Brain damage in preterm babies associated with congenital pneumonia
Currently, thanks to advanced assisted reproductive technologies, as well as improvement of the quality of obstetric and neonatal care, the population of the babies born before 37 weeks of gestation continues to increase. Perinatal brain damage is manifested by disorders affecting the structure and functions of the central nervous system (CNS), and is one of the major factors that contribute to formation of a severe neurological disorder and disability in preterm babies. Intraventricular hemorrhage is the most common form of structural brain damage in premature babies. According to the published data, the incidence of intraventricular hemorrhage is inversely proportional to the gestational age of premature infants and ranges from 15% to 31%. Congenital pneumonia contributes to adverse neurological outcomes of perinatal disorders in preterm babies.Artamkina E.I., Degtyarev D.N., Kvekveskiri M.D., Kirtbaya A.R., Amirkhanova D.Yu. , Beznoschenko O.S., Bykova Yu.K., Golubtsova Yu.M., Balashova E.N, Ionov O.V.
Aim. To define the correlation between congenital pneumonia in preterm babies and the incidence and severity of hypoxic-hemorrhagic brain damage.
Materials and methods. Prospective case-control study was conducted in the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology. The study included 271 premature infants born at 28–36 weeks of gestational age in the period from January, 2017 to February, 2019. The infants were stratified into 2 groups: group 1(the main group) – the babies with the signs of congenital pneumonia (n=115); the group 2 (the comparison group) – the babies without these signs (n=156).
Results. In group1 the incidence and severity of perinatal disorders of CNS in preterm babies with congenital pneumonia was higher than in the comparison group.
Conclusion. According to the obtained data, congenital pneumonia in preterm babies increases the risk of hypoxic-hemorrhagic brain damage.
Keywords
Currently, thanks to the development of assisted reproductive technologies, as well as improvement of the quality of obstetric and neonatological care, the population of babies born before 37 weeks of gestation continues to increase. In 2018, 97,000 of 1.6 million babies were born prematurely (about 6%). In 2018, 5 000 premature infants were born in Moscow [1]. There is a high risk of development of perinatal brain damage in premature babies [2–4]. Perinatal brain damage is manifested by structural and functional disorders of the CNS, and is one of the main factors leading to neurological disorders and disability in premature babies. Most commonly, intraventricular hemorrhage (IVH) is a structural brain injury detected in premature babies. According to the published data, the occurrence rate is inversely proportional to gestational age (GA) and ranges from 15% to 31% [3]. In 74% of cases intraventricular hemorrhage is diagnosed in the first 48 hours in the postnatal period [4]. High incidence rate of intraventricular hemorrhage, especially in very preterm babies is conditioned by the complex of pathogenetic factors: a large number of primitive germinal matrix vessels; cerebral blood flow autoregulation in the immature brain, central hemodynamic abnormalities and hemostasiological disorders [3, 5]. Periventricular leukomalacia (PVL) ranks second among structural brain injuries in premature babies [6].
Some studies showed that the systemic fetal inflammatory response to the threat of preterm birth plays one of the key roles in impaired oligodendrocyte maturation. This has an adverse effect on the development of central nervous system (CNS) after birth. [3, 7, 8].
Infections contribute to the adverse neurological outcomes of perinatal diseases in premature babies. Most often, perinatal infections manifest themselves by clinical laboratory signs of congenital pneumonia in the first days of life, which has bacterial pathogenesis in vast majority of cases. [9]. The incidence rate of congenital pneumonia in infants ranges from 4 to 20 per 1000 live births, and reaches 10:100 among all premature babies and 40:100 among premature babies who need intensive respiratory therapy [9]. Neurosonography (NS) is the most accessible and informative method for diagnosis of structural injuries of the central nervous system in premature babies in inpatient settings [6].
The aim: to define the impact of congenital pneumonia on the incidence, morphofunctional manifestations and severity of Bon brain damage of the CNS in premature babies at 28–36 weeks of gestation.
