The effectiveness of the antenatal examination algorithm for nonimmune hydrops fetalis
Objective. To evaluate the effectiveness of the antenatal examination algorithm for nonimmune hydrops fetalis.Kadyrberdieva F.Z., Shmakov R.G., Bokeriya E.L., Kostyukov K.V., Tetruashvili N.K.
Materials and methods. The study included pregnant women with nonimmune hydrops fetalis delivered at the Center from 2015 to 2020 (n=45), and divided into two groups. Group 1 (prospective group from 2018 to 2020) included 30 pregnant women with nonimmune hydrops fetalis who were antenatally examined and, in some cases, received intrauterine treatment; group 2 (retrospective group from 2015 to 2018) consisted of 15 women who were not antenatally examined and did not receive intrauterine treatment. Pregnant women were selected by the perinatal council of the Center.
Results. According to the examination protocol, in group 1 the etiology of nonimmune hydrops fetalis was antenatally determined in 83.3% (25/30) of cases and 16.7% (5/30) of cases were considered as idiopathic. In group 2 the etiology was established in 86.7% (13/15) of cases: it was determined in 60% (9/15) of cases antenatally (on the basis of fetal ultrasound), and in 26.7% (4/15) of cases postnatally. The live birth rate did not differ statistically between groups, namely 70% (21/30) in group 1 versus 73.3% (11/15) in group 2 (p=0.94). However, the neonatal mortality rate was statistically higher in the group where intrauterine treatment was not performed, namely 73.8% (8/11) versus 23.8% (5/21) (p=0.002). Thus, the overall survival in group 1 was 53.3% (16/30), while in group 2 it was 20% (3/15).
Conclusion. The introduction of the examination protocol into clinical practice has led to an increase in the detection of the causes of nonimmune hydrops fetalis at the antenatal stage, especially in the cases not associated with fetal malformations.
Keywords
Nonimmune hydrops fetalis is known to increase significantly perinatal morbidity and mortality [1].
When detecting nonimmune hydrops fetalis, it is important to determine the cause that led to the development of this complication, because its etiology determines further tactics of pregnancy management and possibility of intrauterine treatment [2]. If the disease is curable, it is possible to conduct antenatal etiopathogenetic and symptomatic therapy, which could help to prolong pregnancy closer to the full term, and in some cases to reduce or completely control nonimmune hydrops fetalis [3].
According to the world recommendations for the management of pregnant women with nonimmune hydrops fetalis [2–4] and clinical and diagnostic capabilities of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, an internal protocol of examination of pregnant women with this fetal pathology was developed and introduced into clinical practice.
We assume that this approach will improve perinatal outcomes and significantly reduce the financial costs of treating and caring for children with nonimmune hydrops fetalis.
The aim of the study was to evaluate the effectiveness of the antenatal examination algorithm for nonimmune hydrops fetalis on the basis of the detected causes and pregnancy outcomes.
Materials and Methods
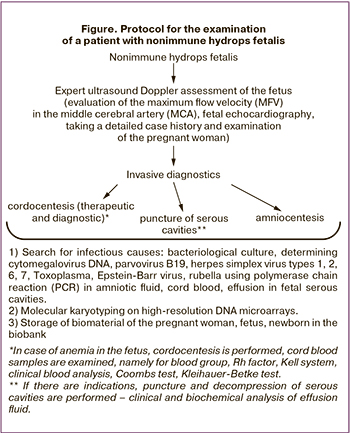 The study was conducted at the obstetric and neonatal departments of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology (hereafter Center), Moscow, Russia. Since 2018, the Center has adopted a protocol for the management of patients with nonimmune hydrops fetalis (Figure), the purpose of which is to determine the cause that led to the development of this complication.
The study was conducted at the obstetric and neonatal departments of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology (hereafter Center), Moscow, Russia. Since 2018, the Center has adopted a protocol for the management of patients with nonimmune hydrops fetalis (Figure), the purpose of which is to determine the cause that led to the development of this complication.
The study included pregnant women with nonimmune hydrops fetalis delivered at the Center from 2015 to 2020 (n = 45), and divided into two groups. Group 1 (prospective group from 2018 to 2020) included 30 pregnant women with nonimmune hydrops fetalis who were antenatally examined and, in some cases, received intrauterine treatment; group 2 (retrospective group from 2015 to 2018) consisted of 15 women who were not antenatally examined and did not receive intrauterine treatment. Pregnant women were selected by the perinatal council of the Center.
