Преэклампсия (ПЭ) – мультисистемное заболевание, осложняющее 2–5% беременностей и приводящее к высокой частоте материнской и перинатальной заболеваемости и смертности [1]. В связи с этим возникает необходимость раннего и эффективного прогнозирования развития данного осложнения у пациенток, относящихся к группам высокого риска по развитию ПЭ [1]. Несомненно, одной из таких групп являются женщины с сахарным диабетом (СД). В настоящее время происходит неуклонный рост заболеваемости СД среди беременных. В 2019 г. состояние гипергликемии в процессе гестации было зарегистрировано у 20 млн (16%) женщин [2].
Несмотря на значимые достижения, связанные с изучением ПЭ, в акушерской практике остается много нерешенных вопросов [3]. Достоверно известно, что развитие ПЭ обусловлено системной эндотелиальной дисфункцией у матери [4]. Недостаточная инвазия цитотрофобласта в спиральные артерии приводит к неполному ремоделированию плацентарных сосудов, ишемически-реперфузионному повреждению плаценты. Вторично возникает оксидативный стресс, в результате которого в материнский кровоток попадает большое количество различных биомаркеров [5]. Имеются убедительные данные, что гипергликемия и патологическая инсулинорезистентность до беременности являются значимыми факторами риска развития ПЭ [4].
ПЭ диагностируется у 25–40% беременных с СД 1 типа и у 20–24% – с СД 2 типа [4]. Системный воспалительный ответ и накопление конечных продуктов гликирования, которые наблюдаются у женщин с СД, значительно усиливают оксидативный стресс [6]. Совокупность этих патологических реакций приводит к развитию ПЭ [4–6].
Сложность предикции ПЭ у беременных с СД заключается в том, что у данной категории женщин симптомы, похожие на ПЭ, наблюдаются и до беременности. Особенно это актуально для пациенток с СД 1 типа и диабетической нефропатией. В связи с этим основные усилия исследователей направлены на оценку валидности различных факторов и биомаркеров как предикторов развития ПЭ.
Оценка материнских факторов
Оценка материнских факторов риска ПЭ – наиболее простой метод скрининга, позволяющий рассчитать риск развития ПЭ у женщин [1]. Новым подходом к рассмотрению ПЭ является конкурирующая модель рисков, которая представлена распределением Гаусса [1]. В исследовании Wright D. et al. (2012) продемонстрировали, что фактор СД 1 типа смещает распределение модели влево на 2–6 недель, тем самым обуславливая раннее начало развития ПЭ у данного контингента женщин [7]426 (2.4%.
Для беременных с прегестационным СД значимыми факторами риска развития ПЭ являются стаж заболевания, наличие микрососудистых осложнений, хроническая артериальная гипертензия и плохой гликемический контроль до и во время беременности [5]. Наличие диабетической нефропатии и микроальбуминурии, по данным Ekbom P. et al. (2001), увеличило вероятность возникновения ПЭ у беременных с СД 1 типа на 64 и 42% соответственно [8]. Проявления диабетической ретинопатии соответствуют 2–3-кратному увеличению риска развития ПЭ [5]. Предшествующая беременности хроническая артериальная гипертензия также значительно увеличивает риск развития ПЭ у беременных с прегестационным СД (OR 3,8–11,7) [9].
Биофизические маркеры
Ряд исследований выявил спектр потенциально полезных биофизических маркеров для прогнозирования ПЭ: пульсационный индекс маточной артерии, среднее артериальное давление. Измеренные параметры в совокупности с априорными материнскими факторами обладают высокой предсказательной ценностью в отношении ПЭ (до 80%) [10].
Определение пульсационного индекса маточных артерий является сильным предиктором ранней ПЭ. Для более высокой частоты выявления ПЭ возможно определение другого эхографического маркера – васкуляризационного индекса плацентарного ложа [11]. Bracero M. et al. (1991), исследуя маточно-плацентарное кровообращение у беременных с СД, обнаружили патологический кровоток в маточных артериях у 15% пациенток, что статистически значимо коррелировало с развитием ПЭ в III триместре [12].