Materials and methods
The study was carried out under the auspices of A.G. Antonov Department of Resuscitation and Intensive Care for Newborns, the Department of Pathology of Newborns and Premature Babies, the Institute of Neonatology and Pediatrics of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of the Ministry of Health of Russia, (further referred to as “the Center”). The study included 271 premature babies born at 28–36 weeks of GA in the period from January 2017 to February 2019. Of them, 132 babies were born in multiple-births pregnancy: 55 twins, 6 – triplets and 1 quadruplet. Inclusion criteria in the main group were premature babies with congenital pneumonia born at 28–36 weeks of GA. Inclusion criteria in the comparison group were premature transient tachypnea of the newborn (TTN) or/and neonatal respiratory distress syndrome (NRDS) born at 28–36 weeks of gestational age. Exclusion criteria were the babies born at > 36 weeks and 6 days of GA; premature babies born at 22–27 weeks of GA; refusal of mothers from participation of their newborn babies in the study, premature babies with multiple malformations; TORCH infections; critical congenital heart disease; purulent meningitis and osteomyelitis; edematous form of hemolytic disease in newborn infants; newborns transferred from the intensive care unit to another hospital and babies admitted from other hospitals.
Standard clinical laboratory tests were carried out, including the blood test, analysis of acid-base status and blood gases in dynamics, microbiological assay of blood for sterility, assessment of plasma C-reactive protein concentration. Neurosonography was performed in all babies in the first 48 hours and next 72–144 hours of life; the subsequent studies (from the 2nd week of life) were prescribed according to clinical indications. Control NS was carried out in all infants at gestationally corrected 38–40 weeks, before discharge from the hospital. Brain damage was found in 87 (32%) premature babies: in 54 (62%) infants – in the main group, 33 (38%) – in the comparison group. Premature babies born at 28–33 weeks of gestational age, who had suspected intraventricular hemorrhage (84 patients), underwent detailed study of hemostasis and platelet functional activity.
To achieve the goal, the infants included in the study were divided into 2 groups: group 1 (the main group) – 115 premature babies born at 28-36 weeks of gestational age, who were transferred from the delivery room to the neonatal intensive care unit (NICU) due to severe respiratory disorders and were diagnosed with pneumonia in the first 72 hours of life; group 2 (the comparison group) – 156 premature babies of similar GA, who were transferred from the delivery room to NICU due to non-infectious causes of respiratory disorders. The diagnosis of congenital pneumonia in infants in group 1 was based on combination of the following clinical laboratory signs: pronounced respiratory disorders and/or focal changes detected on chest x-rays, leukocytosis, neutrophilia and thrombocytopenia according to the clinical blood test, high levels of C-reactive protein and/or positive blood culture result for sterility (Clinical practice guidelines of the Russian Society of Neonatologists (RSN) [10].
In group 2, in 69% babies the cause of respiratory disorders was NRDS, in 31% of babies – TTN. To specify the incidence of CNS damage depending on GA, the babies were divided into two subgroups: premature babies born at 28– and 33 weeks of gestational age and premature babies born at 34–36 weeks. Further, within the subgroups the babies were subdivided taking into account the presence or absence of brain damage according to RSN data (Fig.1).
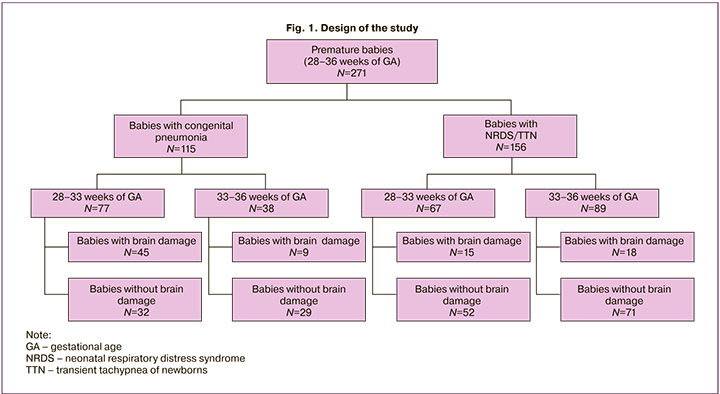
The comparative assessment of the obstetric and gynecological history of mothers, types of delivery, gestational age, Apgar scores at the 1st and the 5th minutes of life, anthropometric parameters of infants at birth, clinical condition in the neonatal period and the results of neurosonography were assessed in each group. Considering the results of NS, all cases of intraventricular hemorrhage depending on the severity were divided into 3 grades according to classification of the Russian association of specialists in perinatal medicine (RASPM) [11]. In each group and subgroups, the neurological status and the results of neurosonography in dynamics, the need for various methods of respiratory, vasopressor and cardiotonic therapy were assessed from the first days of life. Additionally, in babies born at 28–33 weeks of gestational age, the frequency and specific characteristics of hemostasiological disorders were assessed.
