Diagnostic value of determining proinflammatory factors of mitochondrial origin in women with normal pregnancy, threatened and recurrent miscarriages
Objective. To determine the content of proinflammatory factors of mitochondrial origin (mtDAMPs), namely OPA1 and TFAM proteins, in peripheral blood microvesicles in dynamics in women with threatened miscarriage (TM), recurrent miscarriage (RM) and during normal pregnancy (NP).Bulatova Yu.S., Tetruashvili N.K., Mikaelyan A.G., Marey M.V., Sukhanova Yu.A., Vysokikh M.Yu.
Materials and methods. A total of 89 pregnant women were enrolled in the study including 32 patients with RM, 29 patients with TM and 28 women with NP. Peripheral blood was collected from the 6th to 37th week of pregnancy at 3-4-week intervals. To determine the level of mtDAMPs, microvesicles were isolated from the obtained blood plasma and the content of mtDAMPs was analyzed using the Western blot method. The GraphPad Prism 8.0 program was used for statistical processing of the obtained data.
Results. Maximum values of ORA1 and TFAM proteins were registered in patients with RM at 12 weeks, in patients with TM at 18 weeks gestation, and in women with NP at 25 weeks gestation. There was a sharp increase in mtDAMP level in patients with RM at the end of the first trimester which was accompanied by a decrease in the effectiveness of the system for eliminating damaged mitochondria in exogenous oxidative stress associated with inflammation.
Conclusion. Different levels of mtDAMPs in the early stages of pregnancy reflect the various states of the developing placenta in normal and complicated pregnancies, and contribute to the development of placental insufficiency in women with TM and RM.
Keywords
Nowadays, preterm birth is the main cause of neonatal morbidity and mortality worldwide [1]. In the total number of infant deaths, the proportion of premature newborns amounts to 70% or more [2]. Despite a large number of scientific and clinical studies and significant advances in understanding the molecular basis of this pathophysiology, the number of preterm births worldwide remains consistently high [3].
The role of inflammatory factors in the pathogenesis of normal and complicated pregnancy is widely discussed in the modern literature. Pregnant women with a history of recurrent miscarriage (RM) or threatened miscarriage (TM) in the second and third trimesters of pregnancy are more likely to develop placenta-associated complications such as placental insufficiency, fetal growth retardation, which often result in early elective delivery and preterm birth [4–6].
A great number of pregnancy complications are caused by inflammation; its leading role was shown in a study conducted by a group of authors from Canada, United Kingdom, and United States in 2016 [7]. The international team of researchers demonstrated in their work that the proinflammatory factor which appeared in the blood of pregnant women was associated with mitochondria derived damage to molecular pattern (mtDAMP). The appearance of this factor leads to placental disorders and fetal growth retardation. It should be noted that not only inflammation caused by infectious agents is described in the genesis of fetal growth retardation, preeclampsia, and premature birth, but there is also aseptic inflammation resulting from an exposure to mtDAMPs or alarmins (endogenous inflammatory activators), uric acid being one of them [8, 9].
It has been shown that the mechanism of inflammation associated with the introduction of crystals of this substance is implemented via interleukin-1 and causes not only aseptic inflammation, but also apoptosis of placental cells and placental dysfunction, leading to fetal growth retardation.
It is known that risk factors for the development of preterm birth are not specific and the transition from an anti-inflammatory state of the innate immune system to a proinflammatory one plays a key role in pathophysiology of preterm birth [10, 11].
Later, it was shown that proteolipids and peptide fragments of mitochondrial membrane proteins are of vital importance in the cascade of reactions that lead to the development of non-infectious inflammation. Peptide fragments of mitochondrial membrane proteins, containingan N-terminalformyl-methionineresidue, are similar to bacterial proteins in their immunostimulating activity [12, 13].
In normal pregnancy (NP), an imbalance of redox reactions in the placenta arises with increasing gestational age. One of the fundamental mechanisms of biochemical adaptation in the functional system "mother-placenta-fetus" to changing conditions during pregnancy is based on regulated generation of reactive oxygen species (ROS) [14,15]. Despite the relevance of the studies, many questions related to the development of placental disorders under oxidative stress conditions still remain unanswered [16, 17]. In this regard, modern publications increasingly discuss the molecular composition of factors of aseptic inflammation that invade the peripheral bloodstream when cells and tissues are damaged during the complications associated with impaired balance of redox reactions [18–20].