Биохимические маркеры
Изучение новых биохимических маркеров различных патологических состояний является перспективным направлением современного акушерства [10]. Существует множество данных, посвященных оценке валидности различных биомаркеров как предикторов ПЭ. Интересно, что, несмотря на очевидность и необходимость подобных исследований для женщин с CД, таких работ немного, а их данные противоречивы. Трудность диагностики ПЭ у этой группы пациенток связана с тем, что в основе сосудистых осложнений как при ПЭ, так и при СД лежат схожие механизмы.
В настоящее время выделяют несколько семейств биомаркеров, ассоциированных с ПЭ у женщин с СД (рисунок).

В данном обзоре на основании литературных данных был изучен ряд биомаркеров и определена их связь с развитием ПЭ у женщин в I триместре беременности с прегестационным СД. Результаты представлены в таблице.
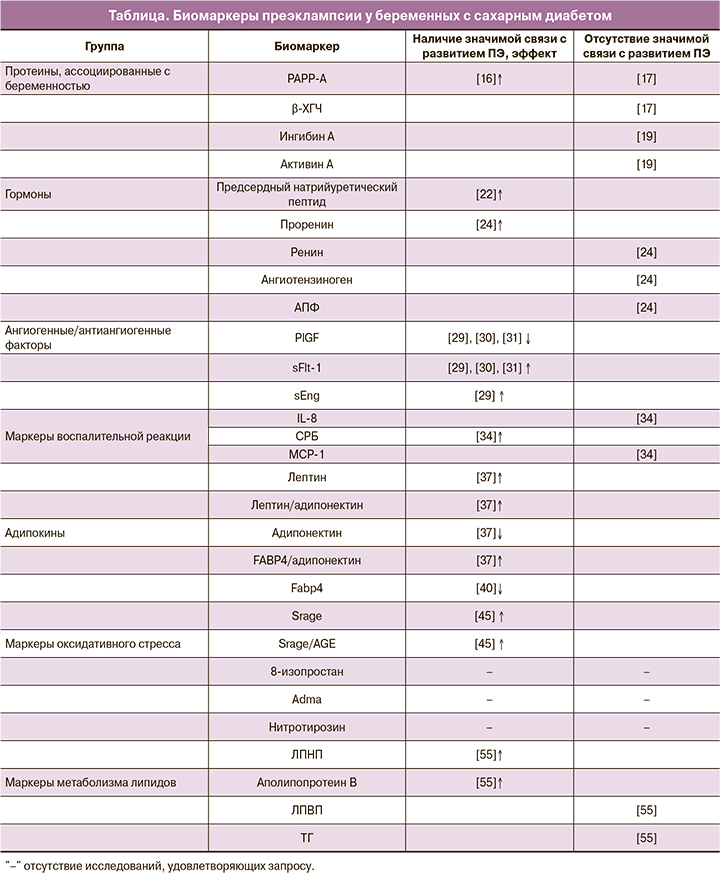
Протеины
Ассоциированный с беременностью протеин-А (PAPP-A) – белок, продуцируемый синцитиотрофобластом, который повышает концентрацию доступного инсулиноподобного фактора роста (IGF). PAPP-A способствует IGF-опосредованной инвазии трофобласта в децидуальную оболочку и транспорту глюкозы и аминокислот в ворсинках хориона [13]. Показано, что снижение уровня PAPP-A при нормальном кариотипе плода ассоциировано с развитием ПЭ и других гестационных осложнений в дальнейшем [14]. Другой биомаркер, также продуцируемый синцитиотрофобластом, – β-хорионический гонадотропин человека (β-ХГЧ). Секреция β-ХГЧ может увеличиваться при аномальной инвазии трофобласта, отражая его реакцию на гипоксию [15].
В немногочисленных работах было изучено изменение уровней этих протеинов у беременных с прегестационным СД в I триместре беременности. Savvidou M. et al. (2012) выявили значительное снижение уровня PAPP-A у группы с СД 2 типа по сравнению с контрольной группой, но какие-либо значимые различия в концентрациях β-ХГЧ отсутствовали [16]. В нашем исследовании мы не установили потенциальную валидность PAPP-A и β-ХГЧ у беременных с СД 1 и 2 типа как предикторов развития ПЭ [17].