In the main group, intensive respiratory therapy was necessary in 112 out of 115 (97%) babies in the first minutes of life. After stabilization of their condition, the babies in the humidicribs were transported from the delivery room to the neonatal intensive care unit (NICU). 5 late preterm infants born at 35–36 weeks of gestational age were in satisfactory condition (3%). They were transferred from the delivery room to the neonatal unit, but in the first 48 hours of life they developed symptoms of respiratory failure (RF), due to which they were transferred to the NICU for intensive therapy. In the comparison group, intensive respiratory therapy was necessary in 140 out of 156 (90%) premature babies in the first days of life. After their condition stabilized, they were transferred from the delivery room to the NICU. 16 (10%) premature babies born at 35–36 weeks of gestational age were in satisfactory condition, and they were transferred from the delivery room to the neonatal unit. However, due to development of respiratory disorders during first 48 hours of life, they were transferred to the NICU.
Depending on the severity of respiratory disorders and changes in blood gases, different types of respiratory therapy were used to treat respiratory depression (RD): high flow air-oxygen mixture delivery at a flow > 4 l/min. through high-flow nasal cannula (HFNC), continuous positive airway pressure (CPAP) for those babies, who were breathing spontaneously, biphasic positive airway pressure (BIPAP), standard artificial lung ventilation (ALV) through the endotracheal tube, high-frequency oscillatory ventilation (HFOV). As a rule, HFNC was used in babies with mild RF born at 34–36 weeks of gestational age, or CPAP – in babies born at 28–33 weeks; biphasic nCPAP – in babies with moderate RF, in babies with severe RF – various regimens of standard ALV, HFOV– in babies with extremely severe RF. When HFNC oxygen therapy and CPAP were ineffective, BIPAP wasused. When BIPAP did not help, standard ALV was used, and when it was ineffective, HFOV was used.
In 43.2% of babies impaired lung function was associated with cardiac insufficiency of various severity, which required cardiotonic and vasopressor therapy.
Statistical analysis
Software statistical package SPSS v.17.0 was used for data processing. Normal distribution of a random variable, median (Me) and quartiles (Q1; Q3) were calculated for each quantitative parameter. The percentage (%) of qualitative data was determined. Before comparative analysis of quantitative data in the studied groups, the Kolmogorov–Smirnov test and graphical test was used to check the compliance with normal distribution. For normally distributed data, the differences in the groups were assessed using parametric Student’s t-test.
The methods of nonparametric statistics was used when distribution differed from normally distributed data. Mann-Whitney U-test was used to compare distribution of differences in paired quantitative data. To compare dichotomous data and assess statistically significant differences, the 2x2 contingency chi-square (χ2), as well as Fisher's exact test for sparse samples was used. Statistically significant differences were at p <0.05 (5% significance level) and at p <0.01 (1% significance level) in a pairwise comparison.
Results and discussion
Taking into account, that severe conditions in pre-mature babies at birth largely depend on obstetric complications during pregnancy and delivery, the unfavorable prenatal factors that influenced fetal condition in the main group (premature babies with congenital pneumonia) and in the comparison group (premature babies with NRDS/ TTN) were analyzed (Table 1).