Due to the presence of the above-mentioned characteristics in their structure, mtDAMPs are the most potent immunological activators that cause aseptic inflammation. These molecules are recognized by specific receptors that are widely represented on the surface of endothelial cells. According to recent data, mtDAMPs can cause generalized vascular changes using mechanisms associated with endothelial activation [7]. In terms of inflammation induction, the most active mtDAMPs are mitochondrial DNA (mtDNA), transcription factor A of mitochondria (TFAM) protein, adenosine triphosphate (ATP), acidic lipids of mitochondrial membranes, and others [8, 10].
Moreover, one of the promising potential mtDAMPs is the protein OPA1 (protein optic atrophy 1), which is responsible for mitochondrial fusion after passing the quality control procedure of this organelle. The OPA1 protein is involved in the protection of cells from apoptosis, in the regulation of calcium levels, and also in binding with high affinity to mtDNA. This protein consists of two domains: transmembrane domain and external domain, which is directed to the intermembrane space of mitochondria [11].
The recent studies have shown that the number of mitochondria in syncytiotrophoblast grows with the increasing gestational age and may cause an increase in the contribution of electron transport chain of the placental mitochondria to the formation of ROS which perform the role of "triggers" of labor [21–23].
Mitochondria play a key role in the physiological development of pregnancy, and mitochondrial dysfunction has a pathogenetic significance in complicated pregnancy, therefore, it is particularly important to develop non-invasive methodological approaches to assess the state of placental mitochondria in normal and pathological conditions in the second trimester of pregnancy.
The aim of the study is to determine the dynamics of mtDAMPs, namely OPA1 and TFAM proteins, in peripheral blood of women with threatened miscarriage, recurrent miscarriage and during normal pregnancy.
Materials and Methods
The prospective study included 89 pregnant women who were divided into three groups. Group I consisted of 32 pregnant women with RM, that is spontaneous loss of two or more pregnancies from the same partner. Group II included 29 patients with TM whose obstetric histories were not remarkable. Group III consisted of 28 patients whose pregnancies were not complicated (NP), and who had full-term birth.
Patients of three groups were included in the study at 6–7 weeks’ gestation from the first day of the last menstruation after confirming the embryo’s heartbeat according to ultrasound data. All the participants met the following inclusion criteria: age of women from 20 to 40 years, spontaneous conception (absence of infertility), absence of severe hormonal disorders, regular menstrual cycle, absence of anatomical causes for TM, and the patient’s signed informed consent for taking part in the study.
When included in the study, patients of group II with TM had some manifestations of TM (bloody discharge from the genital tract, retrochorial hematoma). The patients were admitted to hospital, where they received treatment for prolonging pregnancy, including gestagenic (didrogesterone 40 mg per day), spasmolytic and hemostatic preparations (antifibrinolytic, namely tranexamic acid). If the patients experienced signs of preterm labor, they were hospitalized at the 2nd High Risk Pregnancy Department of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow, Russia. Their treatment was aimed at prolonging pregnancy and included intravenous tocolysis, prevention of fetal respiratory distress syndrome, monitoring indicators of ultrasound fetometry and dopplerometry. If the changes in the fetal condition were critical, preterm delivery was performed (4 cases).
Group III (NP group) consisted of women with a normal course of pregnancy, whose obstetric history was unremarkable, and who signed an informed consent to participate in the study. The management of pregnancy and delivery in women of the control group was carried out in the above-mentioned Center.
The exclusion criteria in all three groups were multiple pregnancies, cancer, severe extragenital, and systemic autoimmune diseases.
Blood of pregnant women in the study groups was collected ten times during the study period at 6, 9, 12, 15, 18, 21, 25, 29, 33, 37 weeks’ gestation.
Special research methods
To determine the level of mtDAMP in patients of the study groups, venous blood was fractionated, microvesicles were isolated from the obtained plasma, and the content of mitochondrial proteins was analyzed using the Western blot method.
Blood sampling was performed on an empty stomach from the ulnar vein of a pregnant woman in a test tube with ethylenediamine tetraacetic acid (EDTA) according to the standard method. Blood plasma was obtained by precipitation of blood corpuscles for 10 minutes at 3000g, 4°C using Eppendorf 5410R centrifuge (USA). Protein content in blood plasma was determined by the biuret method. Plasma microvesicles were obtained by double centrifugation, 30 minutes at 10000g, 4°C using 5430R centrifuge (USA). The precipitate was resuspended in the buffer for electrophoresis.