Важными гормонами, продуцируемыми трофобластом, являются ингибин А и активин А. Они принадлежат к семейству трансформирующего фактора роста-β (ТGF-β) [18]. Плацентарная гипоксия является мощным стимулом выработки активина А [19]. Sebire N. et al. (2000) обнаружили значительное повышение в плазме уровней этих гормонов в I триместре у беременных, у которых в дальнейшем развилась ПЭ [20]. Исследование Ekbom P. et al. (2006) проводилось на беременных с СД 1 типа. Уровень гормонов измерялся на всем протяжении беременности. Отличий в концентрациях ингибина А и активина А у женщин с развитием ПЭ от таковых у беременных без данной патологии выявлено не было [19].
Известным биомаркером, вырабатываемым кардиомиоцитами, является предсердный натрийуретический пептид. Последний значительно увеличивается в крови у женщин с развитием ПЭ. Это изменение ассоциировано с уменьшением объема плазмы, снижением концентрации альдостерона и активности ренина по сравнению со здоровыми беременными [21]. Ringholm L. et al. (2011) исследовали вазоактивные маркеры у женщин с СД 1 типа в 9 недель беременности. Авторами было показано значительное повышение уровня предсердного натрийуретического пептида у беременных с развитием ПЭ. Это говорит о тесной связи патогенеза ПЭ с изменениями в сердечно-сосудистой системе уже в I триместре беременности [22].
Известно значение ренин-ангиотензиновой системы в развитии ПЭ. При данной патологии наблюдается снижение уровней ренина, ангиотензина II и ангиотензиногена по сравнению с нормальной беременностью [3]. В дополнение к этому происходит увеличение чувствительности рецепторов эндотелиальных клеток к ангиотензину II и к другим вазоконстрикторам [23]. В исследовании Ringholm L. et al. (2011) изучались изменения в ренин-ангиотензиновой системе у беременных с СД 1 типа. Было выявлено, что повышение уровня проренина в 8 недель является значимым предиктором развития ПЭ в дальнейшем. Достоверных различий в плазменном содержании ангиотензиногена, ренина и ангиотензинпревращающего фермента (АПФ) у женщин с СД и группой контроля не выявлено [24].
Ангиогенные/антиангиогенные факторы
Дисбаланс синтеза ангиогенных факторов играет ключевую роль в патогенезе ПЭ. Во время беременности основным из них является плацентарный фактор роста (PlGF). PlGF относится к семейству фактора роста эндотелия сосудов (VEGF) и усиливает его ангиогенную активность [25]. Fms-подобная тирозинкиназа-1 (Flt-1) – один из рецепторов, который опосредует действие VEGF. При некоторых условиях Flt-1 может быть синтезирована трофобластом в виде растворимого белка (sFlt-1), связывающегося со всеми изоформами VEGF и являющегося их антагонистом [26]. Еще одним значимым в патогенезе ПЭ антиангиогенным фактором является растворимый эндоглин (sEng) [25]. sEng является ко-рецептором ТGF-β и его антагонистом [14].
При ишемии плаценты запускаются механизмы избыточного синтеза антиангиогенных факторов, которые являются ключевыми медиаторами развития генерализованной эндотелиальной дисфункции [25]. При ПЭ характерно увеличение плазменного содержания sFlt-1 и снижение PlGF. В настоящее время отношение sFlt-1/PlGF является рутинным методом предикции ПЭ, начиная с ранних сроков беременности [27]. В случае избыточной выработки sEng этот фактор нарушает связывание ТGF-β с мембранным рецептором эндоглином и влияет на его основные эффекты [25]. sEng ухудшает рост эндотелия сосудов, усиливает антиангиогенные действия sFlt-1, провоцируя клинические проявления ПЭ [28].
Интересно, но большинство исследований, посвященных этому вопросу, не включало женщин с СД. Yu Y.et al. (2009) изучали сывороточные уровни анти- и ангиогенных факторов у беременных с СД 1 типа на всем протяжении беременности. В I триместре авторы не обнаружили значимых различий в концентрациях sFlt-1, PlGF, sEng у женщин с развитием ПЭ по сравнению с группой контроля. Однако отмечены значимые изменения уровней этих биомаркеров в III триместре у беременных с дальнейшим развитием ПЭ [29]. В более позднем исследовании установлено, что соотношение sFlt-1/PlGF у женщин с предшествующим СД в I триместре было значительно выше относительно женщин без нарушений углеводного обмена [30]. В исследовании Holmes V. et al. (2013) отметили достоверные изменения уровней этих биомаркеров у женщин с СД 1 типа с дальнейшим развитием ПЭ уже во II триместре беременности [31]. Совокупность представленных исследований демонстрирует, что определение концентраций PlGF, sFlt-1, sEng и их соотношений у женщин с СД может быть валидным методом предикции ПЭ.
