Increasing the effectiveness of IVF programs by determining mitochondrial DNA copy number in embryonic trophectoderm
Objective. To improve the effectiveness of in vitro fertilization (IVF) programs in patients of late reproductive age by transferring euploid embryos with normal levels of mtDNA.Korolkova A.I., Mishieva N.G., Martazanova B.A., Bourmenskaya O.V., Ekimov A.N., Trofimov D.Yu., Veyukova M.A., Kirillova A.O., Abubakirov A.N.
Material and methods. This pilot study analyzed the copy number of mitochondrial DNA (mtDNA) in trophectoderm cells of 106 embryos obtained from 50 married couples. After blastocyst trophectoderm biopsy and preimplantation genetic testing for aneuploidy (PGT-A) by a chip-based comparative genomic hybridization (aСGH), the mtDNA copy number was determined using real-time polymerase chain reaction (RT-PCR).
Results. The mtDNA copy number in the trophectoderm of aneuploid embryos (n = 38) was statistically significantly higher than in the trophectoderm of euploid blastocysts (n = 68) (p = 0.003). The ROC analysis resulted in the development of an mtDNA quantity threshold of 0.004 r.u., which was predictive for implantation failure of euploid embryos with above-threshold mtDNA levels with a76.8% sensitivity and 74.9% specificity.
Conclusion. Determining the mtDNA copy number in trophectoderm cells of blastocysts in women undergoing PGT-A is a reliable method for selecting embryos with high implantation potential, which implies the possibility of increasing the effectiveness of infertility treatment in IVF programs.
Keywords
Current demographic trends in industrialized developed nations show a tendency towards late marriage and childbearing due to a change in the social status of women. Access to formal education, economic independence, and a certain social status have become a priority for many women and allowed them to pursue these goals outside of marriage and parenthood. Since women’s fertility is strictly dependent on their age, it is not surprising that delayed motherhood has resulted in a growing demand for the treatment of infertility among women of late reproductive age.
Women over 35 years are at higher risk of diminished ovarian reserve [1], and after 40 years of age, they have a 2-fold rate of follicular atresia [2]. Statistical analysis of fertility, conducted by Smith KE, Buyalos RP, showed that 38 years is the borderline age, at which the live birth rate is reduced by half compared with women younger than 35 years [3]. Besides, women of late reproductive age often have a complicated somatic and gynecological history. However, the main reason for the fertility decline in this patient category is an age-related decrease in the quality of oocytes and, as a result, embryos [4]. According to some foreign authors, chromosomal abnormalities are detected in 50% of cases of reproductive loss in women younger than 35 years, while the corresponding figure for women over 35 years is 75% [5, 6]. Results of preimplantation genetic testing (PGT) of embryos in women of late reproductive age suggest that among them the embryo aneuploidy rate is 80% [6–8].
Assessment of quality of preimplantation embryos is a key component of success in assisted reproductive technology (ART) programs. Existing methods for embryo selection are based on a detailed morphological and genetic assessment of embryos using PGT. However, the effectiveness of ART programs is about 63–64% per thawed embryo transfer cycle [9]. According to Fiorentino F., Rubio C. (2014, 2017), the transfer of morphologically normal and genetically diagnosed euploid blastocyst fails to result in pregnancy in 30% of cases [10, 11]. These data suggest that the transfer of euploid blastocysts of excellent quality, still cannot guarantee the best chance of getting pregnant. Therefore, additional methods of embryo selection are needed. In recent years, studies addressing this issue have been increasingly focused on the mitochondrial function of gametes and embryos.