The content of OPA1, TFAM and VDAC1 proteins (norm/control) in the sediment of plasma microvesicles was determined by immunodetection. It was done after the separation of proteins by electrophoresis under denaturing conditions using the Lammley method in the Biorad MiniProtean system (USA) and subsequent transfer of proteins to the nitrocellulose membrane using the Western blot method. Primary antibodies produced against detectable proteins in the mouse (Abcam, USA) were used in accordance with the manufacturer’s recommendations. Secondary antibodies to mouse IgG conjugated with horseradish peroxidase and the ChemiDOC bioluminescent imaging system (Bio rad, USA) were used for the production of primary antibodies. Identification of mtDAMP components in the band of the corresponding molecular weight of polypeptides on the nitrocellulose membrane was performed using the Geldoc system (USA) with a VDAC normalization.
Statistical analysis
The GraphPad Prism 8.0 program was used for statistical processing of the obtained data.
To evaluate clinical and anamnestic data, the normality of the distribution was evaluated for each sample using the Shapiro–Wilk test. The obtained results were analyzed using the methods of analysis of variance (ANOVA) due to their normal distribution. In the case of reliable influence of the selected factors or their interaction, a subsequent post hoc analysis was performed using the Tukey’s test. The data in the table are presented in the form of the mean and standard deviation.
The comparison of shares in evaluating pregnancy outcomes was performed using the χ2 independence test. Intergroup differences in dynamic parameters (relative content of the studied proteins) were estimated using the restricted maximum likelihood method (REML) and the Tukey’s test was subsequently applied. The data in the figures are presented as a median and an interquartile range. The differences were considered significant at the level of p <0.05.
Results
Clinical characteristics and anamnestic data of women in the study groups are presented in Table 1.
According to the data presented in Table 1, women in the study groups did not differ in age and body mass index (BMI) (Table 2). Differences in the number of pregnancies and miscarriages are due to the criteria for inclusion in the groups. Differences in the number of pregnancies and deliveries were observed in group with RM, compared to TM and NP (p<0.001).
Pregnancy outcomes and the incidence of placental insufficiency in women of the study groups were analyzed (Table 3). It turned out that the largest number of preterm births was observed in the group with TM, 27.5% of cases, as well as the incidence of placental insufficiency was 10.0% of cases requiring preterm operative delivery. Full-term births in this group were observed in 72.5% of patients. In group of patients with NP, in all cases, the women had full-term deliveries, only in one case there was a birth of a low-weight newborn. In group I, the women gave full-term birth in 87.5% of cases due to the therapy; preterm birth occurred in 12.5% of cases. Preterm operative delivery due to placental insufficiency was observed in 3.0% of cases (Table 3).
The results of the analysis of the OPA1 protein using the REML method are presented in Table 4. A significant influence of the ‘week’ factor and the interaction of the ‘week’ x ‘group’ factors were revealed, which can be suggestive of the complex nature of dynamic changes in the OPA1 protein during complicated and normal pregnancy.
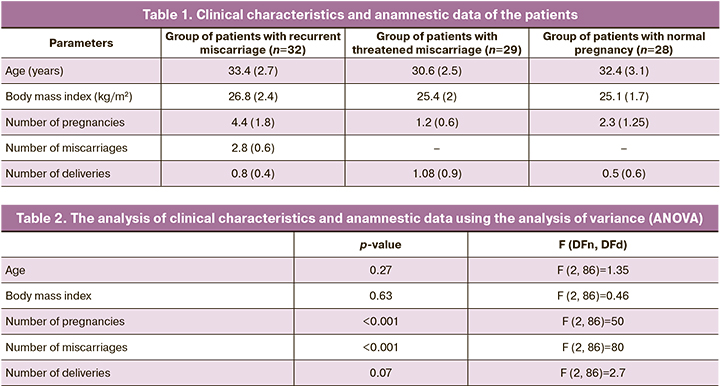
The evaluation of intragroup differences showed that in the group of women with NP, the peak content of OPA1 in plasma microvesicles occurs at week 37. Statistically significant differences (p<0.05) were observed at 6–12, 18–21 and 29 weeks in the patients with NP. In the group with TM, the peak content of OPA1 content was observed at week 18, and statistically significant differences were observed at weeks 9 and 12 (p<0.01). In the group with RM, the maximum OPA1 content was revealed at week 12, and the protein content at this time point was significantly higher (p<0.01) than at weeks 6-9 and weeks 15–33 (Fig. 1).
