second most important clinical symptom of endometriosis was infertility, which occurred in 78% of patients. Of them, 68% and 32% had primary and secondary infertility, respectively.
In patients with EGE, pelvic pain and the severity of dyspareunia were assessed before treatment and six months after the end of treatment. Considering that earlier, we performed a detailed assessment of post-treatment changes in pain severity in patients of these groups [14], this article presents generalized results. Analysis of changes in pelvic pain and severity of dyspareunia in patients with EGE in both groups showed that the use of recombinant IFN-α2b in the comprehensive therapy of EGE was accompanied by a more significant reduction in the pelvic pain severity by 90.5% compared with the comparison group (76 %) (p < 0.001). Pain associated with sexual intercourse also decreased significantly in the group of EGE patients who received GnRHa therapy administrated concurrently with Viferon, compared with the comparison group (85.7% and 80%, respectively, p = 0.04).
Assessment of the peripheral blood composition of lymphocyte subpopulations, NK cell count, and NK cell activity
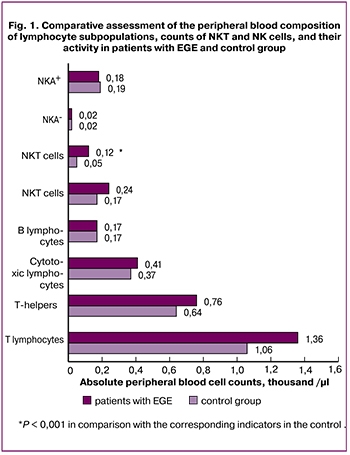 Analysis of the peripheral blood composition of lymphocyte subpopulations at baseline showed that compared with the control group, patients with EGE had a 2.3-fold higher counts of NKT cells with the CD3+CD16+CD56+ phenotype when comparing relative values and a 2.4-fold higher counts when comparing absolute values . No differences were found between both groups in counts of CD3, CD19, CD4, CD8, and NK-cells with the phenotype CD3-CD16+CD56+. Counts of NK-cells expressing 107A both spontaneously activated (NKA) and induced activated (NKA+) also did not differ between the groups (Fig. 1).
Analysis of the peripheral blood composition of lymphocyte subpopulations at baseline showed that compared with the control group, patients with EGE had a 2.3-fold higher counts of NKT cells with the CD3+CD16+CD56+ phenotype when comparing relative values and a 2.4-fold higher counts when comparing absolute values . No differences were found between both groups in counts of CD3, CD19, CD4, CD8, and NK-cells with the phenotype CD3-CD16+CD56+. Counts of NK-cells expressing 107A both spontaneously activated (NKA) and induced activated (NKA+) also did not differ between the groups (Fig. 1).
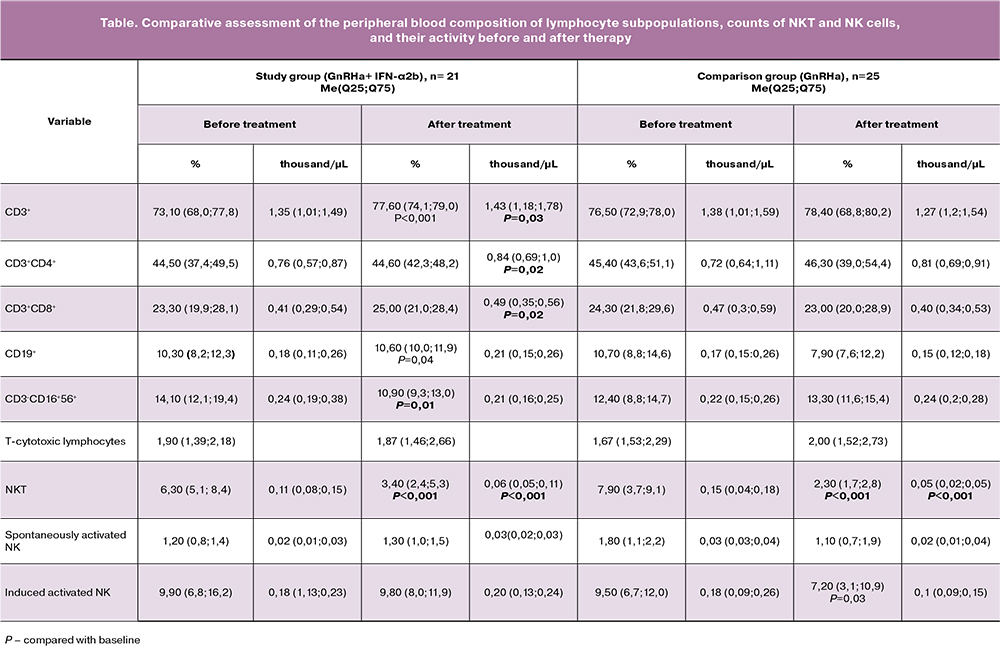