Metabolic rates and adenosine triphosphate (ATP) content of human oocytes and embryos have been shown to vary significantly [11]. It has also been suggested that the fertilization ability of an oocyte and its capacity to support the development the resulting embryo is dependent on its ATP content [11, 12]. Mitochondria are the cellular organelles responsible for ATP production via the biochemical reaction of oxidative phosphorylation (OXPHOS). They are also involved in the regulation of calcium homeostasis, proliferation, apoptosis and control cell metabolism [13, 14]. A feature unique to mitochondria, compared to other cellular organelles, is the presence of the mitochondrial DNA (mtDNA). The human mtDNA encodes for 13 polypeptides, involved in the construction of four of the electron transport chain complexes (I, III, IV, and V). Inheritance of mitochondria and their genome occurs exclusively on the maternal line, while any sperm mitochondria that enter the oocyte undergo degradation and removal by autophagosomes [15].
As soon as fertilization is complete and mitosis begins, oocyte-derived mitochondria are distributed to the newly formed blastomeres. It is believed that replication of mtDNA does not occur during the first three days of preimplantation development and the key cellular processes are carried out by mitochondria, including ATP production and supported by the population of organelles derived from the oocyte [11].
Full mtDNA replication begins at the blastocyst stage when an adequate level of energy is needed for successful division. At this stage, cellular energy metabolism switches from the anaerobic pathway, which was observed in the early stages of embryogenesis, to the aerobic (oxidative phosphorylation) one [13, 14].
Some studies reported a statistically significant correlation between implantation potential, morphology and chromosomal composition of the blastocyst, the age of patients and mtDNA content in cumulus oocytes and trophectoderm of the blastocyst [11, 12, 16–22]. For example, in the study of Fragouli E. et al. (2015) the implantation potential of euploid blastocysts was determined by a relative mtDNA quantity threshold of 0.003 r.u.. Implanted embryos had mtDNA levels below the threshold, while implantation of embryos with mtDNA levels above this threshold was never observed [16]. In more recent studies these authors have changed the threshold from 0.003 to 0.0004 r.u., which was related to the method of determining mtDNA copy number. However, the result of the study confirmed the previously identified correlation between the implantation potential and the mtDNA level in the blastocyst trophectoderm cells[11]. Besides, Fragouli E. et al., 2015, Ravichandran K. et al., 2017, Wells D. et al., 2017 reported a statistically significantly higher mtDNA copy number in tropectoderm among patients of late reproductive age (over 35 years), compared with the younger age group (p = 0.003) [11, 16, 17]. Additionally, it has been shown that mtDNA level is related to the blastocyst ploidy; mtDNA levels were statistically significantly higher in aneuploid than in euploid embryos (p = 0.025) [11, 14, 16, 17, 22]. Therefore, the mtDNA copy number of blastocyst trophectoderm cells has been proposed as a new biomarker of embryo viability to increase the effectiveness of infertility treatment.
Based on the preceding evidence, we conducted a pilot study of the quantity of mtDNA in trophectoderm cells, which allowed us to investigate the relationship between the mtDNA copy number and age of patients, morphology, embryo ploidy, and its implantation potential. To improve outcomes of ART programs, we also proposed a methodology for determining the level of mtDNA,.
This study aimed to increase the effectiveness of in vitro fertilization (IVF) programs by transferring euploid embryos with normal quantities of mtDNA in trophectoderm.
Materials and methods
The study was conducted at the 1st Department of Gynecology and the Laboratory of Molecular Genetics of the V.I. Kulakov NMRC for OG&P of Minzdrav of Russia. The study included 106 embryos obtained from 50 married couples. The inclusion criteria for the study patient selection were as follows: age 25–45 years, body mass index 18–29 kg/m2, basal blood FSH level ≤15 IU/ml; the absence of grade III-IV external genital endometriosis, large uterine fibroids, PCOS; normal karyotype of the husband. The study did not include patients with teratozoospermia (more than 96% of morphologically abnormal sperm, according to WHO criteria).
