2020-й год, несомненно, войдет в историю человечества под знаком пандемии COVID-19 (от англ. Coronavirus Disease 2019). Действительно, первые случаи заболевания, вызванного новым штаммом коронавирусов SARS-CoV-2 (Severe Acute Respiratory Syndrome, Coronavirus-2, коронавирус тяжелого острого респираторного синдрома 2), были отмечены в декабре 2019 г. в городе Ухань – столице Китайской провинции Хубэй [1], а уже 11 марта 2020 г. Всемирная организация здравоохранения обозначила развитие пандемии SARS-CoV-2 [2].
В связи с выраженной контагиозностью, вирулентностью и высоким уровнем смертности в большинстве стран мира были предприняты беспрецедентные меры, включая обязательное ношение масок и перчаток, введение ограничительных и карантинных мер, а также чрезвычайного положения. Согласно Постановлению Правительства Российской Федерации от 31 января 2020 г. № 66, коронавирусная инфекция (2019-nCoV) включена в перечень заболеваний, представляющих опасность для окружающих, наряду с особо опасными инфекциями [3].
По данным литературы, клинические проявления заболевания могут варьировать от бессимптомного течения или с легкими признаками до тяжелых осложнений с развитием летального исхода. Основными симптомами считаются повышение температуры тела, кашель, слабость, утомляемость, одышка и проявления острого респираторного дистресс-синдрома (ОРДС) [4, 5]. Так, на основании анализа 72 000 случаев COVID-19, зарегистрированных в Китайском центре по контролю и профилактике заболеваний, Z. Wu et al. [6] установлено, что в 81% наблюдений отмечались легкие формы острой респираторной инфекции (отсутствие симптомов или пневмония легкого течения), в 14% – имелись тяжелые формы (одышка и поражение более половины легких в течение 24–48 ч), в 5% было критическое состояние (шок, дыхательная недостаточность, полиорганная недостаточность) и в 2,3% наступила смерть.
Несомненно, беременные женщины относятся к группе риска по развитию не только типичных для SARS-CoV-2-инфекции поражений органов и тканей, но и осложнений со стороны плаценты и плода [7, 8]. По данным Q. Wang et al. [9], беременные c COVID в 5 раз чаще поступали в отделение интенсивной терапии и в 4 раза чаще получали искусственную вентиляцию легких по сравнению с небеременными пациентками.
Согласно результатам проведенного систематического обзора данных литературы, содержащих сведения о течении и исходах беременности у 79 женщин, страдавших коронавирусными инфекциями (SARS, MERS или COVID-19), Daniele Di Mascio et al. [10] установили развитие преэклампсии в 16,2%, преждевременного разрыва плодных оболочек – в 20,7%, преждевременных родов – в 24,3%, задержки роста плода – в 11,7% и перинатальной гибели – в 11,1% наблюдений.
Цель работы: анализ данных литературы о механизмах и особенностях развития поражений плаценты у беременных с SARS-CoV-2-инфекцией.
Приступая к анализу данных литературы, следует указать, что коронавирус SARS-CoV-2 является оболочечным РНК-вирусом, который относится к роду Betacoronavirus, подроду Sarbecovirus и виду SARS [11]. К данному роду также относятся вирусы SARS-CoV и MERS-CoV, явившиеся причинами вспышек соответственно тяжелого острого респираторного синдрома (Severe Acute Respiratory Syndrome, SARS) в 2003 г. и ближневосточного респираторного синдрома (Middle East Respiratory Syndrome, MERS) в 2013–2015 гг. [12, 13].
Путями передачи инфекции являются воздушно-капельный, воздушно-пылевой и контактный. Установлено, что SARS-CoV-2 тропен к клеткам, экспрессирующим ангиотензинпревращающий фермент-2 (angiotensin converting enzyme, ACE2) [14]. Следовательно, потенциальными органами-мишенями для SARS-CoV-2 у человека являются легкие, желудочно-кишечный тракт, кровеносные сосуды, мозг, печень, почки, селезенка, кожа [15]. При этом проникновение SARS-CoV-2 в клетки происходит при помощи его структурного S-белка и мембраносвязанной сериновой протеазы TMPRSS2, способствующих слиянию вирусной и клеточной мембран [14]. Кроме того, SARS-CoV-2 способен использовать и другой путь проникновения в клетку – трансмембранный гликопротеин базигин (Basigin, BSG, EMMPRIN, CD147), входящий в суперсемейство иммуноглобулинов [16].
При помощи иммуногистохимических и молекулярно-биологических методов исследования было установлено наличие вышеуказанных рецепторов и в структурах плаценты [17–25], которые были нами суммированы в таблице 1. ACE2 локализовался в синцитиотрофобласте, ворсинковом и вневорсинковом цитотрофобласте, эндотелиоцитах и миоцитах сосудов ворсин, децидуальных клетках. Мембраносвязанная сериновая протеаза (TMPRSS2) и CD147 выявлялись в синцитиотрофобласте и цитотрофобласте ворсин, а также в клетках вневорсинкового трофобласта. Называемые в числе возможных рецепторов для коронавирусов фурин (Paired basic Amino acid Cleaving Enzyme – фермент, расщепляющий белок в месте спаренных основных аминокислот), являющийся сериновой протеазой, катепсин L (CTSL), являющийся внутриклеточной протеазой, и дипептидилпептидаза-4 (DPP4), являющаяся внутримембранным гликопротеином и сериновой экзопептидазой, также определялись в клетках плаценты [17, 18, 20].