Маркеры воспалительной реакции
Системная воспалительная реакция сопровождает любую беременность, но при декомпенсации этого процесса создаются необходимые предпосылки для развития ПЭ. СД, осложняющий беременность, – один из основных факторов, значительно усиливающих системное воспаление [6]. В результате этого дисбаланса в материнском кровотоке повышается уровень провоспалительных цитокинов (интерлейкинов (IL)-6, -8) и снижается уровень противовоспалительных цитокинов (TGF-β, IL-10), что занимает одно из центральных мест в патогенезе ПЭ [3]. В недавнем исследовании Salazar Garcia M. et al. (2018) продемонстрировали эти изменения в периферической крови у женщин на ранних сроках беременности с развитием ПЭ [32]. Однако работ, изучающих эти маркеры на ранних сроках беременности у женщин с прегестационным СД, недостаточно. В ответ на избыточную концентрацию провоспалительных цитокинов в печени вырабатывается С-реактивный белок (СРБ). На ранних сроках беременности повышение уровня СРБ ассоциировано с высоким риском гипертензивных осложнений, в том числе и c риском развития ПЭ [33]. В работе Du M. et al. (2013) оценивались маркеры воспаления у беременных с СД 1 типа и их связь с развитием ПЭ. В I триместре беременности отмечалось значительное увеличение уровня СРБ, отсутствовали достоверные различия в значениях IL-8, моноцитарного хемоаттрактантного протеина-1 (MCP-1) по сравнению с женщинами без ПЭ [34].
Адипокины
Во время беременности плацента является дополнительным источником адипокинов – сигнальных молекул, продуцируемых жировой тканью [35]. Известно, что продукция основных адипокинов – адипонектина, лептина, резистина, висфатина, протеина, связывающего жирные кислоты (FABP4), – нарушается при патологических состояниях, осложняющих беременность. Это имеет особое патофизиологическое и прогностическое значение [36, 37].
СД сопровождается повышением уровня лептина в крови. Гиперлептинемия ассоциирована с увеличенной продукцией других провоспалительных цитокинов, что в совокупности приводит к состоянию хронического субклинического воспаления [35]. При ПЭ также имеет место значительное увеличение уровня лептина, задолго до ее клинических проявлений [36]. Адипонектин, противовоспалительный адипокин, повышает чувствительность к инсулину, обладает антиатерогенными действиями. Низкие концентрации адипонектина тесно связаны с метаболическими нарушениями и нарушениями углеводного обмена [38]. Эти же изменения характерны для I триместра беременности у женщин с развитием ПЭ [38, 39]. Недавние исследования показали связь уровня адипокинов и дальнейшего развития ПЭ у женщин с прегестационным СД. Kelly C. et al. (2017) оценивали содержание различных адипокинов на всем протяжении беременности. Для женщин с СД 1 типа, у которых в дальнейшем развилась ПЭ, было характерно значительное увеличение сывороточного содержания лептина в I триместре по отношению к беременным из группы без ПЭ. Дополнительно установлено увеличение соотношений лептин/адипонектин, FABP4/адипонектин и уменьшение уровня адипонектина у пациенток с СД 1 типа [37]. В исследовании Wotherspoon A. et al. (2016) показано, что увеличение FABP4 на ранних сроках беременности является предиктором дальнейшего развития ПЭ у женщин с СД 1 типа [40]. Эти данные подтверждают то, что адипокины могут являться потенциально валидными биомаркерами для прогнозирования ПЭ в I триместре у женщин с прегестационным СД.
Маркеры оксидативного стресса
СД связан со значительным усилением оксидативного стресса [41]. Состояние гипергликемии стимулирует образование конечных продуктов гликирования (AGE). AGE, взаимодействуя через свои рецепторы (RAGE), запускают образование активных форм кислорода индукцией НАДФH-оксидазы [42]. Оксидативный стресс занимает одно из ключевых мест в патогенезе ПЭ. Повышение уровня маркеров оксидативного стресса и угнетение антиоксидантной системы ассоциировано с развитием ПЭ [43]. Однако исследований, посвященных определению этих биомаркеров у женщин с прегестационным СД для предикции ПЭ, недостаточно.