The patients underwent a full clinical and laboratory examination per the Order No. 107н of the Ministry of Healthcare of Russia, dated 30 August 2012 “On the Procedure for the Use of Assisted Reproductive Technologies, Contraindications and Restrictions to their Application”. The ovarian stimulation protocol with gonadotropin-releasing hormone antagonists included recombinant FSH or human menopausal gonadotropin with dosages selected individually based on the parameters of the ovarian reserve. The ovulation trigger was administered when 3 or more follicles reached ≥17mm in diameter. Either human chorionic gonadotropin or gonadotropin-releasing hormone agonist was used as a trigger. The transvaginal ovarian puncture was performed 36 hours after the injection of the ovulation trigger. Patients were divided into two age groups. The mean age of patients in group I (control group) and group II was 32±2.8 years (25–34) and 38.6 ±3. 6 years (35–45), respectively.
After ICSI and embryo cultivation to the blastocyst stage, a trophectoderm biopsy was performed for aneuploidy testing (PGT-A) using a chip-based comparative genomic hybridization (aСGH). A morphological assessment of blastocyst stage embryos was carried by the Gardner blastocyst grading system [21].
AСGH of the embryo genetic material was carried out using Agilent (USA) equipment. Full-genomic amplification of cellular DNA using the WGA-PCR method (Whole Genome Amplification – Polymerase Chain Reaction) was performed using the PicoPlex SingleCell WGA Kit (Rubicon Genomics, USA) and the GenetiSure Pre-Screen Amplification and Labeling Kit (Agilent, USA) in the case of MDA (Multiple Displacement Amplification). DNA quantity and quality were controlled by electrophoresis in a 1.2% agarose gel. The amplicons were labeled using the SureTag DNA labeling Kit (Agilent) according to the manufacturer’s instructions. Labeled amplicons were applied to a Sure Print G3 8x60 aCGH Biochip Agilent, hybridized for 16 h, and then washed and scanned on a SureScan Microarray Scanner biological chip scanner. The interpretation of the results was performed using the Agilent CytoGenomics software. Then, the obtained amplicons were diluted in a 1:50 ratio and transferred to measure the mtDNA copy number.
A relative quantitative assessment of the mtDNA copy number was performed using real-time polymerase chain reaction (RT-PCR). Specially designed oligonucleotides and TaqMan probes were used in the reaction to amplify and quantify a specific mtDNA fragment (MT-ND2 gene mitochondrially encoded by NADH dehydrogenase 2). Normalization was carried out on genomic DNA (gene LTC4S - leukotriene C4 synthase). TaqMan samples for mitochondrial and genomic DNA fragments were labeled with different fluorophores (FAM and HEX), which allowed the reaction to be performed in one tube (multiplex PCR); the reaction was performed in duplicate for each sample. Paraffin was used to provide a “hot start.” Reagents and detecting amplifiers DTprime (LLC DNA-Technology, Russia) were used by the manufacturer’s instructions and recommendations. Amplification mode: at 80° C for 1 min, at 95 °C for 1 min, and then 50 cycles; at 94°C for 15 s and 64°C for 20 s with measuring fluorescence level at each cycle.
The quantity of mtDNA relative to genome was determined by comparing threshold cycles (2∆Cp) and presented in relative units (r.u.).
The mtDNA quantity thresholds were established using ROC analysis by comparing the trophectoderm samples of implanted and non-implanted embryos, with an area under curve (AUC) > 0.80, which indicates high accuracy. To compare variables of samples with a normal distribution, parametric tests were used (t-test to compare data in 2 groups). If distribution of the data was not normal, the nonparametric methods (Fisher’s exact test) were used to assess differences between groups. Differences were considered statistically significant at p <0.05 (95% level of significance), at p <0.01 (99% level of significance). Statistical analysis was performed using the Microsoft Excel spreadsheet and the SPSS Statistics 17.0 software.