Обращают на себя внимание данные J.L. Hecht et al. [25], указывающие на наличие градиента распределения ACE2 в синцитиотрофобласте с преимущественной его локализацией на стромальной стороне. Кроме того, экспрессия мРНК ACE2 и сериновой протеазы TMPRSS2 была значимо выше в образцах плаценты I триместра по сравнению со II триместром [26].
Важным моментом являются результаты исследований об особенностях локализации самого SARS-CoV-2 в клетках плаценты. В результате иммуногистохимического исследования было показано наличие N-белка SARS-CoV-2 в периворсинковом трофобласте [27] и эндотелии сосудов ворсин [28]. По данным F. Facchetti et al. [29], положительная иммуногистохимическая реакция с маркерами S- и N-белков SARS-CoV-2 наблюдалась не только в цитоплазме синцитиотрофобласта, но и в клетках Гофбауэра и макрофагах стромы ворсин. Методом гибридизации in situ установлено наличие РНК SARS-CoV-2 в синцитиотрофобласте и цитотрофобласте ворсин плаценты. Более того, частицы, соответствующие коронавирусу, были идентифицированы при электронной микроскопии в синцитиотрофобласте, эндотелиоцитах, фибробластах ворсин и, что особенно важно, в моноцитах крови [29].
Закономерно, что наличие вируса и его рецепторов в клетках плаценты указывает на возможность его проникновения в клетки с последующей репликацией и соответствующими изменениями структуры и функции.
В этой связи следует отметить, что плацента, являющаяся, по сути, временным органом, обеспечивает нормальное развитие плода путем обеспечения газообмена и обмена питательных веществ, а также защиты от воздействия вредных факторов. Основным структурным компонентом, образующим фетоплацентарную защитную единицу от патогенной инвазии, является синцитиотрофобласт, выстилающий ворсины плаценты [30]. Именно благодаря своему пограничному расположению между кровеносными системами матери и плода, синцитиотрофобласт препятствует инфицированию, в частности, вирусами полиомиелита, везикулярного стоматита, Коксаки B, простого герпеса, иммунодефицита человека, цитомегаловирусом [31].
Вместе с тем, исходя из особенностей циркуляции SARS-CoV-2 и патогенеза поражений органов и тканей при COVID-19, в литературе в 2020 г. регулярно появляются новые публикации, посвященные изучению поражений плаценты у беременных с COVID-19, а также выяснению ее роли в развитии осложнений беременности и плода, включая особенности вертикальной передачи SARS-CoV-2 от матери к плоду. В основном подобные публикации представляют собой как описания отдельных наблюдений, так и анализ небольших групп.
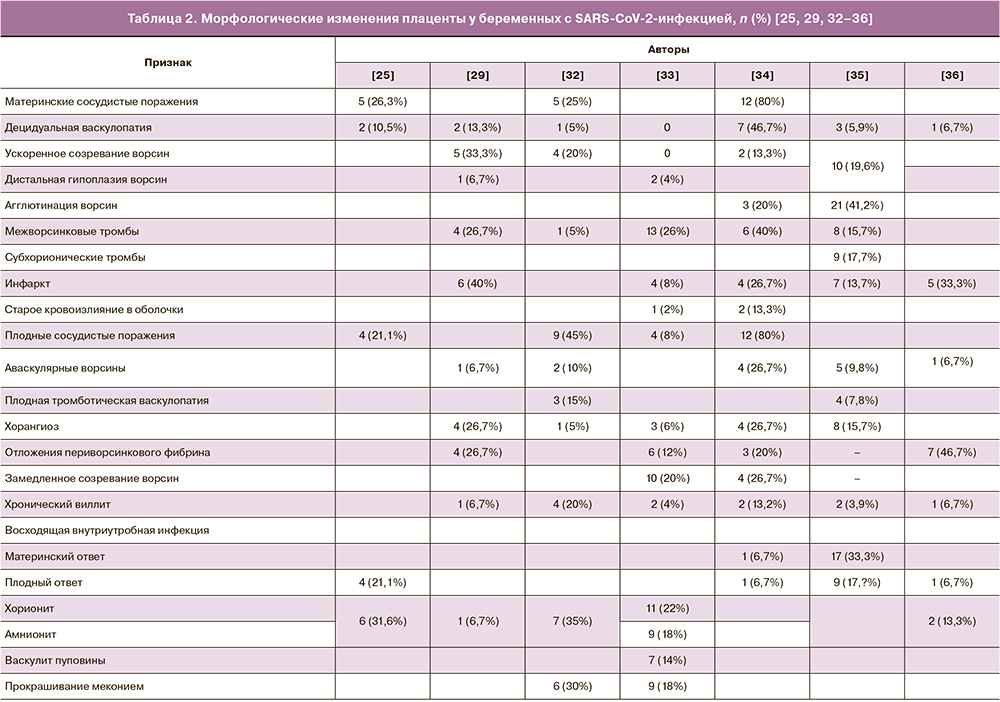
В этой связи нами была составлена сводная таблица 2 данных, представленных в 7 статьях [25, 29, 32–36], содержащих результаты морфологических исследований плацент в группах родильниц, инфицированных SARS-CoV-2. Количество изученных плацент в анализируемых работах варьировало от 15 до 51. Поскольку конкретные морфологические изменения отличались в данных исследованиях, то в таблицу 2 нами были включены основные патологические изменения плаценты в соответствии с наиболее общепринятой современной классификацией ее поражений [37].
