Efficiency of assisted reproductive technology in women with polycystic ovarian syndrome who undergo preimplantation genetic testing for aneuploidy
Objective: To compare the efficiency of assisted reproductive technology (ART) with and without preimplantation genetic testing for aneuploidy (PGT-A) in women with polycystic ovarian syndrome (PCOS).Mikhailova N.D., Aksenenko A.A., Ibragimova M.Kh., Ekimov A.N., Gavisova A.A.
Materials and methods: The study included 80 infertile women with PCOS who underwent assisted reproductive technology (ART) with gonadotropin-releasing hormone (GnRH) antagonists. The patients were divided into Group 1 (n=40) including women who underwent IVF/ICSI/PGT-A and Group 2 (n=40) including women who underwent IVF or IVF/ICSI without PGT-A. In all ovarian stimulation programs, 0.2 mg of GnRH antagonist was used as a trigger instead of human chorionic gonadotropin; a cycle segmentation technique was used. All patients underwent frozen–thawed embryo transfer.
Results: The PGT-A group had a 10% (4/40) higher rate of pregnancies that ended in childbirth [14/40 (35%)] than the group without PGT-A [10/40 (25%)], RR 1.40 (95% CI 0.71–2.77), p=0.46; differences between groups were not statistically significant. The differences in the biochemical pregnancy rate [0/40 in the PGT-A group and 4/40 (10%) in the group without PGT-A, p=0.34] and the miscarriage rate [3/40 (7.5%) in the group with PGT-A and 8/40 (20%) in the group without PGT-A (RR 0.38 (95% CI 0.08–1.31), p=0.10] were also not statistically different. Still, there was a tendency for these complications to decrease in patients who underwent PGT-A. The pregnancy rates were not statistically different between the two study groups: 18/40 (45%) in the group with PGT-A and 22/40 (55%) in the group without PGT-A (RR 0.82 (95% CI 0.53–1.27), p=0.37).
Conclusion: PGT-A in PCOS patients demonstrates its clinical value, but further studies are needed in a larger sample of patients, investigating embryological stage factors and taking into account both the success rates of pregnancy after frozen-thawed embryo transfer and the effectiveness of these programs in this category of patients, and obstetric and perinatal outcomes.
Keywords
Polycystic ovary syndrome (PCOS) is a very common endocrine disorder in women of reproductive age, with a reported prevalence reaching 20% in the female population of developed countries [1]. PCOS is the commonest cause of anovulatory infertility of endocrine origin.
The pathogenesis of PCOS has not yet been fully elucidated. The role of genetic, epigenetic, endocrine, and metabolic factors in the development of the disease is a topic of recent discussions [2–5].
The 2004 Rotterdam consensus workshop suggested that the diagnosis of PCOS should be based on the presence of two of the three proposed characteristics, including clinical or biochemical signs of hyperandrogenism, oligo or anovulation, and polycystic ovaries on ultrasound examination. These criteria have been criticized by specialists, since they leveled the notion of PCOS as a syndrome that includes a number of clinical and laboratory symptoms. The discussion resulted in a new 2012 classification that distinguished PCOS phenotypes [6].
This classification is currently used in clinical practice, although its shortcomings are obvious [7]. No less controversial issue is the choice of the method to achieve pregnancy in this patient population.
Classically, first-line pharmacological treatment for infertile women with PCOS includes clomiphene citrate and letrozole. If they are ineffective and/or impossible to take, gonadotropin ovulation stimulation or surgical treatment (laparoscopic ovarian drilling) are used as second-line therapy [8, 9]. The use of assisted reproductive technology (ART ) in patients with PCOS is a third-line therapy [9].
At the same time, a number of specialists consider the sequential tactics of infertility treatment in patients with PCOS to be outdated in current conditions, when the age of patients is approaching the late reproductive age. Arguments include more rapid achievement of pregnancy by in vitro fertilization (IVF), the possibility of performing genetic testing of the embryos, cryopreservation of "extra" embryos for subsequent childbirth [10].