Groups of patients with EGE in both groups were tested before treatment for homogeneity using the Wilcoxon W-test. It was found that at baseline, the groups of patients with EGE before therapy did not differ in any of the studied parameters. After combination therapy with GnRHa in combination with IFN-α2b, there was a statistically a significant 1.1, 1.1 and 1.2-fold increase in the absolute counts of cells with the phenotype CD3+, CD3+CD4+, CD3+CD8+, respectively, as well as a 1.1 and 1.1-fold increase in the relative counts of cells with the phenotype CD3+, CD19+, respectively, compared with these indicators before surgery. We also found a 1.3 and 1.9 times decrease in the relative counts of NK and NKT cells after treatment, respectively, and a 1.8 times reduction in the absolute counts of NKT cells by after therapy (Table).
In patients receiving GnRHa monotherapy, a three and 3.4-fold decrease in the absolute and relative counts of NKT cells was observed, respectively, and a 1.3 times decrease in the absolute count of NKA+, compared with baseline values. No differences were found in counts of cells with the phenotype CD3+, CD3+CD4+, CD3+CD8+, CD19+, and NK cells before and after the use of monotherapy with the hormonal drug (Table).
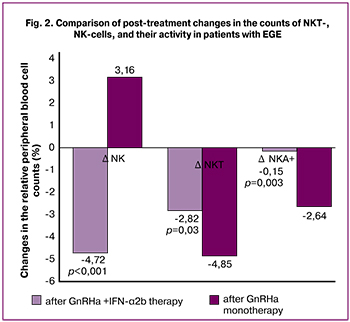 Analysis of changes in the studied parameters after treatment showed that ΔNK cell counts (relative content) in patients after GnRHa and IFN-α2b combination therapy and GnRHa monotherapy was -4.72 95%CI (-7.36; -2.07) and 3.16 95%CI (-0.68; 7.01), respectively. ΔNKT cell counts (relative content) in both groups after treatment had negative dynamics, but to a greater extent in the comparison group and amounted to -4.85 95%CI (-7.15; -2.56). In the study group, ΔNKT cell count was -2.82 95%CI (-4.37; -1.27). ΔNKA+ (relative content) also had a negative dynamics, both when using GnRHa and IFN-α2b combination therapy and GnRHa monotherapy: -0.15 95%CI (-0.42; 0.1) and -2.64 95%CI (-4.38; -1.23) (Fig. 2).
Analysis of changes in the studied parameters after treatment showed that ΔNK cell counts (relative content) in patients after GnRHa and IFN-α2b combination therapy and GnRHa monotherapy was -4.72 95%CI (-7.36; -2.07) and 3.16 95%CI (-0.68; 7.01), respectively. ΔNKT cell counts (relative content) in both groups after treatment had negative dynamics, but to a greater extent in the comparison group and amounted to -4.85 95%CI (-7.15; -2.56). In the study group, ΔNKT cell count was -2.82 95%CI (-4.37; -1.27). ΔNKA+ (relative content) also had a negative dynamics, both when using GnRHa and IFN-α2b combination therapy and GnRHa monotherapy: -0.15 95%CI (-0.42; 0.1) and -2.64 95%CI (-4.38; -1.23) (Fig. 2).
Discussion
Endometriosis is a chronic disease associated with pain and decreased fertility [15]. The critical pathogenic mechanism responsible for the onset of pain is a persistent inflammatory reaction, characterized by disorders in various parts of the immune system and a cytokine imbalance that promotes the pro-inflammatory state [16].