Until 2018, antenatal examination and intrauterine treatment for nonimmune hydrops fetalis were not performed. Only expert fetal ultrasound data were evaluated, in some cases echocardiography data were assessed. Pregnant women from group 2 were admitted to the Center only for delivery.
All patients were performed an expert ultrasound assessment of the fetus. The following parameters were evaluated: the presence and type of fetal structural pathology, the number of serous cavities with effusion, the volume of effusion, the presence and degree of lung hypoplasia, heart compression, the presence, depth and prevalence of soft tissue edema in the fetus, as well as the amount of amniotic fluid and placenta thickness. Maximum flow velocity (MFV) in the middle cerebral artery (MCA) was assessed using Doppler, an increase of more than 1.505 MoM (multiples of median) was a sign of anemia [5]. After 20–22 weeks, an electrocardiography of the fetus was performed using ultrasound machine GE VOLUSON S8 in order to determine the structural or functional pathology of the heart, valves and main vessels, the presence of effusion in the pericardial cavity and its volume, as well as a number of parameters that could assess the functional state of the fetal heart. It should be noted that not all patients in group 2 were performed the assessment of MFV in MCA and echocardiography of the fetus.
After hospitalization in the Center, a detailed somatic, obstetric and gynecological history was taken, as well as a thorough physical examination was performed. When taking the patient’s case history, particular attention was paid to the detection of autoimmune and hematological diseases (especially thalassemia), infectious diseases, especially those associated with fever during pregnancy. These factors could be the causes of nonimmune hydrops fetalis.
From the moment of admission of the pregnant woman to the hospital until delivery, the fetal condition was controlled by dynamic ultrasound, Doppler and cardiac monitoring.
Antenatal examination of patients in group 1
If there were no contraindications, the patients underwent diagnostic amniocentesis. In some cases, if there were indications, curative interventions were carried out at the same time. Thus, if anemia was suspected in the fetus, cordocentesis with transfusion of leukocyte- and platelet-poor red blood cells, thoracocentesis, laparocentesis, thoracoamniotic shunting for the decompression of serous cavities were performed. In case of cordocentesis with transfusion of leukocyte- and platelet-poor red blood cells, there was an assessment of the level of hemoglobin and hematocrit before and after the transfusion. When performing thoracocentesis, laparocentesis, thoracoamniotic shunting, the clinical and biochemical analysis of effusion fluid was carried out in order to determine the nature of the effusion. All the biological material of the fetus was sent for virological and microbiological analysis, as well as biobanking.
Molecular karyotyping was performed on high-resolution DNA microarrays (Cytoscan 750К, Affymetrix). DNA was extracted from fresh whole blood or amniotic fluid using the PureLink Genomic DNA Kit (Invitrogen, USA).
The search for infectious causes was conducted using polymerase chain reaction (PCR). DNA isolation from clinical material samples was performed using the PROBA-CITO kit (LLC, DNA-Technology, Russia) according to the manufacturer’s instructions. Amplification of specific fragments of causative agent DNA was carried out using commercial reagent kits of LLC DNA-Technology in real-time mode and amplifier DT-964 (LLC, DNA-Technology, Russia).
Postnatal examination for inherited metabolic diseases was performed using tandem mass spectrometry. Indications for its implementation were cases of idiopathic nonimmune hydrops fetalis or clinical and laboratory manifestations that are characteristic of the diseases of this group. Depending on the revealed etiology and clinical manifestations of nonimmune hydrops fetalis, the following fetal therapies were used:
- etiopathogenetic therapy: antiarrhythmic therapy, intrauterine transfusion with packed red blood cells, antiviral therapy, immunoglobulin therapy, antibiotic therapy, interstitial laser coagulation of vessels feeding the sacrococcygeal teratoma;
- symptomatic therapy: amnioreduction, thoracocentesis, laparocentesis, thoracoamniotic shunting, transplacental digoxin therapy, corticosteroid administration.
In group 2, postnatal examination was performed individually according to indications. In the case of multiple malformations and signs of dysembryogenesis, karyotyping was performed after the conclusion of a geneticist.
In both groups, autopsy was performed in cases of stillbirth or neonatal death.
Statistical processing of the obtained data was performed using the IBM SPSS Statistica 22 software package. Nonparametric methods of statistical analysis were used. To describe quantitative data, medians and interquartile range (Me (Q1; Q3)) were estimated. The qualitative parameters of the groups were compared using the χ2 criterion. The statistical significance of difference in quantitative parameters between the groups was assessed using the p-value of the Mann-Whitney test. The difference was considered statistically significant at p≤ 0.05.