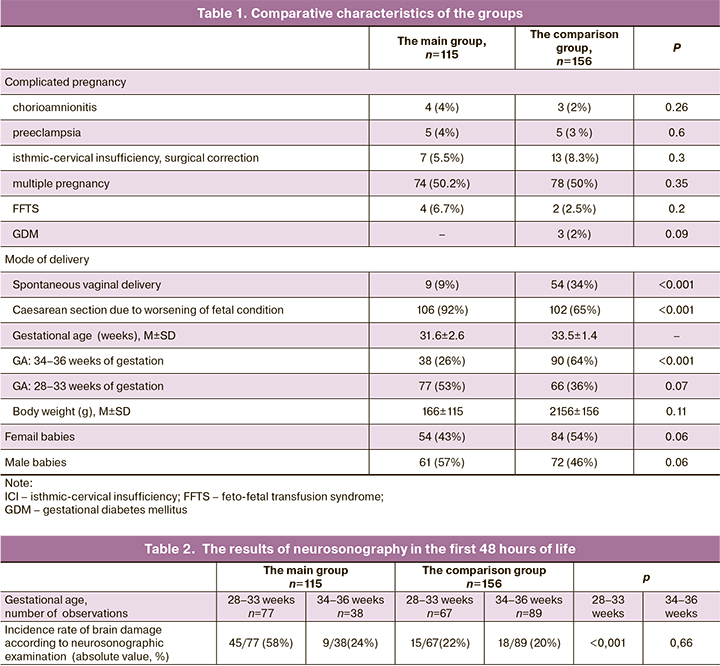
The data presented in Table 1 shows that most of factors complicating pregnancy and childbirth had no statistically significant differences. The most frequent complication during pregnancy was isthmic-cervical insufficiency, which required surgical correction during pregnancy (in the main group – 5.5% of infants, in the comparison group – in 8.3%, the differences were not statistically significant). Due to fetal distress, most women underwent cesarean section to deliver their babies. At the same time, a statistically significant difference in the rate of emergency caesarean section was determined: 88% in the main group versus 62% in the comparison group. The mean values of birth weight and the percentage of female babies was higher in the comparison group, but the differences were not statistically significant.
Comparison of NS results in premature babies with congenital pneumonia (the main group) in the first 48 hours of life and in babies with NRDS/TTN (in the comparison group) considering GA is shown in Table 2.
It should be noted that, according to the results of the first neurosonographic examination in 4 babies born at 28–33 weeks of GA in the main group, a combination of structural brain injuries were detected (intraventricular hemmoraghe (IVH) + cerebellar hemorrhage was in 1 baby, IVH + cerebral venous sinus thrombosis (CVST) – in 2, PVL + parenchymal hemorrhage in 1).
The incidence rate of different types of structural brain injuries detected in the first 48 hours of life and the ratio between the main and the comparison groups considering gestational age is shown in Fig. 2.
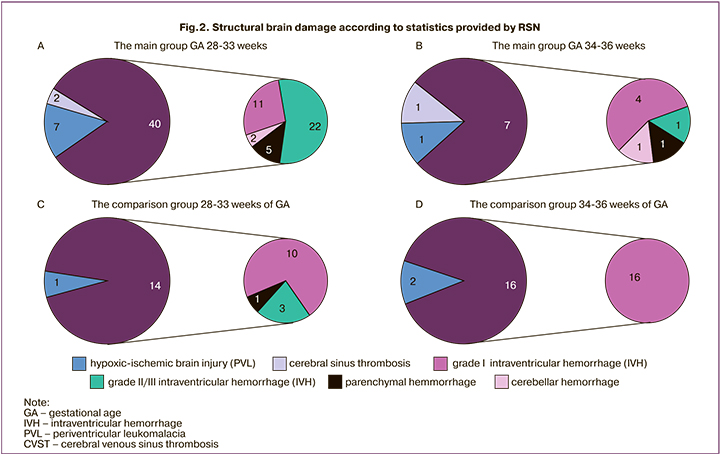
The results of NS in the main group were compared with the results in the comparison group. The incidence rate of perinatal brain injuries detected in premature babies born at 28–33 weeks of gestational age in the first two days of life was 2.5 times higher in babies with congenital pneumonia than in babies with NRDS/TTN (the differences were statistically significant, p<0,001). At the same time, in infants in the main group, both severe intraventricular hemorrhage (grade II–III IVH) and hypoxic ischemic brain injury (PVL) statistically significantly occurred more often.
It is important to note that in the majority of infants, brain damage was manifested by depression of neural reflex activity of varying intensity. In 15 infants (13%) in the main group and in 6 infants (4%) in the comparison group, convulsive syndrome was noted.
Taking into account high incidence and characteristics of structural brain injuries, premature infants received treatment in the NICU. To identify the factors that contribute to the development of brain injuries, considering GA, all patients were divided into two subgroups: sub-group A – with brain injury and subgroup B – without brain injury. The frequency of various types of respiratory therapy, the use of which reflected the severity of RD in the midst of the disease in babies in the studied subgroups is presented in Table 3.