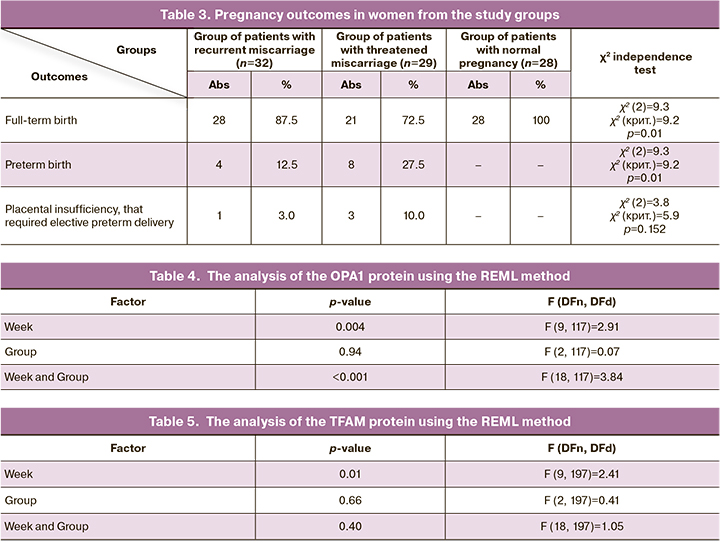
The evaluation of intergroup differences showed a significant increase in the level of relative content of OPA1 at week 12 in the group of patients with RM, compared to the women with NP and TM (p<0.001). The peak content of this protein in the group of patients with TM occurs at 18-week gestation and this result is significantly higher, compared to the group of patients with NP and RM (p<0.007). The OPA1 content at 37 weeks in patients with NP is significantly higher, compared to the indicators in patients with TM (p=0.006) (Fig. 2).
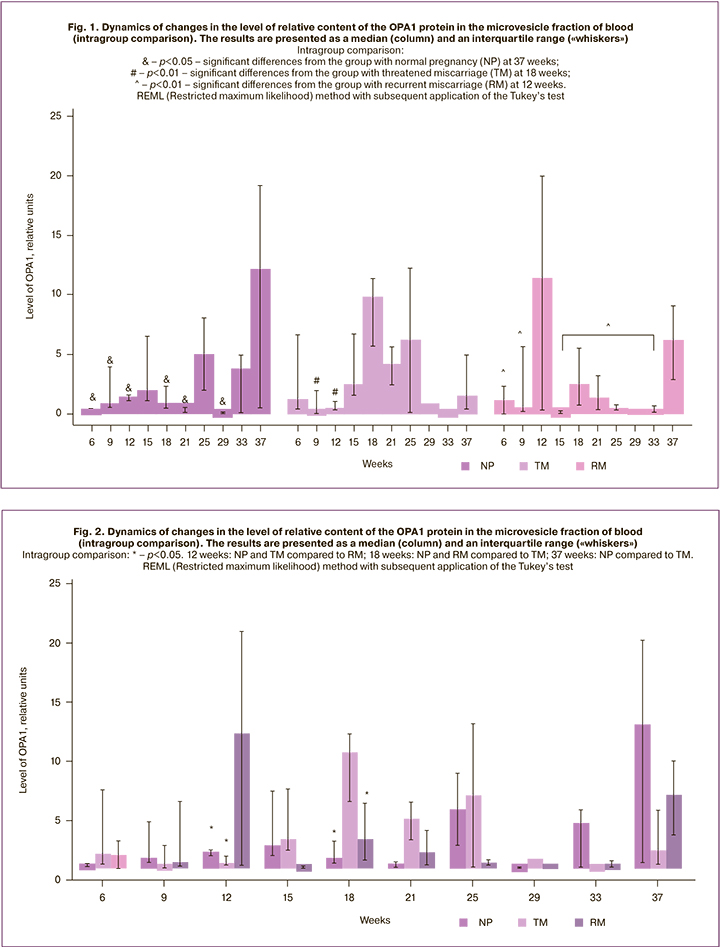
The results of the analysis of the TFAM protein using the REML method are presented in Table 5.
A significant influence of factor ‘week’ on the content of the TFAM protein in the microvesicle fraction of the blood was revealed in women of the study groups; however, the factor ‘group’ and the interaction of factors ‘week’ and ‘group’ did not affect the level of the TFAM protein. Thus, this protein is characterized by dynamic changes during pregnancy, which were not affected by obstetric complications (Fig. 3).
Discussion
Various directions of the OPA1 protein at different gestational age reflect different states of the placenta in normal condition and in placental insufficiency. If the mitochondrial selection process is blocked or significantly inhibited in the early stages of gestation, damaged mitochondria will accumulate in the placenta cells, which can contribute to the state of oxidative stress, followed by the induction of apoptosis of placental cells. The increasing dysfunction of the placenta leads to a termination or complication of pregnancy. At an early gestational age, in normal pregnancy we observed a low level of this protein in the mother’s secretome (1.79 (0.38)), which controls the state of the placenta; women with RM showed a sharp increase in this protein, leading to a decrease in the effectiveness of the elimination system of damaged mitochondria (8.86 (5.08)) which are the sources of ROS accompanied by exogenous oxidative stress and associated with inflammation (Fig. 4).