Certain limitations of ovarian stimulation in PCOS patients, namely an increased risk of ovarian hyperstimulation syndrome, lead to the development of a technique when immature oocytes are collected in an unstimulated cycle and mature outside the body by in vitro maturation (IVM). Proponents of this technique argue for its safety and low cost due to the absence of gonadotropic stimulation [11, 12]. However, IVM efficiency proved to be significantly lower than classical IVF, and modification of stimulation protocols, replacement of ovulation trigger and cryopreservation of all obtained embryos, significantly reduces the risk of ovarian hyperstimulation syndrome [13, 14].
Nevertheless, IVF in patients with PCOS is associated with a number of difficulties, both at the stage of ovarian stimulation and at the embryological stage. There are reports that patients with PCOS undergoing ART are characterized by more obtained oocytes compared to patients with normal ovarian reserve. However, a significant portion of the obtained oocytes are characterized as immature. Abnormalities in early embryogenesis and blastogenesis have been observed, when there were no good quality blastocysts while a sufficient number of mature oocytes was obtained and adequate fertilization was performed [15, 16].
These facts, as well as the possible genetic nature of PCOS formation, lead to the opinion of some specialists on the necessity of obligatory preimplantation genetic testing for aneuploidy (PGT-A) in PCOS patients undergoing IVF [17].
In general, the impact of PGT-A on the effectiveness of ART in infertile patients remains under discussion. Regarding PCOS patients, there is evidence that PGT-A is particularly effective in these patients of young age (up to 35 years) [18], and reduces the risk of miscarriage in this category of patients [19].
However, these questions are far from being finally resolved, which is why this study was aimed to compare the efficiency ART with and without PGT-A in patients with PCOS.
Materials and methods
This study is a nonrandomized clinical trial conducted in two groups of subjects to demonstrate the efficiency of PGT-A in patients with PCOS. The study was conducted at the V.I. Kulakov NMRC for OG&P of the Ministry of Health of Russia. The study enrolled 80 women aged 18-40 years with PCOS diagnosed according to Rotterdam criteria who were treated for infertility (after failed first- and second-line therapy for anovulatory infertility) in the 1st Gynecology Department of the Center from September 2020 to March 2022.
Group 1 (n=40) included women treated with IVF/intracytoplasmic sperm injection (ICSI) with PGT-A.
Group 2 (n=40) consisted of women treated with IVF or IVF/ICSI without PGT-A.
The inclusion criteria were age between 18 and 40 years, the presence of PCOS, and indications for ART. Exclusion criteria were contraindications for ART, severe male factor, uterine abnormalities, cancer, HIV infection, and other immunodeficiency conditions.
Evaluation of baseline clinical and laboratory examinations before the IVF and IVF/ICSI included ultrasound, hormonal investigations and anamnestic data according to the Order of the Russian Ministry of Health (#107n, 803n).
Stimulation of ovarian function in both groups was performed in protocols with gonadotropin-releasing hormone (GnRH) antagonists from days 2–3 of the menstrual cycle using recombinant follicle stimulating hormone (FSH), human menopausal gonadotropins containing equal amounts of FSH and luteinizing hormone (LH). The starting dose of gonadotropins was selected according to the individual parameters of the patient, including age, body mass index, hormonal status, and ovarian reserve (antral follicle count (AFC), anti-Müllerian hormone (AMH), and ovarian volume), and ranged from 100 to 225 IU/day.
To inhibit the premature LH peak upon reaching a follicle diameter of 13–14 mm, GnRH antagonist was given daily at a dose of 0.25 mg/day subcutaneously until the day of ovulation trigger administration.
When the leading follicles reached 17 mm in diameter, an ovulation trigger was administered for the final maturation of the oocyte. Given the presence of PCOS and the high risk of ovarian hyperstimulation syndrome, 0.2 mg of subcutaneous triptorelin acetate was used as an ovulation trigger.
Thirty-six hours after ovulation triggering, a transvaginal ovarian puncture was performed and oocytes were harvested and the maturity of the aspirated oocyte-cumulus complexes evaluated. In the group without PGT-A, the obtained oocytes were fertilized with the partner's sperm by in vitro insemination (n=17, 42.5%) or by ICSI (n=23, 57.5%) according to the indications (depending on the sperm quality). In the group of patients with PGT-A, fertilization was performed by ICSI in 100% of the patients.