Results and discussion
There were no statistically significant differences between the groups regarding the rates of somatic and gynecological diseases. The mean age of patients in group I and group II was 32±2.8 years (25–34 years) and 38.6±3.6 years (35–45 years), respectively. The mean total dose of gonadotropins was significantly higher in the group of patients of older reproductive age than among younger patients (1398.3±207 ME and 2508.1±480 ME, in groups I and II, respectively, p = 0.03). Treatment length in group I was significantly shorter than in group II (9.02±1.2 and 12.7±1.3 days in group I and group II, respectively, p = 0.02). The number of mature oocytes in the group of patients of late reproductive age was significantly lower than in group I (7.2 ±1. 0 and 4.4±0.9 in groups I and II, respectively, p = 0.04). Among the 106 embryos that were analyzed using the aCGH, 38 and 68 blastocysts were aneuploid and euploid, respectively.
To determine the mtDNA copy number, products of genome-wide amplification were used. Forty-one euploid blastocysts were transferred. Of these, 27 transfers resulted in pregnancy (27/41); the pregnancy rate was 65.8% (table). The ROC analysis allowed us to develop an mtDNA quantity threshold of 0.004 r.u., above which euploid blastocysts failed to implant with a76.8% sensitivity, 74.9% specificity, and the area under the ROC curve 0.823 (95% CI, 0.710–0.935 ), which indicates a high predictive value. Among all transferred embryos, 32 blastocysts had mtDNA levels below the identified threshold, and the implantation rate of euploid embryos with subthreshold mtDNA levels was 84.4% (27/32) (95% CI: 0.710–0.97) (table). All aneuploid blastocysts with mtDNA levels above the threshold failed to implant (p <0.0001). The negative predictive value for determining the mtDNA copy number in this cohort was 100% (9/9) (95% CI: 93–100%).
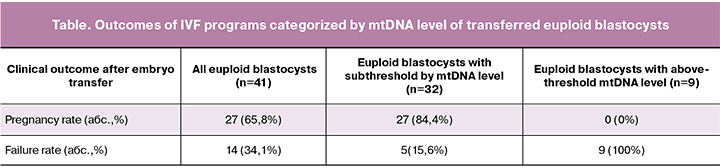
Therefore, implantation failures of euploid embryos may be associated with abnormally high levels of mtDNA.
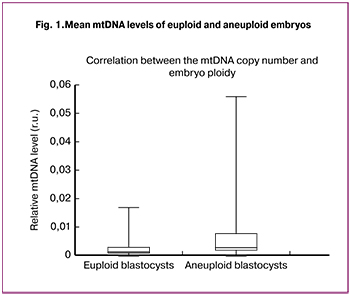 Findings of real-time PCR analysis of mtDNA in the blastocyst trophectoderm cells showed that among aneuploid embryos (n = 38) mtDNA level was statistically significantly higher than in the trophectoderm of euploid blastocysts (n = 68) (p = 0.003) (Fig. 1).
Findings of real-time PCR analysis of mtDNA in the blastocyst trophectoderm cells showed that among aneuploid embryos (n = 38) mtDNA level was statistically significantly higher than in the trophectoderm of euploid blastocysts (n = 68) (p = 0.003) (Fig. 1).
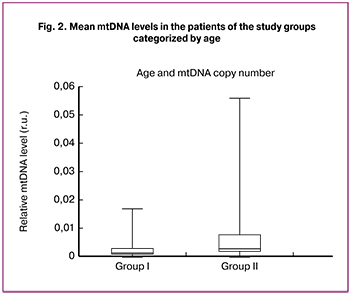
Additionally, the age of the patients undergoing IVF/ICSI + PGT-A was found to be positively correlated with the relative level of mtDNA in the embryo trophectoderm (p = 0.0038) (Fig. 2).
It is important to note that in our study, age-associated changes in the mtDNA copy number were observed in the trophectoderm of both euploid and aneuploid embryos (Fig. 3).
At this stage of the study, no correlation was found between mtDNA levels in the blastocyst trophectoderm cells and their morphology. Similarly, no statistically significant differences were found between trophectoderm mtDNA copy number and the trophectoderm morphology according to the Gardner grading system. Also, there were no significant differences in mtDNA levels between male and female embryos.