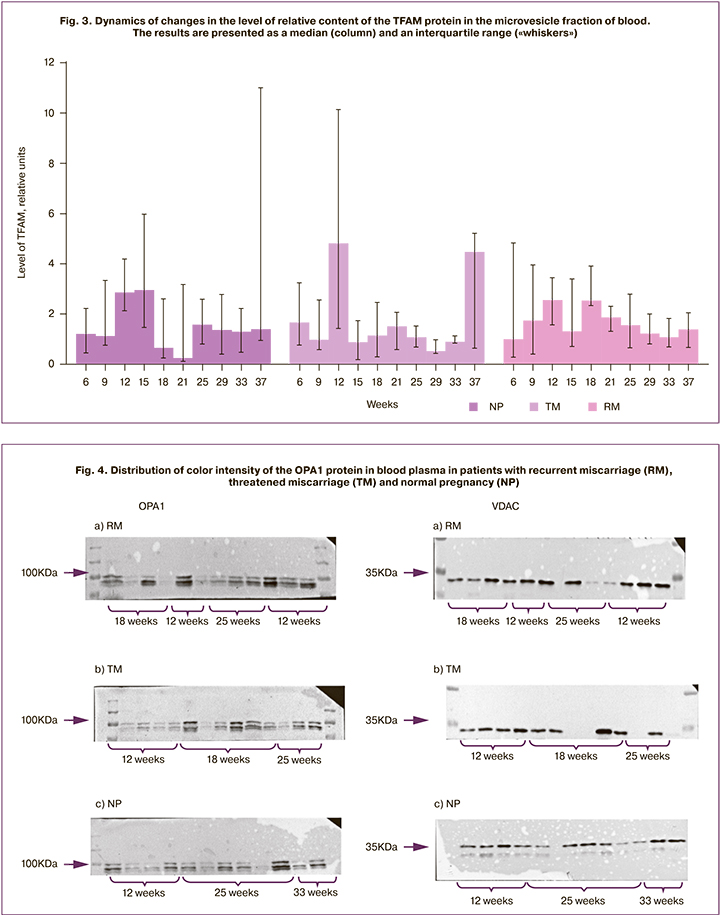
The reverse situation occurs at 25 weeks’ gestation, when due to the aging of placental cells, the quality of mitochondria is likely to decrease, and even in normal conditions it is necessary to lower the barrier level of mitochondrial selection, which leads to an increase in OPA1. High level of damage and low level of OPA1 in patients with RM (0.73 (0.19)), unlike ones with NP (7.79 (3.33)) enhance the elimination of mitochondrial mass and a sharp drop in the placenta’s energy supply and ability to transport nutrients. We registered statistically significant differences in the levels of OPA1 in women with NP, TM and RM. At the same time, the maximum values were noted at the early stages of pregnancy in the patients with RM, at 18 weeks in the TM group, and at 37 weeks of pregnancy in the NP group.
Preterm birth is well known to be associated with a systemic inflammatory response, which is accompanied by an imbalance of redox reactions, leading to disorders at both cellular and subcellular levels, an increase in the level of apoptosis in the placenta, resulting in the development of placental insufficiency [24].
The clinical significance of aseptic inflammation in preterm birth was determined in the study carried out by R. Romero et al., which was devoted to the investigation of the possibility of determining inflammatory markers in the amniotic fluid in preterm birth associated with rupture of membranes [25]. It should be noted that preterm birth in women with aseptic inflammation was observed at the same gestational age and was accompanied by severe inflammatory changes in the placenta and adverse neonatal outcomes with the same frequency as in women with inflammation caused by microorganisms.
Our results clearly demonstrate that there were statistically significant differences in markers of aseptic inflammation in women with TM and RM in the early stages of pregnancy. Women with RM had the peak level of the OPA1 protein at 12 weeks’ gestation, which is statistically significantly different from the indicators in both groups with TM and NP; however, under the influence of gestagen therapy, this indicator decreases and remains at the level corresponding to NP until 37 weeks. In the group of women whose obstetric history was unremarkable, gestagen therapy was short-term, and the peak of inflammatory markers was noted at 18 weeks’ gestation.
In the literature, there is no information about both the structural features and the ratio of ROS formation and ROS elimination in the mitochondria of cells of different placental functional zones in the normal and complicated course of pregnancy. At the same time, a large number of experimental findings have been accumulated to substantiate the idea of the leading role of mitochondria in the generation of ROS in the cell [26, 27].