At the systemic level, the primary cells that play a role in the recognition and subsequent elimination of endometrioid heterotopia are NK cells and effector lymphoid cells that directly provide an immune response: T-helpers, cytotoxic T-lymphocytes, and B-lymphocytes [3].
According to our data, before treatment initiation, patients with EGE had higher counts of NKT cells than patients in the control group. NKT cells are a minor subpopulation of T-lymphocytes expressing both NK-cell markers (CD16, CD56) and T-cell antigens (CD3), which plays a key role in the regulation of the immune response to organ damage, neoplasms, and autoimmune reactions [17].
NKT cells induce a quick cytotoxic cell-mediated response by secretion of IFN-γ, which activates nonspecific cytotoxicity of other innate immunity cells, NK cells, and macrophages. Besides, IFN-γ I activate Th1-dependent adaptive immune response with the formation of effector cytotoxic lymphocytes inducing apoptosis of foreign cells [18]. According to our findings, NKT cell counts in patients with endometriosis were higher than those in the control group. However, NK cell count, their activity (NKA and NKA+), and the count of cytotoxic lymphocytes was similar to that in the control group. This observation may indicate insufficient stimulation of cytotoxic lymphocytes by NKT cells and NK cell activity, which impairs timely recognition and elimination of endometriotic heterotopia.
Being one of the critical factors of innate immunity, IFN-α is primarily involved in the nonspecific immune response, forming the defense against viruses and bacteria, and also stops the proliferation of tumor cells much earlier than specific protective immune responses [19]. Type I IFNs were found to promote the synthesis of IL-15 by macrophages for the subsequent proliferation and differentiation of NK cells. Interferon-α enhances the taxis of NK and CD8 lymphocytes into the infection site, increases the expression of perforin mRNA in them, and stimulates their lytic activity against tumor cells [20]. Type I interferons also participate in a specific immune response. In viral infections and oncological diseases, IFN-α/β primarily controls the development of the immune response along the Th1 pathway [20, 21]. According to the literature, type I IFN plays an essential role in the differentiation and activation of CD4+, CD8+ lymphocytes [22]. We found an increase in the total counts of T-lymphocytes (CD3 +), T-helpers (CD3+CD4+), cytotoxic (CD3+CD8+) and B-lymphocytes (CD19+) during combination therapy with IFN-α2b-containing drug and no changes in these parameters in patients after GnRHa monotherapy. This observation does not contradict the above data on the effect of type 1 IFN on a specific immune response. Together with our earlier data regarding the impact of the IFN-α2b-containing medication on the severity of EGE-associated pain [14], changes in the composition of lymphocyte subpopulation suggest an adequate immune response toward the cells of endometriotic heterotopy.
NK cells are lymphocytes of the innate immune system that are capable of killing target cells without prior immunization and secrete cytokines that are involved in the formation of an adaptive immune response and tissue repair. Until now, there is no consensus regarding the quantitative content of NK cells, both in the peritoneal fluid and in peripheral blood in patients with EGE [23]. However, patients with EGE have been shown to have reduced cytotoxic activity of natural killers, both in the peritoneal fluid and in the PC, and this decrease correlates with the stages of the disease [24]. The reduced NK cell activity is an essential factor contributing to the implantation, survival, and proliferation of endometriotic cells migrated into the abdominal cavity [25]. Our study found a decrease in NK cell counts in patients receiving genetically engineered IFN, which may indicate a more favorable redistribution of effector cells from the peripheral circulation to the pathological foci in the pelvic area. Preservation of the cytotoxic activity (NKA+) of natural killer cells in patients of the study group, compared with a decrease in the number of NKA+ in women who received GnRHa monotherapy, indicates that fewer NK cells have higher cytotoxic activity, which, together with our earlier clinical data [14], supports an adequate antitumor immune response. This may be one of the reasons for a lower recurrence rate of the disease later. These results contradict the earlier literature on the effect of GnRHa therapy on NK cells, according to which treatment with these drugs increased the count and activity of natural killer cells in peripheral blood of patients with EGE.