Results and Discussion
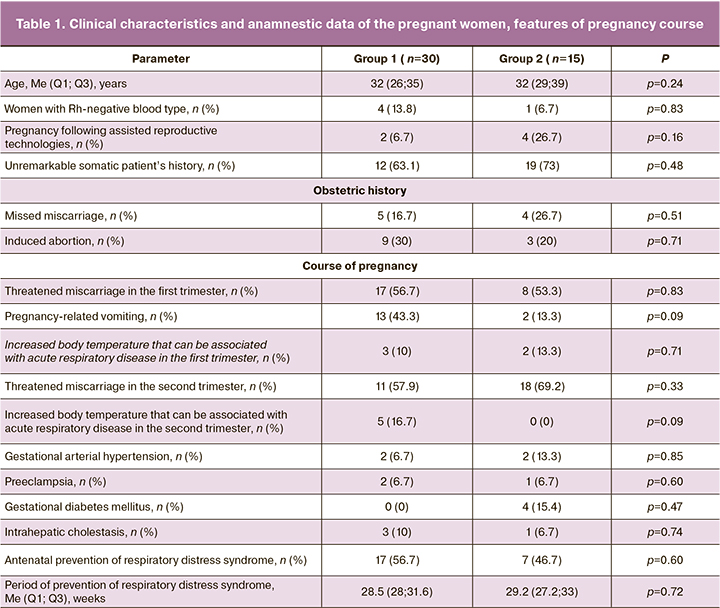
The analysis of somatic, obstetric and gynecological history of the pregnant women and the course of the present pregnancy did not reveal any statistically significant differences between the groups (p≥0.05) (Table 1).
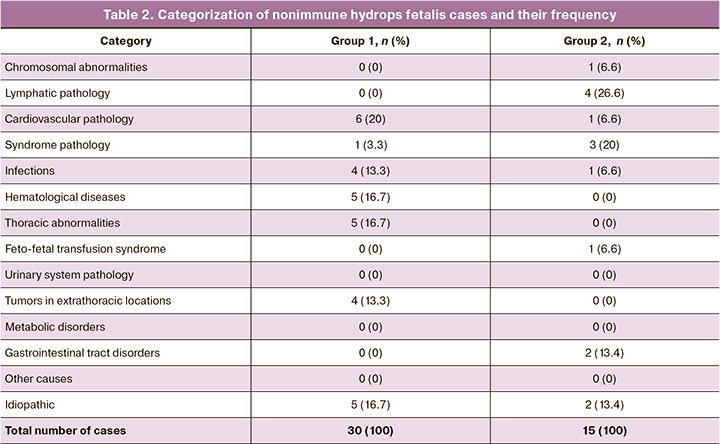
Depending on the data of ultrasound, fetal echocardiography, antenatalandpostnatalexaminations, all cases were referred to one of the 14 categories of diseases proposed by C. Bellini et al. (2015) [6]. The categorization is presented in Table 2.
According to the protocol of examination, in group 1, etiology of nonimmune hydrops fetalis was established antenatally in 83.3% (25/30) of cases, and 16.7% (5/30) of cases were referred to the idiopathic category. The causes that were revealed antenatally were confirmed by postnatal examination. In group 2, etiology was established antenatally in 60% (9/15) of cases (based on fetal ultrasound), and it was found postnatally in 26.7% (4/15) of cases. Postnatally, the following causes of nonimmune hydrops fetalis were identified in this group: herpetic infection in one case, Noonan syndrome in one case, trisomy 18 in one case, and chylothorax in one case.
The frequency of congenital malformations in the fetus did not differ significantly between the groups: 33.3% (10/30) in group 1 versus 46.7% (7/15) in group 2 (p=0.58). These congenital malformations included congenital cystic adenomatous malformation of the lung (CCAM) – 13.3% (4/30), sacrococcygeal teratoma (SCT) – 13.3% (4/30), anterior mediastinal teratoma – 3.3% (1/30) and were found in group 1. Structural defects and their frequency detected by ultrasound data are presented in Table 3. Differences in diagnoses of congenital malformations at the antenatal and postnatal stages were not detected in the groups.