Some studies [15, 28] have shown that increased production of ROS in placental mitochondria can lead to depletion of certain links of antioxidant protection and to the development of a systemic imbalance of redox reactions in cells, accompanied by an increase in cell death (apoptosis and syncytiotrophoblast necrosis) and the development of placental insufficiency, leading to miscarriage.
By 25 weeks, markers of placental aseptic inflammation in women with RM probably do not reach the values characteristic of NP. The reserve capacity of the placenta in women with RM and TM appears to be determined by a narrower range of adaptive response in uncomplicated pregnancy. A proinflammatory condition corresponding to NP at 25 weeks has been described in previous studies. Thus, the content of NK cells with the CD3-CD16 + phenotype in women with NP at 25 weeks was significantly higher than in pregnant women with RM, similar to the levels of CD200+cells [29]. It is not clear what evolutionary task dictated the necessity of proinflammatory changes in this particular gestational period, namely 25 weeks. We assume that at this time there are structural changes in the placenta caused by the beginning of intensive fetal growth, accompanied by an increase in the threshold of quality and functional state of the mitochondria, which leads to the selection and destruction of a significant part of the mitochondria and to the release of mitochondrial OPA1 proteins into the systemic bloodstream. Dysregulation of the described processes and an increase in the cutoff threshold leads to a critically high level of selection and elimination of mitochondria at a low level of OPA1. In patients with RM, this contributes to a decrease in the mass of mitochondria and a sharp drop in the energy supply of the placenta, creating the conditions for the formation of placental insufficiency. Therefore, there are statistically significant differences in OPA1 levels in women with NP, TM, and RM.
Conclusion
The development of non-invasive methods for evaluating the placenta, measuring the intensity of the proinflammatory response, predicting pregnancy complications, and monitoring the effectiveness of therapy will be possible due to the further research into this area.
References
- Born Too Soon: the global action report in preterm birth. New York; 2 May 2012.
- Di Renzo G.C., Roura L.C., Faccbintti F., Antsaklis A., Breborowicz G., Gratacos E. et al. Guidelines for the management of spontaneous preterm labor: identification of spontaneous preterm labor, diagnosis of preterm premature rupture of membranes, and preventive tools for preterm birth. J. Matern. Fetal Neonatal Med. 2017; 24(5): 659-67. https://dx.doi.org/10.3109/14767058.2011.553694.
- Савельева Г.М., Шалина Р.И., Курцер М.А., Клименко П.А., Сичинава Л.Г.,Панина О.Б., Плеханова Е.Р., Выхристюк Ю.В., Лебедев Е.В. Преждевременные роды как важнейшая проблема современного акушерства. Акушерство и гинекология. 2012; 8-2: 4-10. [Savelyeva G.M.,Shalina R.I., Kurtzer M.A. Preterm birth as the most important problem of modern obstetrics. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2012; 8-2: 4-10. (in Russian)].
- van Oppenraaij R.H., Jauniaux E., Christiansen O.B., Horcajadas J.A., Farquharson R.G., Exalto N.; ESHRE Special Interest Group for Early Pregnancy (SIGEP). Predicting adverse obstetric outcome after early pregnancy events and complications: a review. Hum. Reprod.Update. 2009; 15(4): 409-21. https://dx.doi.org/10.1093/humupd/dmp009.
- Сидельникова В.М., Сухих Г.Т. Невынашивание беременности. М.: МИА; 2010. 536 с. [Sidelnikova V.M., Sukhykh G.T. Miscarriage. M.:MIA, 2010. 536 p. (in Russian)].
- Краснопольский В.И., Серова О.Ф., Титченко Л.И., Петрухин В.А., Туманова В.А., Зароченцева Н.В., Капустина М.В., Мельник Т.Н., Липовенко Л.Н., Бакотина И.В., Марченко С.Ю., Тамазян Г.В., Гридчик А.Л. Ведение беременности у женщин с невынашиванием в анамнезе. Пособие для врачей. М.; 2007. [Krasnopolskiy V.I., Serova O.F., Titchenko L.I., Petruhin V.A., Tumanova V.A., Zarochentseva N.V., Kapustina M.V.,Melnik T.N., Lipovenko L.N., Bakotina I.V., Marchenko S.Yu., Tamazyan G.V.,Gridchik A.L. Conducting pregnancy in women with a history of miscarriage. A manual for doctors. Moscow; 2007: 24 р. (in Russian)].
- Brien V.E., Duval C., Palacios J., Boufaied I., Hudon-Thibeault A.A., Nadeau-Vallée M. et al. Uric acid crystals induce placental inflammation and alter trophoblast function via an IL-1-dependent pathway: implications for fetal growth restriction. J. Immunol. 2017; 198(1): 443-51. https://dx.doi.org/10.4049/jimmunol.1601179.