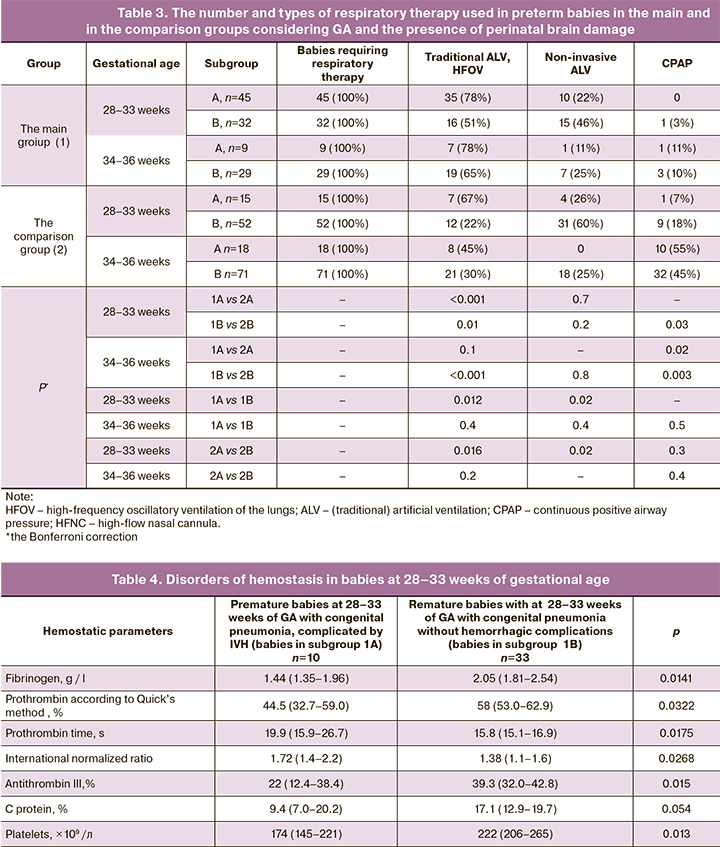
The data in Table 3 shows, that due to severe form of respiratory distress, the babies born at 28–33 weeks of gestational age required standard ALV and HFOV statistically significantly more often compared to babies born at 34–36 weeks. Necessity in these types of respiratory therapy was statistically significantly higher in infants with congenital pneumonia with congenital pneumonia compared to babies, in whom NRDS/TTN was the cause of respiratory distress. At the same time, the frequency of standard ALV and HFOV performed in babies between 28–33 weeks of gestation with congenital pneumonia was significantly higher in the presence of structural brain injuries. The obtained results indicate that severe RD in the first hours of life contributes to the development of severe perinatal hypoxic-ischemic and hypoxic-hemorrhagic CNS injuries in premature babies.
Additionally, it was found, that 72 out of 115 (62%) infants in the main group and 45 out of 156 (29%) infants in the comparison group had the signs of arterial hypotension, due to which they underwent vasopressor and cardiotonic therapy. At the same time, statistically, cardiac insufficiency statistically significantly was most often noted in babies with brain damage: in 30 (66%) and 7 (53%) cases, in subgroup B – 17 (77%) and in 18 (62%) cases respectively (statistical significance was at level р<0,05).
The obtained data showed that arterial hypotension and shock were twice as common in premature infants with congenital pneumonia as in babies with severe condition caused by NRDS/TTN. These data are consistent with the findings of other researchers who found that pronounced changes in central hemodynamic parameters were significant pathogenetic factors for IVH development in premature babies in early neonatal period [5]. According to published data [2, 4, 5], the other significant pathogenetic mechanisms that caused IVH in babies born at ˂ 34 weeks of gestational age were pronounced hemostasiological disorders.
To clarify the role of disorders of hemostasis in babies at 28–33 weeks of GA, the results of coagulometric tests were assessed in 43 babies in the first day of life. The main hemostatic parameters in the first hours of life in 10 very preterm babies with IVH in early neonatal period and associated congenital pneumonia were compared with similar parameters in 33 very premature infants, who did not have hemorrhagic complications in early neonatal period (Table 4).
A statistically significant decrease in concentration of fibrinogen was found in newborns with congenital pneumonia complicated by intraventricular hemorrhage. In addition, statistically significant differences in parameters of hemostasis characterizing the extrinsic pathway of coagulation were detected: the newborns with congenital pneumonia complicated by IVH had low percentage of prothrombin according to the Quick PT method, prolonged prothrombin time, and increased values of International normalized ratio (INR). Activated partial thromboplastin time, characterizing the intrinsic pathway of coagulation, also tended to increase in babies with IVH.