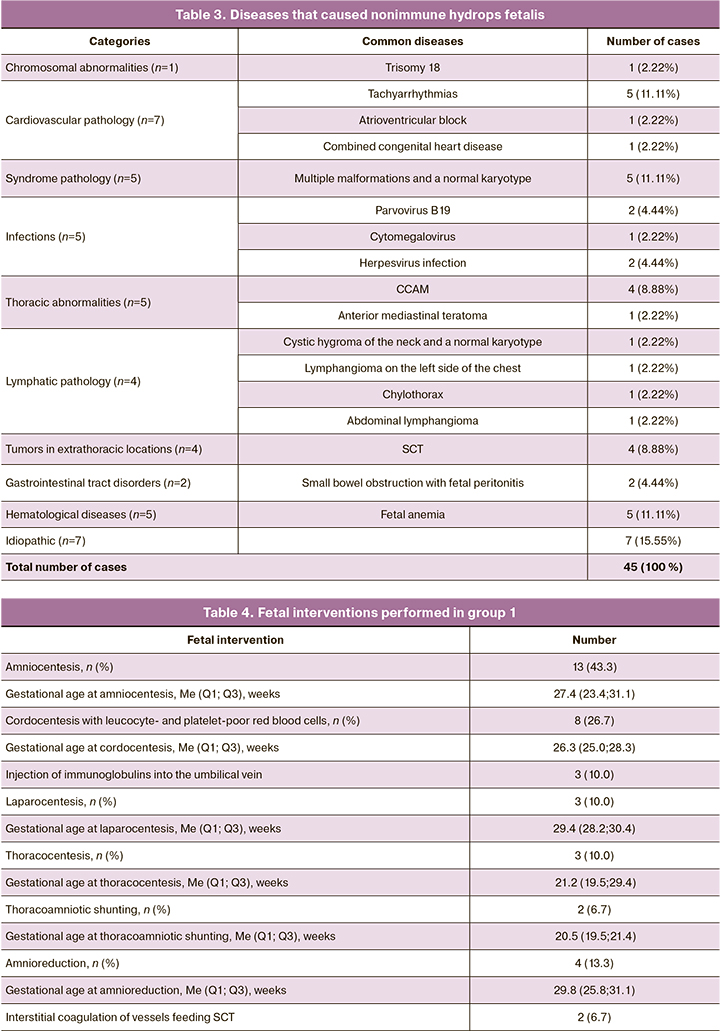
The category of cardiovascular pathology in group 1 includes cases of heart rhythm disorders in the fetus and isolated congenital heart disease: tachyarrhythmia was found in 13.3% (4/30) of cases, atrioventricular block (AV block) in 3.3% (1/30) of cases, and hypoplasia of the left heart with a restrictive oval window in 3.3% (1/30) of cases. AV block in the fetus resulted from an autoimmune disease of the pregnant woman (Sjogren’s syndrome, rheumatoid arthritis); there were detected antibodies to SSA/Ro in high titers and anti-nuclear antibodies. After passing through the placenta, maternal SSA/Ro antibodies and anti-nuclear antibodies cross react with L-types of calcium channels in fetal heart cells, resulting in a slowdown in atrioventricular conduction (AV block). In group 2, this category includes a case of fetal tachyarrhythmia, which was revealed in 6.7% (1/15) of patients.
Congenital heart defects were found both in isolation and in combination with congenital malformations. In group 1, coronary heart defects were detected antenatally in 23.3% (7/30) of cases, the main share of which was due to a ventricular septal defect (VSD) that occurred in 16.7% (5/30) of cases. VSD was detected in isolation in 3.3% (1/30) of cases, and in combination with congenital malformation – in 13.3% (4/30) of cases: in 6.6% (2/30) – with SCT, and in 6.6% (2/30) – with CCAM. The combined congenital heart defects were found in 6.6% (2/30) of patients (hypoplasia of the left heart with a restrictive oval window, VSD and coarctation of the aorta, VSD). In group 2, congenital heart defects were detected antenatally in 40% (6/15) of cases, of which 33.3% (5/15) were diagnosed with VSD. In most cases, VSD was observed in combination with congenital malformations and other heart defects: cystic neck hygroma was observed in 6.7% (1/15) of cases; pathology of the aorta, aortic valve, and the central nervous system was detected in 6.7% (1/15) of cases; combined congenital heart disease (left ventricular hypoplasia, aortic coarctation, and VSD) was found in 6.7% (1/15) of cases; and congenital malformations of the central nervous system were observed in 6.7% (1/15) of cases. Isolated VSD was revealed in 6.7% (1/15) of cases.