When fertilization was confirmed by the presence of two pronuclei on days 1 and 5 in culture, we performed a trophectoderm biopsy of all morphologically suitable embryos (from 3ВВ and 3AB and higher according to Gardner grading system) for subsequent PGT-A (in group 1) and cryopreservation of blastocysts in both groups.
PGT-A on 46 chromosomes was performed in the molecular genetics by next-generation sequencing (NGS).
All patients from both groups entered the program of frozen-thawed embryo transfer against the backdrop of hormone replacement therapy in the next menstrual cycle. Ultrasound monitoring was performed from days 3–5 of the menstrual cycle and cyclic hormonal therapy was administered, provided there were no functional cysts in the ovaries and the endometrium was no thicker than 4–5 mm. The endometrium was prepared using oral estradiol valerate 8 mg/day; micronized progesterone or didrogesterone 30 mg was added on days 15–16 of the menstrual cycle. The embryo was transferred into the uterine cavity on day 6 of progesterone therapy. All patients received a single top-quality embryo transfer. In the posttransfer period, the administration of the above-mentioned drugs was continued. On day 14 after embryo transfer, serum beta-chorionic gonadotropin concentration was measured.
The efficiency of ART was evaluated by the number of achieved pregnancies, the number of biochemical pregnancies, the number of ectopic pregnancies, the number of miscarriages, and failed pregnancies, and the live birth rate.
Statistical analysis
Statistical analysis was performed using Statistica 8.0 software (StatSoft Inc., USA). Quantitative variables were reported as mean (М), standard deviation (SD), 95% confidence interval (CI), median (Me), and interquartile range (Q25%–Q75%). Categorical variables were presented as counts and percentages. The distribution of continuous variables was tested for normality using Shapiro–Wilk test. Quantitative variables that showed normal distribution were expressed as means (M) and standard deviation (SD) and presented as M (SD); otherwise, medians (Me) with interquartile range (Q1; Q3) were reported. Normally distributed continuous variables were compared with a Student’s t-test. Variables not meeting normality assumptions were compared by the nonparametric Mann–Whitney U-test.
Categorical variables were compared with the chi-square (χ2), which was calculated using 2×2 tables, and Fisher's exact test for small samples. When the χ2 test could not be used (expected counts >5), the Z-test (analog of Student's t-test for fractions) was used, and for 0% and 100%, endpoint adjusted. The critical level of significance when testing statistical hypotheses was considered at p<0.05. The relative risk (RR) with 95% confidence interval (CI) for outcomes was calculated according to the Woolf method. For binary outcomes, the effect size was calculated as the risk ratio and the absolute risk reduction (95%CI).
The hypothesis was to obtain at least as good an efficacy of pregnancy initiation and maintenance. The sample size calculation was based on an analysis of the main outcome variable, in this study, the clinical pregnancy rate and the delivery rate. The plan was to compare the effectiveness of IVF in reducing the risk of pregnancy loss in groups 1 and 2.
Previous studies have shown that the clinical pregnancy rate ranges from 0 to 50% and the loss rate is up to 35%. We believe that reducing the pregnancy loss rate to 10% would be a clinically significant outcome.
Results
The clinical characteristics of the patients of the study groups are presented in Table 1.
As can be seen from the data presented in the table, all patients were of reproductive age. Although the patients in the PGT-A group were somewhat older and had higher body weight, no statistically significant differences between the groups were detected (p>0.05). The groups were representative of the main clinical characteristics.
In the PGT-A group, 18/40 (45%) patients had secondary infertility. There were 25 pregnancies, including 3/25 (12%) miscarriages, 6/25 (24%) births, 1/25 (4%) abortions and 15/25 (60%) did not conceive.