Evaluation of natural anticoagulants revealed that the levels of antithrombin III, C protein decreased in patients in the subgroup 1A compared to the subgroup 1B. At the same time, there were no differences in the levels of plasma D-dimer (a marker of intravascular coagulation) in the groups.
Despite the limited sample size, the obtained data indicate that IVH developed in babies with low potential for blood coagulation, primarily due to impaired liver protein synthesis. An additional factor was a decrease in peripheral blood platelet counts.
Conclusion
Congenital infection and a combination of pathological factors in the early neonatal period predispose to severe perinatal brain injuries in premature babies.
The analysis of postnatal factors revealed that the incidence rate of perinatal brain injuries in premature babies at 28–33 weeks of GA was 2.5 times higher in infants with congenital pneumonia than in infants with NRDS/TTN. At the same time, the babies in the main group statistically significantly most often had both hypoxic-hemorrhagic CNS injuries (grade II–III IVH) and severe hypoxicischemic injuries (PVL). Also, in babies with congenital pneumonia, statistically significantly, severe respiratory distress developed more often than in babies with NRDS/TTN, which required HFOV and traditional ALV. Severe cardiac insufficiency in infants in the main group statistically significantly occurred 2 times more often in infants with brain damage. These common factors were found both in late preterm (at 34–36 weeks of GA) and very preterm infants (at 28–33 weeks of GA).
Thus, the obtained data demonstrate that high incidence rate of brain damage (hypoxic-hemorrhagic injury) in premature babies with congenital pneumonia is associated with a greater severity of respiratory distress and cardiac insufficiency. An additional factor contributing to the development of severe IVH in very preterm babies is initial decrease in the coagulation potential of blood, which is probably associated with impaired liver protein synthesis in babies with congenital pneumonia.
References
- https://www.ncagp.ru/index.php?_t8=381
- Volpe J.J., Inder T., Darras B., de Vries L.S., du Plessis A., Neil J. et al. Volpe’s neurology of the newborn. 6th ed. Elsevier; 2018. 1240 p.
- Шабалов Н.П. Неонатология. Учебное пособие. Т. 1-2. М.: ГЭОТАР-Медиа; 2019. 736 с. [Shabalov N.P. Neonatology. Tutorial. In 2 volumes; Moscow: GEOTAR-Media; 2019. 736 р. (in Russian)].
- Veldman A., Fischer D., Nold M.F., Wong F.Y. Disseminated intravascular coagulation in term and preterm neonates. Semin. Thromb. Hemost. 2010; 36(4): 419-28. https://dx.doi.org/10.1055/s-0030-1254050.
- Puthiyachirakkal M. Pathophysiology and management of intraventricular hemorrhage in preterm infants. EC Paediatrics. 2018; 7(6): 537-45. https://dx.doi.org/10.1203/PDR.0b013e3181c1b176.
- Зубарева Е.А., Улезко Е.А. Нейросонография у детей раннего возраста. М.: НИИ неврологии РАМН; 2012. 188 c. [Zubareva E.A, Ulezko E.A. Neurosonography in infants. Moscow: Research Institute of Neurology of the RAS; 2012. 188 p. (in Russian)].
- Kollmann T.R., Kampmann B., Mazmanian S.K., Marchant A., Levy O. Protecting the newborn and young infant from infectious diseases: lessons from immune ontogeny. Immunity. 2017; 46(3): 350-63. https://dx.doi.org/ 10.1016/j.immuni.2017.03.009.
- Гузева В.И., Скрипченко Н.В., Батышева Т.Т., Вильниц А.А., Васильев В.В., Щугарева Л.М., Горелик Е.Ю., Иванова Г.П., Медведев М.Н., Дегтярева М.Г., Заваденко А.И., Чутко Л.С., Мелашенко Т.В., Пальчик А.Б., Назджанова З.Г., Евстафеева И.В., Легонькова С.В., Софронова Г.И., Федорова Л.А., Шабалов Н.П., Ассунца С.З., Юрьева Д.С., Гузева В.В., Гузева О.В., Понятишин А.Е., Охрим И.В., Иванов Д.О., Иова А.С., Шалькевич Л.В., Львова О.А., Кулагин А.Е., Талабаев М.В., Ивашина И.Н., Сулимов А.В., Куренков А.Л., Кузенкова Л.М., Никитин С.С.. Намазова-Баранова Л.С., Бурсагова Б.И., Мамедъяров А.М. Детская неврология. Клинические рекомендации. Вып. 3. М.: «МК»; 2015. 336 c. [Guzeva V.I., Skripchenko N.V., Batysheva T.T., Vilnic A.A., Vasilev V.V., Shugareva L.M. et al. Pediatric neurology. Clinical practice guidelines. Issue 3. Мoscow; 2015. 336 р. (in Russian)].