RAGE занимают одно из центральных мест в патогенезе сосудистых заболеваний. Растворимая форма RAGE (sRAGE), связываясь с AGE, ингибирует действия, опосредованные RAGE [44]. Yu Y.et al. (2012) показали, что уровень sRAGE и соотношение sRAGE/AGE были значимо ниже у женщин с СД 1 типа, у которых впоследствии развилась ПЭ, относительно группы с СД без ПЭ [45]. Таким образом, более низкий уровень sRAGE предположительно ассоциирован с васкулопатиями [45].
Стабильными маркерами оксидативного стресса являются 8-изопростан и нитротирозин [46, 47]. Известна связь уровней этих соединений с развитием ПЭ. Harsem N. et al. (2009) оценивали содержание 8-изопростана у беременных с СД 1 типа и у беременных с ПЭ. В обеих группах наблюдалось увеличение уровня этого биомаркера относительно группы контроля [48]. Johnston Р. et al. (2013) исследовали плаценты женщин с СД 1 типа и различными исходами беременности. Нитротирозин синтезировался как в плацентах женщин с ПЭ, так и в плацентах беременных с СД, но без ПЭ. При этом значимых различий между этими группами не наблюдалось [49]. Еще одним важным медиатором избыточной активации оксидативного стресса является асимметричный диметиларгинин (ADMA) [50]. Имеются данные об уровне ADMA при беременностях, осложненных СД. При всех типах диабета наблюдается повышение плазменного содержания ADMA. Однако существенные различия между группами СД отсутствуют [51]. Но исследований нитротирозина, 8-изопростана и ADMA как предикторов ПЭ у женщин с СД в I триместре беременности мы не обнаружили.
Маркеры метаболизма липидов
Во время нормальной беременности изменяется метаболизм липидов в сторону некоторого увеличения уровня триглицеридов (ТГ), повышения липопротеидов низкой плотности (ЛПНП) и общего холестерина [52]. Изменение липидного профиля во время беременности также связано с эндотелиальной дисфункцией и активацией процессов оксидативного стресса. Увеличение концентрации ЛПНП способствует стимуляции синтеза тромбоксана эндотелиоцитами и снижению синтеза простациклина [52]. Нарушение экспрессии данных факторов приводит к развитию генерализованной вазоконстрикции и ПЭ [53]. Состояние инсулинорезистентности снижает активность липопротеинлипазы, вследствие чего концентрации липопротеидов очень низкой плотности и ТГ у женщин повышаются [54]. Эти изменения во время беременности на фоне СД значительно повышают риск ПЭ. Basu A. et al. (2012) оценили связь маркеров липидного профиля с развитием ПЭ у женщин с СД 1 типа в I триместре беременности. У беременных с развитием ПЭ были значимо увеличены фракции ЛПНП, аполипопротеина B и снижены показатели периферического липолиза. Однако корреляция с концентрациями ТГ и липопротеидов высокой плотности не установлена [55].
Заключение
Таким образом, на основании приведенных данных можно констатировать, что роль биомаркеров в оценке ПЭ в I триместре беременности достаточно высока. Но в группе женщин с прегестационным СД имеющиеся знания и опыт их применения требуют более глубокого и повсеместного изучения этого перспективного направления современного акушерства. На данный момент потенциальную ценность для предикции ПЭ у женщин с СД, помимо оценки биофизических показателей, представляет определение концентрации протеинов (sFlT-1, PlGF, sEng), некоторых гормонов (предсердный натрийуретический пептид, проренин), маркеров воспалительной реакции (IL-8, СРБ) и оксидативного стресса (sRAGE, AGE). Безусловно, интерес возникает и к предикции ПЭ у беременных с гестационным СД. Но методологически это требует иных подходов по причине развития данного типа СД во II триместре беременности. Бесспорно, что для оценки валидности и активного внедрения биомаркеров в практическую деятельность актуальной является задача по оптимизации дизайна исследований и сведения их в единую систему мониторинга.