 Taking into account the study findings, it can be assumed that blastocysts with elevated mtDNA levels either experience some form of cellular stress and require additional energy or an increase in mitochondria and mtDNA is a compensatory mechanism, which is triggered when mitochondria functioning is disturbed due to the accumulated mutations in the mitochondrial genome. The presented data are consistent with the hypothesis about the role of mitochondrial biogenesis in the embryo implantation potential. The “quiet embryo” hypothesis proposed by Leese (2002) suggests that the viability of embryos is associated with a metabolism that is “quiet” rather than “active,” while embryos that are under stress and have a lower developmental potential are usually more metabolically active [23].
Taking into account the study findings, it can be assumed that blastocysts with elevated mtDNA levels either experience some form of cellular stress and require additional energy or an increase in mitochondria and mtDNA is a compensatory mechanism, which is triggered when mitochondria functioning is disturbed due to the accumulated mutations in the mitochondrial genome. The presented data are consistent with the hypothesis about the role of mitochondrial biogenesis in the embryo implantation potential. The “quiet embryo” hypothesis proposed by Leese (2002) suggests that the viability of embryos is associated with a metabolism that is “quiet” rather than “active,” while embryos that are under stress and have a lower developmental potential are usually more metabolically active [23].
Fragouli E. et al. (2015, 2017) reported the relationship between implantation potential and blastocyst trophectoderm mtDNA level [11, 16]. Initially, the mtDNA quantity threshold was set at 0.003 r.u., above which all embryos failed to implant. Later, a switch from using aStepOne Real-Time PCR System (ThermoFisher), which was initially used, to a Viia7 RT-PCR machine (ThermoFisher) led to an alteration in mtDNA quantity thresholds to 0.0004 r.u., allowing the implantation potential of the embryo to be determined. The findings of our study allowed the establishment of the mtDNA quantity thresholds at 0.004 r.u., above which all embryos failed to implant with a sensitivity of 76.8% and specificity of 74.9%. Different threshold values seem to depend on the quantification equipment for determining the relative level of mtDNA. Thus, in our study, specially developed oligonucleotides and TaqMan samples were used in RT-PCR to amplify and quantify a specific mtDNA fragment (MT-ND2 gene mitochondrially encoded NADH dehydrogenase 2), normalization was carried out on genomic DNA (LTC4S gene - leukotriene C4 synth). Fragouli E. et al. (2015, 2017) used a specially designed TaqMan-analysis (AATTTAACTGTTAGTCCAAAGAG, Life Technologies) to detect and amplify a specific fragment of mtDNA (mitochondrial 16S ribosomal RNA sequences), normalization was carried out on a multicopy sequence of Alu (AGCTACTCGGGAGGCTGAAGGCAGGA, Life Technologies). However, according to all previous studies, embryos with above-threshold mtDNA copy numbers have a low implantation potential. Besides, many studies have reported that the trophectoderm mtDNA levels in aneuploid embryos were significantly higher than that in euploid embryos [11, 12, 22], which is consistent with the results of our study. Thus, the mean relative levels of mtDNA were statistically significantly higher in the trophectoderm of aneuploid than euploid embryos (p = 0.003).

Further, during the study, the relationship between the mtDNA copy number and the age of patients was investigated. Our data disagree with the results of Santos M. et al. (2017), which did not reveal a statistically significant correlation between the mtDNA copy number and patient age, but the authors themselves noted that it might be associated with the very narrow age range of their sample (38.8 ± 3.2 years) [12]. Our results indicate a positive correlation between the mtDNA copy number and the age of patients (p = 0.0038). This finding supports the hypothesis offered by Fragouli E. et al. (2015, 2017) and Ravichandran K. et al., (2017), suggesting that patients of late reproductive age may have a compensatory increase in mtDNA level due to age-related accumulation of mutations in the mitochondrial genome [11, 16, 17].