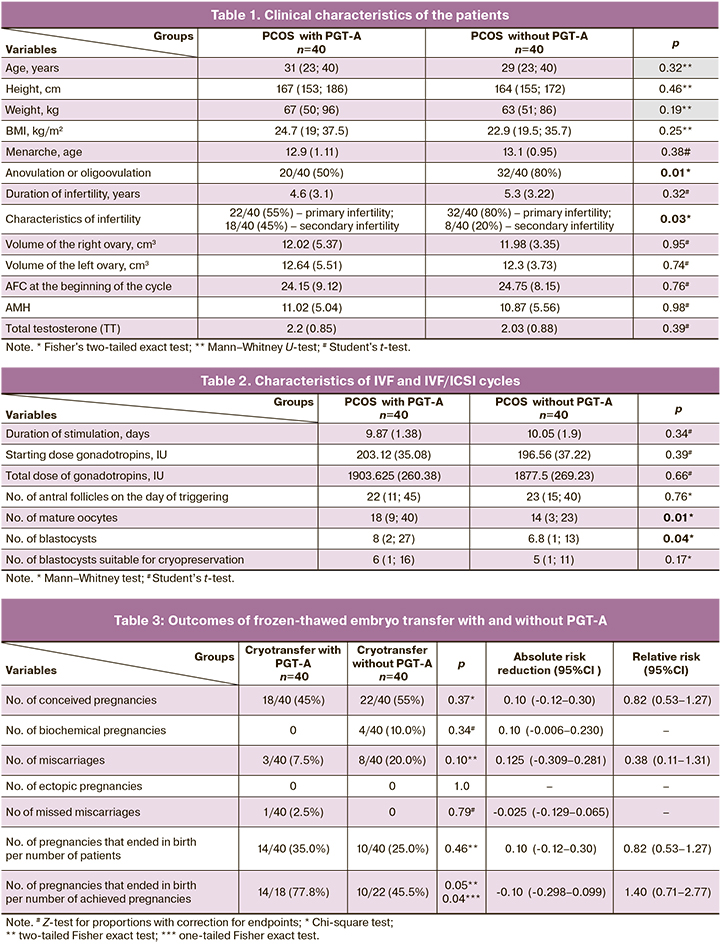
In the group without PGT-A, 8/40 (20%) patients had secondary infertility. There were 10 pregnancies, including 4/10 (40%) miscarriages, 1/10 (10%) abortions, 2/10 (20%) births, 1/10 (10%) ectopic pregnancies and 2/10 (20%) did not conceive.
It was impossible to calculate the mean duration of the menstrual cycle in the patients of both groups, because most of them had chronic anovulation or oligoovulation (menstrual cycle lasting from 28 days to 6 months) in 20/40 (50%) women with PGT-A and 32/40 (80%) women without PGT-A and/or long term use of combined oral contraceptives in 15/40 (35%) women with PGT-A and 6/40 (15%) women without PGT-A (p=0.04 two-tailed Fisher exact test).
In terms of total testosterone levels, hyperandrogenism was diagnosed in 25/40 (62.5%) women in PGT-A group and 17/40 (42.5%) women in the group without PGT-A (p=0.12 two-tailed Fisher's exact test).
A statistically significantly higher number of mature oocytes and the number of blastocysts (p<0.05) were recorded in the PGT-A group of patients.
All embryos suitable for cryopreservation (excellent and good quality) were vitrified. In the group of patients with PGT-A, embryos of 5–6 days of age with a cell content in the trophectoderm > 120, with a well-formed intracellular mass were biopsied - the biopsy specimen contained 7–8 cells. According to Gardner's classification, these were embryos from 3B and 3AB and above.
In the PGT-A group, the median number of embryos retrieved was 5 (1;12), the median number of embryos recommended for transfer was 3 (1;7). A total of 195 embryos were sent for genetic testing; 111/195 (56.9%) were recommended for transfer, 13/195 (6.7%) were mosaic embryos that required genetic counseling before transfer. The remaining 71/195 (36.4%) were not recommended for transfer, of which 53/71 (74.6%) had aneuploidies including 18/53 (33.96%) with trisomy, 17/53 (32.08%) with monosomy, and 18/53 (33.96%) with other aneuploidies. In 1/53 (1.4%) of all embryos not recommended for transfer, a chromosome deletion was detected. Additionally, 4 embryos were excluded from genetic testing due to lack of signal passage.