- Wenceslau C.F., McCarthy C.G., Szasz T. Mitochondrial damage-associated molecular patterns and vascular function. Eur. Heart J. 2014; 35(18): 1172-7.
- Bianchi M.E. DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 2007; 81: 1-5. https://dx.doi.org/10.1189/jlb.0306164.
- Chen G.Y., Nun˜ez G. Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 2013; 10(12): 826-37. 10.1038/nri2873.
- Arenas-Hernandez M., Romero R., St Louis D., Hassan S.S., Kaye E.B., Gomez-Lopez N. An imbalance between innate and adaptive immune cells at the maternal-fetal interface occurs prior to endotoxin-induced preterm birth. Cell. Mol. Immunol. 2016; 13(4): 462-73. https://dx.doi.org/10.1038/cmi.2015.22.
- Charnock-Jones D.S., Kaufmann P., Mayhew T.M. Aspects of human fetoplacental vasculogenesis and angiogenesis. I. Molecular regulation. Placenta. 2004;25(2-3): 103-13.
- Доброхотова Ю.Э., Ганковская Л.В., Бахарева И.В., Свитич О.А., Малушенко С.В., Магомедова А.М. Роль иммунных механизмов в патогенезе невынашивания беременности. Акушерство и гинекология. 2016; 7: 5-10. [Dobrokhotova Yu.E., Gankovskaya L.V., Bakhareva I.V., Svitich O.A.,Malushenko S.V., Magomedova A.M. Role of immune mechanisms in the pathogenesis of miscarriage. Akusherstvo i ginekologiya/Obstetrics and Gynecology. 2016; (7): 5-10. (in Russian)].
- Доброхотова Ю.Э. Окислительный стресс в плаценте при физиологической и патологически протекающей беременности. Российский вестник акушера-гинеколога. 2008; 8(6): 33-5. [Dobrokhotova Yu.E. Placental oxidative stress during physiological and abnormal pregnancies. Ross. Vestn. Akushera-Ginekol. 2008; 8(6): 33-5. (in Russian)].
- Burton G.J. Oxygen, the Janus gas; its effects on human placental development and function. J. Anat. 2009; 215(1): 27-35. https://dx.doi.org/10.1111/j.1469-7580.2008.00978.x.
- Wang Y., Walsh S.M. Placental mitochondria as a source of oxidative stress in pre-eclampsia. Placenta. 2010; 19(8): 581-6. https://dx.doi.org/10.1016/s0143-4004(98)90018-2.
- Myatt L. Review: Reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta. 2010; 31(Suppl.): 566-9. https://dx.doi.org/10.1016/j.placenta.2009.12.021.
- Howard J.A. Carp, ed. Reccurent pregnancy loss: causes, controversies, and treatment. 2nd ed. CRС Press; 2015.
- Zhang Q., Raoof M., Chen Y., Sumi Y., Sursal T. et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010; 464: 104-7.
- Rzepka R., Dołęgowska B., Rajewska A., Kwiatkowski S. On the significance of new biochemical markers for the diagnosis of premature labour. Mediators Inflamm. 2014; 2014: 251451. https://dx.doi.org/10.1155/2014/251451.
- Bilban M., Ghaffari-Tabrizi N., Hintermann E., Bauer S., Molzer S., Zoratti C. et al. Kisspeptin-10, a KiSS-1/metastin-derived decapeptide, is a physiological invasion inhibitor of primary human trophoblasts. J. Cell Sci. 2004; 117(Pt 8): 1319-28. https://dx.doi.org/10.1242/jcs.00971.
- Burnstock G. Blood cells: an historical account of the roles of purinergic signaling. Purinergic Signal. 2015; 11(4): 411-34. https://dx.doi.org/10.1007/s11302-015-9462-7.
- Spaans F., de Vos P., Bakker W.W., van Goor H., Faas M.M. Danger signals from ATP and adenosine in pregnancy and preeclampsia. Hypertension. 2014; 63(6): 1154-60. https://dx.doi.org/10.1161/HYPERTENSIONAHA.114.03240.
- Gozal D., Kheirandish-Gozal L. Cardiovascular morbidity in obstructive sleep apnea oxidative stress, inflammation, and much more. Am. J. Respir. Crit. Care Med. 2008; 177(4): 369-75. https://dx.doi.org/10.1164/rccm.200608-1190PP.