Additionally, we did not find a connection between mtDNA level and the morphological quality of embryos, while Santos M. et al. (2017) reported a positive correlation between the mtDNA copy number in trophectoderm cells and the blastocyst morphology. According to the results of the study, the mtDNA level is significantly lower in the blastocysts of “excellent” and “good” quality according to Gardner’s grading system [12, 21].
Most chromosomal abnormalities result from the errors occurring during oogenesis (meiosis), but impaired chromosome segregation is also common during the first cell divisions after fertilization (mitotic division) [24]. However, the causes of high levels of meiosis and mitosis errors are still not fully understood. Based on the study results, changes in the quantity or functionality of mtDNA may affect chromosome segregation accuracy.
Conclusion
The study findings suggest that determining the mtDNA copy number in trophectoderm cells of blastocysts in women undergoing PGT-A is a reliable method for selecting embryos with high implantation potential, which implies the possibility of increasing the effectiveness of infertility treatment in IVF programs.
References
- Meczekalski B., Czyzyk A., Kunicki M., Podfigurna-Stopa A., Plociennik L., Jakiel G. et al. Fertility in women of late reproductive age: the role of serum anti-Müllerian hormone (AMH) levels in its assessment. J. Endocrinol. Invest. 2016; 39(11): 1259-65. https://dx.doi.org/10.1007/s40618-016-0497-6.
- Мишиева Н.Г. Лечение бесплодия у женщин позднего репродуктивного возраста. Российский вестник акушера-гинеколога. 2008; 8(5): 51-5. [Mishieva N.G. Treatment of infertility in women of late reproductive age. Russian Bulletin of the obstetrician-gynecologist. 2008; 8 (5): 51-5. (in Russian)]
- Smith K.E., Buyalos R.P. The profound impact of patient age on pregnancy outcome after early detection of fetal cardiac activity. Fertil. Steril. 1996; 65(1): 35-40. https://dx.doi.org/10.1016/S0015-0282(2016)58024-8.
- Королькова А.И., Мишиева Н.Г., Абубакиров А.Н., Павлова Ю.С., Имиева Т.Б. Антимюллеров гормон как показатель фертильности женщин позднего репродуктивного возраста. Проблемы репродукции. 2018; 24(2): 23-7. [Korolkova A.I., Mishieva N.G., Abubakirov A.N., Pavlova Yu.S., Imieva T.B. Anti-Muller hormone as an indicator of fertility in women of late reproductive age. Reproduction problems. 2018; 24 (2): 23-7. (in Russian)]. https://dx.doi.org/10.17116/repro201824223-27.
- Tsutsumi M., Fujiwara R., Nishizawa H., Ito M., Kogo H., Inagaki H. et al. Age-related decrease of meiotic cohesins in human oocytes. PLoS One. 2014; 9(5): e96710. https://dx.doi.org/10.1371/journal.pone.0096710. eCollection 2014.
- Lukaszuk K., Jakiel G., Kuczynski W., Pukszta S., Liss J., Plociennik L. et al. Next generation sequencing for preimplantation genetic testing of blastocysts aneuploidies in women of different ages. Ann. Agric. Environ. Med. 2016; 23(1): 163-6.
- Brezina P.R., Anchan R., Kearns W.G. Preimplantation genetic testing for aneuploidy: what technology should you use and what are the differences? J. Assist. Reprod. Genet. 2016; 33(7): 823-32.
- Ma G.C., Chen H.F., Yang Y.S., Lin W.H., Tsai F.P., Lin C.F. et al. A pilot proof-of-principle study to compare fresh and vitrified cycle preimplantation genetic screening by chromosome microarray and next generation sequencing. Mol. Cytogenet. 2016; 9: 25.
- Fiorentino F., Bono S., Biricik A., Nuccitelli A., Cotroneo E., Cottone G. et al. Application of next-generation sequencing technology for comprehensive aneuploidy screening of blastocysts in clinical preimplantation genetic screening cycles. Hum. Reprod. 2014; 29(12): 2802-13.