The rate of achieved pregnancies was 18/40 (45%) in PGT-A group and 22/40 (55%) in the group without PGT-A; the difference was statistically insignificant (RR 0.82 (95% CI 0.53–1.27), p=0.37). Pregnancy rates in the two study groups were not statistically different.
There were no statistically significant differences in the number of missed miscarriages between the two groups (only one case was recorded in the PGT-A group (1/40 (2.5%)) and in the number of biochemical pregnancies (4 cases were recorded in the group without PGT-A (4/40 (10.0%)). No ectopic pregnancies were observed in both groups. The number of miscarriages in PGT-A group was 3/40 (7.5%) and 8/40 (20%) in the group without PGT-A (RR 0.38 (95% CI 0.08–1.31), p=0.10).
In the PGT-A group, the number of pregnancies that ended in birth was 14/40 (35%) and 10/40 (25%) in the group without PGT-A (RR 1.40 (95% CI 0.71; 2.77), p=0.46).
At the same time, the proportion of pregnancies that ended with births was higher in PGT-A group [14/18 (77.8%)] compared to the group without PGT-A [10/22 (45.5%), (RR 1.71 (95% CI 1.02–2.88), p=0.05)], (0.05 two-tailed Fisher's exact test or 0.04 one-tailed Fisher exact test).
Discussion
The study findings did not show statistically significant differences in the number of pregnancies that ended in childbirth between the studied patient groups; however, there was a tendency for an increased number of live births in the PGT-A group, which is the final treatment outcome.
Biochemical pregnancy rates and miscarriage rates also did not differ significantly, but there was a tendency for lower rates of these complications in patients undergoing PGT-A.
There is evidence that even with euploid embryo transfer, patients with PCOS and normal body weight have a higher risk of early reproductive loss than patients without PCOS [20], but genetic testing can reduce the risk of miscarriage in patients with PCOS [19].
Despite the advantages of PGT-A, according to the literature, a biopsy of only part of the trophectoderm may not be representative of all cells and intracellular masses, so genetic diagnosis is not a guarantee of euploid blastocyst transfer [21]. This may explain the relatively small percentage of early reproductive losses in patients undergoing PGT-A.
The results of a recent study by Li Y. et al. showed that chromosomal abnormalities are more common in PCOS based on the study of chorionic villi after reproductive losses in ART programs [22].
There is also evidence that PGT-A in PCOS patients reduces the risk of pregnancy failure and demonstrates a high rate of clinical pregnancy [23].
Conclusion
Summing up the results of our study, it can be concluded that PGT-A in patients with PCOS has clinical value, but further studies are needed in a larger sample of patients. They should investigate factors of embryological stage and take into account both the success rates of pregnancy after frozen-thawed embryo transfer and the effectiveness of these programs in this category of patients, and obstetric and perinatal outcomes.
References
- Ding T., Hardiman P.J., Petersen I., Wang FF., Qu F., Baio G. The prevalence of polycystic ovary syndrome in reproductive-aged women of different ethnicity: a systematic review and meta-analysis. Oncotarget. 2017; 56(8): 96351-8.https://dx.doi.org/10.18632/oncotarget.19180.
- Назаренко Т.А. Синдром поликистозных яичников: современные подходы к диагностике и лечению бесплодия. М.: МЕДпресс-информ; 2005. 208с. [Nazarenko T.A. Polycystic ovary syndrome: modern approaches to the diagnosis and treatment of infertility. M.: MEDpress-inform; 2005. 208 p.(in Russian)].
- Aversa A., La Vignera S., Rago R., Gambineri A., Nappi R.E., Calogero A.E.et al. Fundamental concepts and novel aspects of polycystic ovarian syndrome: Expert consensus resolutions. Front. Endocrinol. 2020; 11: 516.https://dx.doi.org/10.3389/fendo.2020.00516.
- Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertilю Steril. 2004; 81(1): 19-25. https://dx.doi.org/10.1016/j.fertnstert.2003.10.004.
- Михайлова Н.Д., Мишиева Н.Г., Кириллова А.О., Джинчарадзе Л.Г. Современные методы лечения бесплодия у пациенок с синдромом поликистозных яичников. Акушерство и гинекология. 2021; 7: 37-44. [Mikhailova N.D., Mishieva N.G., Kirillova A.O., Dzhincharadze L.G. Modern methods of infertility treatment in patients with polycystic ovary syndrome. Obstetrics and Gynecology. 2021; 7: 37-44. (in Russian)].https://dx.doi.org/10.18565/aig.2021.7.37-44.