In group 1, molecular karyotyping was performed on high-resolution DNA microarrays using the biological material of the fetus, and no aneuploidies were detected. In group 2, karyotyping was performed postnatally when clinically indicated; one case of trisomy 18 in a newborn with multiple congenital malformations was revealed.
The assessment of MFV in the fetal MCA plays an important role in making a diagnosis [5]. Thus, fetal anemia was detected in 26.7% (8/30) of cases in group 1, among them anemia was caused by infectious agents in 10% (3/30) of patients: parvovirus B19 was detected in two cases, cytomegalovirus - in one case. In group 2, antenatal assessment of MFV in MCA was not performed in all cases, and severe congenital anemia was diagnosed at birth in 27.3% (3/11) of cases according to clinical blood analysis; its treatment was necessary in the early neonatal period. Among these cases, anemia was observed in two newborns with multiple malformations, and in one case it was detected in a newborn with a congenital herpetic infection, which was also diagnosed postnatally.
Therefore, when an antenatal examination was performed in group 1, the spectrum of causes of nonimmune hydrops fetalis turned out to be wider, and contained not only fetal malformations, but also infections and hematological pathology. In group 2, the causes of nonimmune hydrops fetalis that were detected antenatally include only fetal malformations, and only a more detailed postnatal examination made it possible to clarify and complete the etiological structure by diagnosing infectious, chromosomal, syndrome, and hematological factors. Timely detection of the causes of nonimmune hydrops fetalis could change the management tactics of pregnant women in this group.
After the examination, a differentiated approach to the intrauterine treatment was applied in group 1. On the basis of etiology and clinical manifestations, different methods of intrauterine treatment were applied in 56.7% (17/30) of cases. These fetal interventions are presented in Table 4.
Tachyarrhythmia and cardiac decompensation in the fetus were indications for the administration of transplacental drug therapy [7–9]. Antiarrhythmic therapy was performed with digoxin and sotalol with simultaneous administration of potassium preparations.
An important sign that characterizes the severity of intrauterine heart failure is tricuspid valve insufficiency [9]. In group 1, this sign was detected in 56.7% (17/30) of cases, digoxin was used in the treatment of 43.3% (13/30) of them.
The duration, multiplicity and effectiveness of intrauterine treatment methods were determined in dynamics based on the data of ultrasound and fetal echocardiography.
The main characteristics of infants at birth are shown in Table 5. Signs of hydrops fetalis were more common in newborns from group 2 in comparison with group 1, namely, 15/15 (100%) newborns versus 19/30 (63.3%), respectively (p=0.02). The most successful intrauterine regression of nonimmune hydrops fetalis was observed when using etiopathogenetic therapy for tachyarrhythmia and fetal anemia.
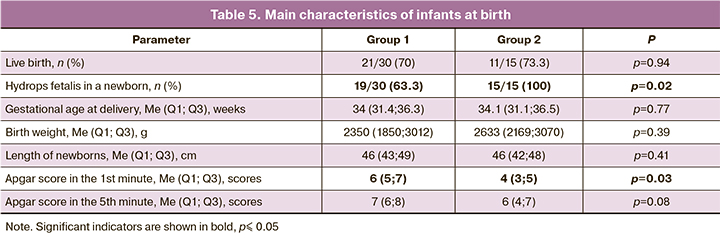
Live birth rate did not differ significantly between the groups, 70% (21/30) in group 1 versus 73.3% (11/15) in group2, respectively (p=0.94). However, the rate of neonatal mortality was significantly higher in group 2 compared to group 1, 73.8% (8/11) versus 23.8% (5/21), respectively (p=0.002). Thus, the overall survival rate in group 1 was 53.3% (16/30), while in group 2 it was 20% (3/15).
Conclusion
It should be noted that the introduction of the examination protocol has led to an increase in the detection of causes of nonimmune hydrops fetalis at the antenatal stage, especially those causes that are not associated with fetal malformations. This protocol allowed us to evaluate the morphological and functional state of the fetus in more detail, to conduct therapeutic measures, and to assess their effectiveness. Further work in this direction will make it possible to reach a rational decision on the management tactics of pregnant women, taking into account the prognosis for the life of the fetus and newborn.
References
- Ota S., Sahara J., Mabuchi A., Yamamoto R., Ishii K., Mitsuda N. Perinatal and one-year outcomes of non-immune hydrops fetalis by etiology and age at diagnosis. J. Obstet. Gynaecol. Res. 2015; 42(4): 385-91. https://dx.doi.org/10.1111/jog.12922.