- Rubio C., Bellver J., Rodrigoa L., Castillon G., Guillen A., Vidal C. et al. In vitro fertilization with preimplantation genetic diagnosis for aneuploidies in advanced maternal age: a randomized controlled study. Fertil. Steril. 2017; 107(5): 1122-9.
- Fragouli E., McCaffrey C., Ravichandran K., Spath K., Grifo J.A., Munné S., Wells D. Clinical implications of mitochondrial DNA quantification on pregnancy outcomes: a blinded prospective non-selection study. Hum. Reprod. 2017; 32(11): 2340-7. https://dx.doi.org/10.1093/humrep/dex292.
- de Los Santos M.J., Diez Juan A., Mifsud A., Mercader A., Meseguer M., Rubio C., Pellicer A. Variables associated with mitochondrial copy number in human blastocysts: what can we learn from trophectoderm biopsies? Fertil. Steril. 2018; 109(1): 110-7. https://dx.doi.org/10.1016/j.fertnstert.2017.11.007.
- Cagnone G.L., Tsai T.S., Makanji Y., Matthews P., Gould J., Bonkowski M.S. et al. Restoration of normal embryogenesis by mitochondrial supplementation in pig oocytes exhibiting mitochondrial DNA deficiency. Sci. Rep. 2016; 6: 23229.
- Королькова А.И., Мишиева Н.Г., Бурменская О.В., Екимов А.Н., Абубакиров А.Н., Богатырева Х.А. Современные методы селекции эмбрионов при проведении программ вспомогательных репродуктивных технологий. Акушерство и гинекология. 2018; 2: 13-8. [Korolkova A.I., Mishieva N.G., Burmenskaya O.V., Ekimov A.N., Abubakirov A.N., Bogatyreva Kh.A. Modern methods of embryo selection when conducting programs of assisted reproductive technologies. Obstetrics and gynecology. 2018; 2: 13-8. (in Russian)] https://dx.doi.org/10.18565/aig.2018.2.13-18.
- Zhou Q., Li H., Li H., Nakagawa A., Lin J.L., Lee E.S. et al. Mitochondrial endonuclease G mediates breakdown of paternal mitochondria upon fertilization. Science. 2016; 353(6297): 394-9. https://dx.doi.org/10.1126/science.aaf4777.
- Fragouli E., Spath K., Alfarawati S., Kaper F., Craig A., Michel C.E. et al. Altered levels of mitochondrial DNA are associated with female age, aneuploidy, and provide an independent measure of embryonic implantation potential. PLoS Genet. 2015; 11(6): e1005241. https://dx.doi.org/10.1371/journal.pgen.1005241.
- Ravichandran K., McCaffrey C., Grifo J., Morales A., Perloe M., S. Munne S. et al. Mitochondrial DNA quantification as a tool for embryo viability assessment: retrospective analysis of data from single euploid blastocyst transfers. Hum. Reprod. 2017; 32(6): 1282-92. https://dx.doi.org/10.1093/humrep/dex070.
- Ogino M., Tsubamoto H., Sakata K., Oohama N., Hayakawa H., Kojima T. et al. Mitochondrial DNA copy number in cumulus cells is a strong predictor of obtaining good-quality embryos after IVF. J. Assist. Reprod. Genet. 2016; 33(3): 367-71.
- Boucret L., Chao de la Barca J.M., Morinière C., Desquiret V., Ferré-L’Hôtellier V., Descamps P. et al. Relationship between diminished ovarian reserve and mitochondrial biogenesis in cumulus cells. Hum. Reprod. 2015; 30(7): 1653-64.
- Desquiret-Dumas V., Clément A., Seegers V., Boucret L., Ferré-L’Hotellier V., Bouet P.E. et al. The mitochondrial DNA content of cumulus granulosa cells is linked to embryo quality. Hum. Reprod. 2017; 32(3): 607-14. https://dx.doi.org/10.1093/humrep/dew341.