- Зубков В.В., Байбарина Е.Н., Рюмина И.И., Дегтярев Д.Н. Диагностическая значимость признаков пневмонии у новорожденных детей. Акушерство и гинекология. 2012; 7: 68-73. [Zubkov V.V., Baybarina E.N., Ryumin I.I., Degtyarev D.N. Diagnostic value of the signs of neonatal pneumonia. Obstetrics and gynecology. 2012; 7: 68-73 (in Russian)].
- Антонов А.Г., Байбарина Е.Н., Балашова Е.Н., Дегтярев Д.Н., Зубков В.В.,Иванов Д.О., Ионов О.В., Карпова А.Л., Киртбая А.Р., Крохина К.Н., Крючко Д.С., Ленюшкина А.А., Ли А.Г., Малютина Л.В., Мебелова И.Н.,Никитина И.В., Петренко Ю.В., Рындин А.Ю., Рюмина И.И., Романенко А.В.Врожденная пневмония (Клинические рекомендации). Неонатология: новости, мнения, обучение. 2017; 4: 133-48. Доступно по: https://cyberleninka.ru/article/n/vrozhdennaya-pnevmoniya-klinicheskie-rekomendatsii. [Antonov A.G.,Baybarina E.N., Balashova E.N., Degtyarev D.N., Zubkov V.V. et al. Congenital pneumonia (Clinical guidelines). Neonatology: news, opinions, training. 2017; 4: 133-48. (in Russian)]. Available at: https://cyberleninka.ru/article/n/vrozhdennaya-pnevmoniya-klinicheskie-rekomendatsii.
- Володин Н.Н., Горелышев С.К., Попов В.Е., ред. Оказание медицинской помощи новорожденным детям с внутрижелудочковыми кровоизлияниями и постгеморрагической гидроцефалией. Клинические рекомендации. М.: Российская Ассоциация специалистов перинатальной медицины, Ассоциация детских нейрохирургов России; 2014. [Volodin N.N., Gorelyshev S.K., Popov V.E., ed. Providing of medical care and treatment options in newborns with intraventricular hemorrhage and posthemorrhagic hydrocephalus. Clinical guidelines. Moscow: Russian Association of Perinatal Medicine Specialists, Association of Pediatric Neurosurgeons of Russia; 2014. (in Russian)].
Received 14.10.2020
Accepted 17.11.2020
About the Authors
Ekaterina I. Artamkina, PhD student, Department of Neonatology, N.F. Filatov Clinical Institute of Children’s Health, I.M. Sechenov First Moscow State Medical University of the Ministry of Health of the Russian Federation (Sechenov University); doctor-neonatologist of the A.G. Antonov Intensive Care Unit and Intensive Care of Newborns, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation.Tel.: +7(916)813-14-43. E-mail: artamkina.e@gmail.com. ORCID:0000-0002-2920-6382. 119991, Russia, Moscow, Trubetskaya str., 8-2; 117997, Russia, Moscow, Oparina str., 4.
Dmitry N. Degtyarev, Dr.Med.Sci., Professor, Head of the Department of Neonatology, N.F. Filatov Clinical Institute of Children’s Health, I.M. Sechenov First Moscow State Medical University of the Ministry of Health of the Russian Federation (Sechenov University); Deputy Director for Research, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation.
Tel.: +7(499)248-42-00; +7(495)531-44-44. E-mail: d_degtiarev@oparina4.ru. ORCID: 0000-0001-8975-2425.
119991, Russia, Moscow, Trubetskaya str., 8-2; 117997, Russia, Moscow, Oparina str., 4.
Milana D. Kvekveskiri, PhD student, Department of Neonatology, N.F. Filatov Clinical Institute of Children’s Health, I.M. Sechenov First Moscow State Medical University of the Ministry of Health of the Russian Federation (Sechenov University); doctor-neonatologist of the A.G. Antonov Intensive Care Unit and Intensive Care of Newborns, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation.