- Romero R., Miranda J., Chaiworapongsa T., Korzeniewski S.J., Chaemsaithong P., Gotsch F. et al. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am. J. Reprod. Immunol. 2014; 72(5): 458-74. https://dx.doi.org/10.1111/aji.12296.
- Андреев А.А., Картавенко В.И. Метаболизм активных форм кислорода в митохондриях. Биохимия. 2005; 70(2): 246-64. [Andreev A.A., Kartavenko V.I.Metabolism of active oxygen forms in mitochondria. Biochemistry. 2005; 70(2): 246-64. (in Russian)].
- Арутюнян А.В., Дубинина Е.Е., Н.Н. Зыбина Н.Н. Методы оценки свободнорадикального окисления и антиоксидантной системы организма. Методические рекомендации. СПб.; 2008. 104 с. [Arutyunyan A.V., Dubinina E.E., Zybina N.N. Methods for evaluating free radical oxidation and the body’s antioxidant system. Guidelines. Saint Petersburg; 2008. 104 p. (in Russian)].
- Прокопенко В.М., Павлова Н.Г., Арутюнян А.В. Прооксидантная и антиоксидантная системы в митохондриях плаценты при ее дисфункции. Журнал акушерства и женских болезней. 2010; 59(5): 56-62.[Prokopenko V.M., Pavlova N.G., Arutyunyan A.V. Prooxidant and antioxidant systems in the mitochondria of the placenta during its dysfunction. Journal of obstetrics and women's diseases. 2010; 59(5): 56-62. (in Russian)].
- Кречетова Л.В., Тетруашвили Н.К., Сарибегова В.А., Вторушина В.В., Голубева Е.Л., Хачатрян Н.А., Агаджанова А.А. Динамика субпопуляционного состава лимфоцитов в периферической крови у пациенток с привычным выкидышем аллоиммунного генеза в течение беременности. Акушерство и гинекология. 2015; 6: 59-66. [Krechetova L.V.,Tetruashvili N.K., Saribegova V.A., Vtorushina V.V., Golubeva E.L.,Khachatryan N.A., Agadzhanova A.A. Dynamics of the subpopulation composition of lymphocytes in peripheral blood in patients with recurrent miscarriage of alloimmune genesis during pregnancy. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2015; 6: 59-66. (in Russian)].
Received 29.11.2019
Accepted 22.07.2020
About the Authors
Yulia S. Bulatova, Graduate student, Department of Pregnancy Loss Prevention and Therapy, Academician V.I. Kulakov Research Center of Obstetrics, Gynecology, and Perinatology Ministry of Health of Russia. Tel.: +7(495)438-14-77. E-mail: yu.bulatova@mail.ru/ 117997, Russia, Moscow, Ac. Oparina str., 4.Nana K. Тetruashvili, Doctor of Medicine, Head of the Department of Pregnancy Loss Prevention and Therapy, Academician V.I. Kulakov Research Center of Obstetrics, Gynecology, and Perinatology Ministry of Health of Russia. Tel.: +7(495)438-14-77. E-mail: tetrauly@mail.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Аsmik G. Mikaelyan, Graduate student, Department of Pregnancy Loss Prevention and Therapy, Academician V.I. Kulakov Research Center of Obstetrics, Gynecology, and Perinatology Ministry of Health of Russia. Tel.: +7(495)438-14-77. E-mail: mikaelyan_asmik@bk.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Maria V. Marey, researcher at the Laboratory of mitochondrial medicine, Academician V.I. Kulakov Research Center of Obstetrics, Gynecology, and Perinatology Ministry of Health of Russia. E-mail: m_marey@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Julia A. Suhanova, researcher at the Laboratory of mitochondrial medicine, Academician V.I. Kulakov Research Center of Obstetrics, Gynecology, and Perinatology Ministry of Health of Russia. E-mail: suhanova_julia@hotmail.com. 117997, Russia, Moscow, Ac. Oparina str., 4.
Mikhail Y. Vysokikh, PhD, the Head of mitochondrial medicine research group, Academician V.I. Kulakov Research Center of Obstetrics, Gynecology, and Perinatology Ministry of Health of Russia. Tel.: +7(495)438-76-33 (1472). E-mail: m_vysokikh@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
For citation: Bulatova Yu.S., Tetruashvili N.K., Mikaelyan A.G., Marey M.V., Sukhanova Yu.A., Vysokikh M.Yu. Diagnostic value of determining proinflammatory factors of mitochondrial origin in women with normal pregnancy, threatened and recurrent miscarriages.
Akusherstvo i Ginekologiya / Obstetrics and gynecology. 2020; 8: 47-56 (in Russian)
https://dx.doi.org/10.18565/aig.2020.8.47-56