- http://prevention.nih.gov/workshops/2012/pcos/docs/FinalReport.pdf
- Министерство здравоохранения Российской Федерации. Клинические рекомендации (протокол лечения) «Синдром поликистозных яичников в репродуктивном возрасте (современные подходы к диагностике и лечению)». М.; 2015. [Ministry of Health of the Russian Federation. Clinical recommendations (treatment protocol) "Polycystic ovary syndrome in reproductive age (modern approaches to diagnosis and treatment)". M.; 2015. (in Russian)].
- Legro R.S., Arslanian S.A., Ehrmann D.A, Hoeger K.M., Murad M.H., Pasquali R. et al. Diagnosis and treatment of polycystic ovary syndrome: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2013; 98(12): 4565-9. https://dx.doi.org/10.1210/jc.2013-2350.
- Costello M.F., Garad R.M., Hart R., Homer H., Johnson L., Jordan C. et al. A review of second- and third-line infertility treatments and supporting evidence in women with polycystic ovary syndrome. Med. Sci. (Basel). 2019; 7(7): 75. https://dx.doi.org/10.3390/medsci7070075.
- Yang W., Yang R., Yang S., Li J., Tu B., Gao C. et al. Infertile polycystic ovary syndrome patients undergoing in vitro fertilization with the gonadotropin-releasing hormone-antagonist protocol: role of hyperandrogenism. Gynecol. Endocrinol. 2018; 34(8): 715-8. https://dx.doi.org/10.1080/09513590.2018.1431773.
- Ho V.N.A., Braam S.C., Pham T.D., Mol B.W., Vuong L.N. The effectiveness and safety of in vitro maturation of oocytes versus in vitro fertilization in women with a high antral follicle count. Hum. Reprod. 2019; 34(6): 1055-64.https://dx.doi.org/10.1093/humrep/dez060.
- Михайлова Н.Д., Мишиева Н.Г., Кириллова А.О., Мартазанова Б.А., Джинчарадзе Л.Г. Дозревание ооцитов in vitro. Акушерство и гинекология. 2021; 11: 64-70. [Mikhailova N.D., Mishieva N.G., Kirillova A.O., Martazanova B.A., Dzhincharade L.G. In vitro oocyte maturation. Obstetrics and Gynecology. 2021; 11: 64-70. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.11.64-70.
- Yang Z.Y., Chian R.C. Development of in vitro maturation techniques for clinical applications. Fertil. Steril. 2017; 108(4): 577-84. https://dx.doi.org/10.1016/j.fertnstert.2017.08.020.
- Vuong L.N., Ho V.N.A., Ho T.M., Dang V.Q., Phung T.H., Giang N.H. et al. In-vitro maturation of oocytes versus conventional IVF in women with infertility and a high antral follicle count: a randomized non-inferiority controlled trial. Hum. Reprod. 2020; 35(11): 2537-47. https://dx.doi.org/10.1093/humrep/deaa240.
- Dumesic D.A., Padmanabhan V., Abbott D.H. Polycystic ovary syndrome and oocyte developmental competence. Obstet. Gynecol. Surv. 2008; 63(1): 39-48. https://dx.doi.org/10.1097/OGX.0b013e31815e85fc.
- Qiao J., Feng H.L. Extra- and intra-ovarian factors in polycystic ovary syndrome: impact on oocyte maturation and embryo developmental competence. Hum. Reprod. Update. 2011; 17(1): 17-33. https://dx.doi.org/10.1093/humupd/dmq032.
- Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. The use of preimplantation genetic testing for aneuploidy (PGT-A): a committee opinion. Fertil. Steril. 2018; 109(3): 429-36. https://dx.doi.org/10.1016/j.fertnstert.2018.01.002.
- Pearson H., Abittan B., Goldman R.H., Mullin C. Preimplantation genetic testing for aneuploidy confers greater benefit to young patients with polycystic ovarian syndrome. Fertil. Steril. 2020; 114(3): e422-3. https://dx.doi.org/10.1016/j.fertnstert.2020.08.1229.