- Gardner D.K., Schoolcraft W.B. In vitro culture of human blastocysts. In: Jansen R., Mortimer D., eds. Toward reproductive gertainty: fertility and genetics beyond 1999 . London: Parthenon Publishing; 1999: 378-88.
- Dagan Wells. Mitochondrial DNA quantity as a biomarker for blastocyst implantation potential. Fertil. Steril. 2017; 108(5): 742-7. https://doi.org/10.1016/j.fertnstert.2017.10.007.
- Leese H.J. Quiet please, do not disturb: a hypothesis of embryo metabolism and viability. Bioessays. 2002: 24(9): 845-9.
- Баранов В.С., Кузнецова Т.В. Цитогенетика эмбрионального развития человека. СПб.: Н-Л; 2007: 78-9. [Baranov V.S., Kuznetsova T.V. Cytogenetics of human embryonic development. SPb .: NH; 2007: 78-9. (in Russian)]
Received 30.11.2018
Accepted 07.12.2018
About the Authors
Korolkova, Anna I., PhD, junior researcher, 1st gynecology Department of Gynecology, National Medical Research Center for Obstetrics, Gynecology and Perinatologynamed after Academician V.I. Kulakov Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Тel.: +79153220879. E- mail: zaikinaai@icloud.com
Mishieva, Nona G., MD, senior researcher, 1st Department of Gynecology, National Medical Research Center for Obstetrics, Gynecology and Perinatology named
after Academician V.I. Kulakov Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Тel.: +79104244197. E- mail: nondoc555@mail.ru
Martazanova, Bella A., PhD, junior researcher, 1st Department of Gynecology, National Medical Research Center for Obstetrics, Gynecology and Perinatology named
after Academician V.I. Kulakov Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Тel.: +79104244197. E-mail: bellamart88@mail.ru
Bourmenskaya, Olga V., MD, researcher, Laboratory of Molecular Genetics, National Medical Research Center for Obstetrics, Gynecology and Perinatology
named after Academician V.I. Kulakov Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954382292. E-mail: o_bourmenskaya@oparina4.ru
Ekimov, Aleksey N., MD, geneticist, Laboratory of Molecular Genetics, National Medical Research Center for Obstetrics, Gynecology and Perinatology
named after Academician V.I. Kulakov Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. E-mail: a_ekimov@oparina4.ru
Trofimov, Dmitry Y., MD, head of the Laboratory of Molecular Genetics, National Medical Research Center for Obstetrics, Gynecology and Perinatology
named after Academician V.I. Kulakov Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. E-mail: d_trofimov@oparina4.ru
Veyukova, Maria A., PhD, embryologist, 1st Department of Gynecology, National Medical Research Center for Obstetrics, Gynecology and Perinatology
named after Academician V.I. Kulakov Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Тel.: +74382622. Е-mail: veymary@gmail.com
Kirillova, Anastasia O., PhD, embryologist,1st Department of Gynecology, National Medical Research Center for Obstetrics, Gynecology and Perinatology named
after Academician V.I. Kulakov Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Тel.: +74382622. Е-mail: a_kozyreva@oparina.ru
Abubakirov, Aidar N., PhD, head of the 1st Department of Gynecology, National Medical Research Center for Obstetrics, Gynecology and Perinatology named
after Academician V.I. Kulakov Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Тel.: +74382622. Е-mail: nondoc555@yahoo.com.
For citation: Korolkova A.I., Mishieva N.G., Martazanova B.A., Bourmenskaya O.V., Ekimov A.N., Trofimov D.Yu., Veyukova M.A., Kirillova A.O., Abubakirov A.N. Increasing the effectiveness of IVF programs by determining mitochondrial DNA copy number in embryonic trophectoderm. Akusherstvo i Ginekologiya/Obstetrics and Gynecology.2019; (3): 98-104. (in Russian)
https://dx.doi.org/10.18565/aig.2019.3.98-104