Tel.: +7(495)438-22-77; +7(926)994-22-27. E-mail: milly_apsny@mail.ru. ORCID: 0000-0002-5175-8570.
119991, Russia, Moscow, Trubetskaya str., 8-2; 117997, Russia, Moscow, Oparina str., 4.
Anna R. Kirtbaya, Cand.Med.Sci., associate professor of the Department of Neonatology, N.F. Filatov Clinical Institute of Children’s Health, I.M. Sechenov First Moscow State Medical University of the Ministry of Health of the Russian Federation (Sechenov University); Head of the clinical work of the A.G. Antonov Intensive Care Unit and Intensive Care of Newborns, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation. Tel.: +7(495)438-22-77. E-mail: a_kirtbaya@oparina4.ru. ORCID: 0000-0002-7628-8157.
119991, Russia, Moscow, Trubetskaya str., 8-2; 117997, Russia, Moscow, Oparina str., 4.
Dzhеnneta Yu. Amirkhanova, neurologist at the consultative pediatric department, pathology department of newborns and premature children, Institute of Neonatology and Pediatrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation.
Tel: +7(495)438-22-77. E-mail: djenn@mail.ru. ORCID: 0000-0002-5923-6646. 117997, Russia, Moscow, Oparina str., 4.
Olga S. Beznoshenco, doctor of clinical diagnostics, Laboratory of clinical immunology, Institute of Neonatology and Pediatrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation. Tel: +7(919)721-43-29.
E-mail: ospa-a@yandex.ru. ORCID: 0000-0003-4645-8976. 117997, Russia, Moscow, Oparina str., 4.
Julia K. Bykova, Ph.D., researcher and doctor of the Department of Ultrasound Diagnostics in Neonatology and Pediatrics; Associate Professor, Department of Radiation Diagnostics of Children, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation. Tel.: +7(495)438-22-77; +7(903)770-00-10. E-mail: Yulia.bykova@mail.ru, yu_bykova@oparina4.ru. ORCID: 0000-0003-2423-9123.
117997, Russia, Moscow, Oparina str., 4.
Yulia M. Golubtsova, Cand.Med.Sci., Associate Professor, Department of Neonatology, N.F. Filatov Clinical Institute of Children’s Health, I.M. Sechenov First Moscow State Medical University of the Ministry of Health of the Russian Federation (Sechenov University); doctor-neonatologist of the Scientific Advisory Pediatric Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation.
Tel.: +7(495)438-25-00. E-mail: yugolubtsova@yandex.ru. ORCID: 0000-0002-2288-1721.
119991, Russia, Moscow, Trubetskaya str., 8-2; 117997, Russia, Moscow, Oparina str., 4.
Ekaterina N. Balashova, Cand.Med.Sci., leading researcher of the A.G. Antonov Intensive Care Unit and Intensive Care of Newborns, Academician V.I. Kulakov
National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation.
Tel.: +7(495)438-22-77. E-mail: e_balashova@oparina4.ru. ORCID: 0000-0002-3741-0770. 117997, Russia, Moscow, Oparina str., 4.
Oleg V. Ionov, Cand.Med.Sci., Associate Professor, Department of Neonatology, N.F. Fil atov Clinical Institute of Children’s Health, I.M. Sechenov First Moscow State Medical University of the Ministry of Health of the Russian Federation (Sechenov University); Head of the A.G. Antonov Intensive Care Unit and Intensive Care of Newborns, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology,
Ministry of Health of the Russian Federation. Tel.: +7(495)438-22-77. E-mail: dr.ionov@hotmail.com, o_ionov@oparina4.ru. ORCID: 0000-0002-4153-133x.
119991, Russia, Moscow, Trubetskaya str., 8-2; 117997, Russia, Moscow, Oparina str., 4.
For citation: Artamkina E.I., Degtyarev D.N., Kvekveskiri M.D. , Kirtbaya A.R., Amirkhanova D. Yu. , Beznoschenko O.S., Bykova Yu.K., Golubtsova Yu.M., Balashova E.N., Ionov O.V. Brain damage in preterm babies associated with congenital pneumonia.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2020; 12: 159-168 (in Russian)
https://dx.doi.org/10.18565/aig.2020.12.159-168