- Pearson H., Abittan B., Frankel R., Goldman R.H. Preimplantation genetic testing for aneuploidy (PGT-a) eliminates the increased miscarriage rate in patients with pcos. Fertil. Steril. 2020; 113(4, Suppl.): e39.https://dx.doi.org/10.1016/j.fertnstert.2020.02.087.
- Luo L., Gu F., Jie H., Ding C., Zhao Q., Wang Q. et al. Early miscarriage rate in lean polycystic ovary syndrome women after euploid embryo transfer - a matched-pair study. Reprod. Biomed. Online. 2017; 35(5): 576-82.https://dx.doi.org/10.1016/j.rbmo.2017.07.010.
- Макарова Н.П., Екимов А.Н., Кулакова Е.В., Драпкина Ю.С., Сысоева А.П., Краснова Н.А., Калинина Е.А. Особенности мозаицизма у эмбрионов человека в программах лечения бесплодия методами вспомогательных репродуктивных технологий. Акушерство и гинекология. 2021; 7: 144-51. [Makarova N.P., Ekimov A.N., Kulakova E.V., Drapkina Yu.S., Sysoeva A.P., Krasnova N.A., Kalinina E.A. Characteristics of embryonic mosaicism in infertility treatment with assisted reproductive technologies. Obstetrics and Gynecology. 2021; 7: 144-51. (in Russian)].https://dx.doi.org/10.18565/aig.2021.7.144-151.
- Li Y., Wang L., Xu J., Niu W., Shi H., Hu L. et al. Higher chromosomal aberration rate in miscarried conceptus from polycystic ovary syndrome women undergoing assisted reproductive treatment. Fertil. Steril. 2019; 111(5): 936-43.e2. https://dx.doi.org/10.1016/j.fertnstert.2019.01.026.
- Колода Ю.А., Подзолкова Н.М., Петриченко Ю.Г. Прогнозирование исходов и выбор оптимальной тактики в программах вспомогательных репродуктивных технологий при синдроме поликистозных яичников. Акушерство и гинекология. 2021; 2: 84-9. [Koloda Yu.A., Podzolkova N.M., Petrichenko Yu.G. Prediction of ART outcomes and treatment of choice in PCOS patients. Obstetrics and Gynecology. 2021; 2: 84-9. (in Russian)].https://dx.doi.org/10.18565/aig.2021.2.84-89.
Received 31.03.2022
Accepted 23.06.2022
About the Authors
Nina D. Mikhailova, Ph.D. Student at the 1st Gynecology Department, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, mihailnina@mail.ru,117997, Russia, Moscow, Ac. Oparina str., 4.
Artem A. Aksenenko, Gynecologist at the 1st Gynecology Department, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, a_axenenko@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Muminat Kh. Ibragimova, Ph.D., Gynecologist at the 1st Gynecology Department, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
m_ibragimova@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Alexey N. Ekimov, Laboratory geneticist at the Laboratory of Molecular Genetics, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
a_ekimov@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Alla A. Gavisova, Ph.D., Senior Researcher at the 1st Gynecology Department, National Medical Research Center for Obstetrics, Academician V.I. Kulakov NMRC
for OG&P, Ministry of Health of Russia, gavialla@yandex.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Authors' contributions: Gavisova A.A., Aksenenko A.A., Ibragimova M.Kh. – conception and design of the study;
Ekimov A.N. – preimplantation genetic testing; Mikhailova N.D. – data collection and analysis, statistical analysis, manuscript drafting; Gavisova A.A., Aksenenko A.A. – manuscript editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study, which was conducted as part of the dissertation work of Mikhailova N.D.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Mikhailova N.D., Aksenenko A.A., Ibragimova M.Kh., Ekimov A.N., Gavisova A.A. Efficiency of assisted reproductive technology in women with polycystic ovarian syndrome who undergo preimplantation genetic testing for aneuploidy.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 7: 60-67 (in Russian)
https://dx.doi.org/10.18565/aig.2022.7.60-67