However, the same study showed that the low activity of NK cells during GnRHa therapy and six months after its completion was associated with a high recurrence rate [4]. Besides, the literature reports a decrease in the cytotoxic activity of NK cells upon exposure to GnRHa in vitro [26]. IFN-α2b has an immunomodulatory function; namely, it increases the activity of NK, NKT cells, and cytotoxic lymphocytes. This is reflected in our data on NKT cells. Thus, the decrease in NKT cell count after therapy in the study group is less pronounced than in the comparison group (p = 0.03). This suggests the possible implementation of IFN biological properties.
Conclusion
The findings of this study once again demonstrated that the immune system plays a critical role in the development of endometriosis due to disruption in the functioning of NKT cells, thus impairing timely recognition and elimination of endometriotic heterotopia. An increase in the total count of T-lymphocytes, T-helpers, cytotoxic and B-lymphocytes, as well as a decrease in the number of NK cells while maintaining their activity as a result of combination therapy with IFN-containing medication, suggest an adequate immune response towards cells of endometriotic heterotopia, as well as the possible protective effect of IFN against recurrent endometriosis. Therefore, the use of immunomodulatory IFN-α2b-containing drug as part of EGE combination therapy is pathogenetically substantiated.
References
- Bulun S.E., Yilmaz B.D., Sison C., Miyazaki K., Bernardi L., Liu S. et al. Endometriosis. Endocr. Rev. 2019; 40(4): 1048-9. https://dx.doi.org/10.1210/er.2018-00242.
- Culley L., Law C., Hudson N., Denny E., Mitchell H., Baumgarten M. et al. The social and psychological impact of endometriosis on women’s lives: a critical narrative review. Hum. Reprod. Update. 2013; 19(6): 625-39. https://dx.doi.org/10.1093/humupd/dmt027.
- Symons L.K., Miller J.E., Kay V.R., Marks R.M., Liblik K., Koti M. et al. The immunopathophysiology of endometriosis. Trends Mol. Med. 2018; 24(9): 748-62. https://dx.doi.org/10.1016/j.molmed.2018.07.004.
- Osuga Y., Koga K., Hirota Y., Hirata T., Yoshino O., Taketani Y. Lymphocytes in endometriosis. Am. J. Reprod. Immunol. 2011; 65(1): 1-10. https://dx.doi.org/10.1111/j.1600-0897.2010.00887.x.
- Sourial S., Tempest N., Hapangama D.K. Theories on the pathogenesis of endometriosis. Int. J. Reprod. Med. 2014; 2014: 179515. https://dx.doi.org/10.1155/2014/179515.
- Dicitore A., Castiglioni S., Saronni D., Gentilini D., Borghi M.O., Stabile S. et al. Effects of human recombinant type I IFNs (IFN-alpha2b and IFN-beta1a) on growth and migration of primary endometrial stromal cells from women with deeply infiltrating endometriosis: A preliminary study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018; 230: 192-8. https://dx.doi.org/10.1016/j.ejogrb.2018.10.004.
- Gonzalez-Navajas J.M., Lee J., David M., Raz E. Immunomodulatory functions of type I interferons. Nat. Rev. Immunol. 2012; 12(2): 125-35. https://dx.doi.org/10.1038/nri3133.
- Ingelmo J.M., Quereda F., Acien P. Effect of human interferon-alpha-2b on experimental endometriosis in rats: comparison between short and long series of treatment. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013; 167(2): 190-3. https://dx.doi.org/10.1016/j.ejogrb.2012.11.019.
- Badawy S.Z., Etman A., Cuenca V., Montante A., Kaufman L. Effect of interferon alpha-2b on endometrioma cells in vitro. Obstet. Gynecol. 2001; 98(3): 417-20. https://dx.doi.org/10.1016/S0029-7844(01)01395-3.
- Ingelmo J.M., Quereda F., Acien P. Intraperitoneal and subcutaneous treatment of experimental endometriosis with recombinant human interferon-alpha-2b in a murine model. Fertil. Steril. 1999; 71(5): 907-11. https://dx.doi.org/10.1016/S0015-0282(99)00087-4.