- Mary E.N., Suneet P.C., Jodi S.D. Society for Maternal-Fetal Medicine (SMFM) Clinical Guideline No.7: nonimmune hydrops fetalis. Am. J. Obstet. Gynecol. 2015; 212(2): 127-39. https://dx.doi.org/10.1016/j.ajog.2014.12.018.
- Désilets V., De Bie I., Audibert F. No.36–investigation and management of non-immune fetal hydrops. J. Obstet. Gynaecol. Can. 2018; 40(8): 1077-90. https://dx.doi.org/10.1016/j.jogc.2017.12.011.
- Laterre M., Bernard P., Vikkula M., Sznajer Y. Improved diagnosis in non-immune hydrops fetalis using a standardized algorithm. Prenat. Diagn. 2018; 38(5): 337-43. https://dx.doi.org/10.1002/pd.5243.
- Berry S.M., Stone J., Norton M.E., Johnson D., Berghella V. Fetal blood sampling. Am. J. Obstet. Gynecol. 2013; 209(3): 170-80. https://dx.doi.org/10.1016/j.ajog.2013.07.014.
- Bellini C., Donarini G., Paladini D., Calevo M.G., Bellini T., Ramenghi L.A., Hennekam R.C. Etiology of non-immune hydrops fetalis: An update. Am. J. Med. Genet. A. 2015; 167A(5): 1082-8. https://dx.doi.org/10.1002/ajmg.a.36988.
- Бокерия Е.Л., Беспалова Е.Д., Суратова О.Г. Фетальные органические тахиаритмии: опыт лечения. Анналы аритмологии. 2011; 2: 36-44. [Bockeria E.L., Bespalova E.D., Suratova O.G. Fetal organic tachyarrhythmias: treatment experience. Annaly Arithmologii (Annals of Arrhythmology). 2011; 2: 36-44. (in Russian)].
- Беспалова Е.Д., Бокерия Е.Л. Перинатальный кардиологический скрининг. Методические рекомендации для врачей неонатологов, педиатров, акушеров-гинекологов, врачей функциональной диагностики. Бокерия Л.А., Володин Н.Н., ред. М.; 2010. [Bespalova E.D., Bockeria E.L. Perinatal cardiological screening: guidelines for neonatologists, pediatricians, obstetricians/gynecologists, functional diagnosticians, Moscow, 2010. (in Russian)].
- Huhta J.C. Guidelines for the evaluation of heart failure in the fetus with or without hydrops. Pediatr. Cardiol. 2004; 25(3): 274-86. https://dx.doi.org/10.1007/s00246-003-0591-3.
Received 05.02.2020
Accepted 07.07.2020
About the Authors
Faina Z. Kadyrberdieva, postgraduate student, National Medical Research Center of Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. Tel.: +7(909)916-58-52. E-mail: f_kadyrberdieva@oparina4.ru.4, Ac. Oparina str., Moscow, 117997, Russia.
Roman G. Shmakov, MD, professor of the Russian Academy of Sciences, National Medical Research Center of Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. Tel.: +7(495)438-72-00. E-mail: r_shmakov@oparina4.ru.
4, Ac. Oparina str., Moscow, 117997, Russia.
Ekaterina L. Bokeriya, MD, professor, head of the department of pathology of newborn and premature babies, National Medical Research Center of Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. Tel.: +7(495)438-27-05. E-mail: e_bokeriya@oparina4.ru.
4, Ac. Oparina str., Moscow, 117997, Russia.
Kirill V. Kostyukov, PhD, doctor of the Department of the functional diagnosis, National Medical Research Center of Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. Tel.: +7(495)438-25-29. E-mail: k_kostyukov@oparina4.ru.
4, Ac. Oparina str., Moscow, 117997, Russia.
Nana K. Tetruashvili, MD, Head of the Department of pregnancy loss prevention and therapy, National Medical Research Center of Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. Tel.: +7(495)438-14-77. E-mail: n_tetruashvili@oparina4.ru.
4, Ac. Oparina str., Moscow, 117997, Russia.
For citation: Kadyrberdieva F.Z., Shmakov R.G., Bokeriya E.L., Kostyukov K.V., Tetruashvili N.K. The effectiveness of the antenatal examination algorithm for nonimmune hydrops fetalis.
Akusherstvo i Ginekologiya / Obstetrics and gynecology. 2020; 7: 71-78 (in Russian)
https://dx.doi.org/10.18565/aig.2020.7.71-78