- Павлов Р.В., Сельков С.А. Эффективность внутрибрюшинного применения интерферона в терапии экспериментального эндометриоза у крыс. Медицинская иммунология. 2006; 8(5-8): 721-6. [Pavlov RV, Sel’kov SA. Efficacy of intraperitoneal use of interferon in the treatment of experimental endometriosis in rats. Medical Immunology. 2006; 8(5-6): 721-6.(in Russian)]
- Ali A.F.M., Fateen B., Ezzet A., Badawy H., Ramadan A., El-tobge A. Laparoscopic intraperitoneal injection of human interferon-a2b in the treatment of pelvic endometriosis: a new modality. Obstet. Gynecol. 2000; 95(4, Suppl. 1): 547--8. https://dx.doi.org/10.1016/S0029-7844(00)00684-0.
- Acien P., Quereda F., Campos A., Gomez-Torres M.J., Velasco I., Gutierrez M. Use of intraperitoneal interferon alpha-2b therapy after conservative surgery for endometriosis and postoperative medical treatment with depot gonadotropin-releasing hormone analog: a randomized clinical trial. Fertil. Steril. 2002; 78(4): 705-11. https://dx.doi.org/10.1016/S0015-0282(02)03330-7.
- Дурнева Е.И., Ярмолинская М.И., Соколов Д.И., Сельков С.А., Селютин А.В. Клиническая эффективность и патогенетическое обоснование применения человеческого рекомбинантного интерферона альфа-2 b в комбинированном лечении больных наружным генитальным эндометриозом. Вопросы гинекологии, акушерства и перинатологии. 2019; 18(2): 61-8. https://dx.doi.org/10.20953/1726-1678-2019-2-61-68. [Durneva E.I.,Yarmolinskaya M.I., Sokolov D.I., Sel’kov S.A., Selyutin A.V.The clinical effectiveness and pathogenetic mechanisms of using human recombinant interferon alpha-2b in combined treatment of patients with external genital endometriosis. Gynecology, Obstetrics and Perinatology. 2019; 18(2): 61-8. (in Russian)]. https://dx.doi.org/10.20953/1726-1678-2019-2-61-68
- Alter G., Malenfant J.M., Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods. 2004; 294(1-2): 15-22. https://dx.doi.org/10.1016/j.jim.2004.08.008.
- Dunselman G.A., Vermeulen N., Becker C., Calhaz-Jorge C., D’Hooghe T., De Bie B. et al. ESHRE guideline: management of women with endometriosis. Hum. Reprod. 2014; 29(3): 400-12. https//dx.doi.org/10.1093/humrep/det457.
- Bishop L.A. Management of chronic pelvic pain. Clin. Obstet. Gynecol. 2017; 60(3): 524-30. https://dx.doi.org/10.1097/GRF.0000000000000299.
- Акинфиева О.В., Бубнова Л.Н. NKT-клетки: характерные свойства и функциональная значимость для регуляции иммунного ответа. Онкогематология. 2010; 4: 39-47. [Akinfieva O.V., Bubnova L.N. NKT cells: characteristic features and functional significance in the immune response regulation. Oncohematology. 2010; 4: 39-47.(in Russian)].
- Negishi Y., Takahashi H., Kuwabara Y., Takeshita T. Innate immune cells in reproduction. J. Obstet. Gynaecol. Res. 2018; 44(11): 2025-36. https://dx.doi.org/10.1111/jog.13759.
- Spaccarelli N., Rook A. The use of interferons in the treatment of cutaneous T-cell lymphoma. Dermatol. Clin. 2015; 33(4): 731-45. https://dx.doi.org/10.1016/j.det.2015.05.008.
- Lapenta C., Donati S., Spadaro F., Castaldo P., Belardelli F., Cox M.C.,Santini S.M. NK cell activation in the antitumor response induced by IFN-alpha dendritic cells loaded with apoptotic cells from follicular lymphoma patients. J. Immunol. 2016; 197(3): 795-806. https://dx.doi.org/10.4049/jimmunol.1600262.
- Бекетова Г.В. Интерфероны в лечении острых респираторных инфекций у детей. Лики Украины. 2011; 3: 106-9. [Beketova GV. Interferony v lechenii ostrykh respiratornykh infektsiy u detey. Lіki Ukraїni. 2011; 3: 106-9. (in Russian)].
- Thiruchelvam U., Wingfield M., O’Farrelly C. Natural killer cells: key players in endometriosis. Am. J. Reprod. Immunol. 2015; 74(4): 291-301. https://dx.doi.org/10.1111/aji.12408.
- Riccio L., Santulli P., Marcellin L., Abrao M.S., Batteux F., Chapron C. Immunology of endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2018; 50: 39-49. https://dx.doi.org/10.1016/j.bpobgyn.2018.01.010.
- Ярмолинская М.И., Айламазян Э.К. Генитальный эндометриоз. Различные грани проблемы. СПб.: Эко-Вектор; 2017: 150-1. [Yarmolinskaya M.I., Aylamazyan E.K. Genital’nyy endometrioz. Razlichnye grani problemy. SPb.: Eko-Vektor; 2017: 150-1. (in Russian)].
- Wong K.H., Simon J.A. In vitro effect of gonadotropin-releasing hormone agonist on natural killer cell cytolysis in women with and without endometriosis. Am. J. Obstet. Gynecol. 2014; 190(1): 44-9. https://dx.doi.org/10.1016/j.ajog.2003.08.032.
Received 26.12.2019
Accepted 07.02.2020
About the Authors
Mariya I. Yarmolinskaya, Dr.Med.Sci., Professor of the RAS, Head of the Department of Gynecology and Endocrinology, Head of the Center of Diagnosis and Treatmentof Endometriosis, D.O. Ott Research Institute for Obstetrics and Gynecology, Professor at the Department of Obstetrics and Gynecology,
I.I. Mechnikov North-Western State Medical University. Tel.: +7(812)334-35-85. E-mail: m.yarmolinskaya@gmail.com. ORCID: 0000-0002-6551-4147.
3 Mendeleyevskaya Line, Saint Petersburg, 199034, Russian Federation; 41 Kirochnaya str., Saint Petersburg, 191015, Russian Federation.
Elena I. Durneva, Ph.D. Student at the Department of Gynecology and Endocrinology, D.O. Ott Research Institute for Obstetrics and Gynecology. Tel.: +7(812)334-35-85. Email: elenadurneva1303@gmail.com. ORCID: 0000-0001-5819-7772. 3 Mendeleyevskaya Line, Saint Petersburg, 199034, Russian Federation
Sergey A. Sel’kov, Merited Scholar of the Russian Federation, Professor, Head of the Department of Immunology and Intercellular Interactions, D.O. Ott Research Institute for Obstetrics and Gynecology. Tel.: +7(812)328-98-50. E-mail: selkovsa@mail.ru. ORCID: 0000-0003-1560-7529.
3 Mendeleyevskaya Line, Saint Petersburg, 199034, Russian Federation.
Sergey V. Chepanov, Ph.D., Senior Researcher at the Proteomic Immunoregulation Group, Department of Immunology and Intercellular Interactions, D.O. Ott Research Institute for Obstetrics and Gynecology. Tel.: +7(812)328-98-50. E-mail: chepanovsv@gmail.com.
3 Mendeleyevskaya Line, Saint Petersburg, 199034, Russian Federation.
Alexander V. Selutin, Ph.D. (biol.sci.), Senior Researcher at the Immunology Laboratory with AIDS Diagnostics Group, Department of Immunology and Intercellular Interactions, D.O. Ott Research Institute for Obstetrics and Gynecology . Tel.: +7(812)328-98-50. Email: a_selutin@yahoo.com.
3 Mendeleyevskaya Line, Saint Petersburg, 199034, Russian Federation.
Dmitriy I. Sokolov, Dr.Biol.Sci., Head of the Laboratory of Intercellular Interactions, Department of Immunology and Intercellular Interactions, D.O. Ott Research Institute
for Obstetrics and Gynecology. Tel.: +7(812)328-98-50. E-mail: falcojugger@yandex.ru. 3 Mendeleyevskaya Line, Saint Petersburg, 199034, Russian Federation.
For citation: Yarmolinskaya M.I., Durneva E.I., Sel’kov S.A.,
Chepanov S.V., Selyutin A.V., Sokolov D.I. The effectiveness of targeted interferon replacement therapy in patients with extragenital endometriosis
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2020; 5: 105-12 (In Russian):
https://dx.doi.org/10.18565/aig.2020.5.105-12