Данная классификация и рекомендации по морфологической диагностике поражений плаценты были представлены Amsterdam Placenta Workshop Group в 2014 г., на русском языке были опубликованы на страницах журнала «Акушерство и гинекология» в 2016 г. [38].
Наиболее частыми поражениями плаценты, согласно данным литературы, являлись сосудистые нарушения, которые в классификации поражений плаценты подразделяют на две подгруппы: нарушения со стороны матери и со стороны плода.
На основании анализа данных литературы об изменениях плаценты у родильниц, инфицированных SARS-CoV-2, можно заключить, что во всех исследованиях авторы указывают на выявление признаков нарушения материнского кровообращения. Так, при исследовании 15 плацент, полученных от родильниц, инфицированных SARS-CoV-2, сосудистые нарушения со стороны матери выявлены в 12 (80%) наблюдениях E.D. Shanes et al. [34]. Согласно данным литературы [23–25], ранее подобные изменения обозначались как материнская сосудистая недостаточность и были связаны с маловодием, задержкой роста плода и преждевременными родами [39].
Одним из морфологических признаков материнской сосудистой недостаточности является децидуальная васкулопатия (артериопатия), проявляющаяся в виде атероза и фибриноидного некроза децидуальных артерий, гипертрофии стенки артериол и отсутствия ремоделированных спиральных артерий. Основным же фактором риска развития децидуальной васкулопатии считается преэклампсия [40].
Можно также добавить, что атероз спиральных артерий, считающийся специфичным морфологическим признаком поражения плаценты при преэклампсии, относится к одной из наиболее частых причин развития внутриутробной гибели плода и преждевременных родов [41].
В то же время практически во всех работах по изучению плацент родильниц, инфицированных SARS-CoV-2, имеются указания на наличие признаков децидуальной васкулопатии, частота выявления которой варьировала от 5% до 46,7% (табл. 2). Наиболее часто (в 46,7% наблюдений) подобные изменения были зарегистрированы E.D. Shanes et al. [34], тогда как гипертоническая болезнь была лишь у одной пациентки.
Другим морфологическим признаком сосудистых нарушений со стороны матери, выявляемых в плацентах родильниц, инфицированных SARS-CoV-2, является ускоренное созревание ворсин. Согласно данным литературы (табл. 2), частота выявления такого признака при SARS-CoV-2-инфекции достигает 33,3% изученных наблюдений [29].
Ускоренное созревание ворсин отражает общие или частичные нарушения материнской циркуляции крови, в частности, кровотока с пониженной скоростью, но бóльшим объемом по сравнению с нормальными показателями. К морфологическим проявлениям ускоренного созревания ворсин относят наличие участков агглютинированных ворсин с повышенным количеством синцитиальных узелков на фоне уменьшения количества разветвленных ворсин [42]. Примечательно, что увеличение количества синцитиальных узелков отражает развитие гипоксии ткани плаценты и происходит при преэклампсии и задержке роста плода. Более того, cогласно данным литературы [43] и результатам проведенных нами исследований [44], большее количество синцитиальных узелков в терминальных ворсинах плаценты отмечалось в наблюдениях поздней преэклампсии [45].
Помимо признаков ускоренного созревания ворсин в плаценте беременных, инфицированных SARS-CoV-2, также отмечается дистальная гипоплазия ворсин [29, 33]. По данным M.C. Smithgall et al. [35], ускоренное созревание и дистальная гипоплазия ворсин наблюдались в 10 (19,6%) из 51 изученной плаценты, полученной от родильниц, инфицированных SARS-CoV-2.
Достаточно частым поражением плаценты у беременных с SARS-CoV-2-инфекцией является развитие межворсинкового тромбоза и инфаркта ворсин (табл. 2). Последние встречались в 40% изученных плацент в исследованиях E.D. Shanes et al. [34] и Facchetti et al. [29] соответственно. Учитывая клинические данные о развитии тромбоэмболических осложнений и синдрома диссеминированного внутрисосудистого свертывания у больных с COVID-19 [46], подобные изменения, видимо, отражают проявление таких осложнений на территории плаценты и, соответственно, могут трактоваться как весьма специфические осложнения со стороны плаценты в ответ на вирус SARS-CoV-2.
В этой связи заслуживает внимания описание J.E. Mongula et al. [47] отдельного наблюдения беременной, страдающей COVID-19 с признаками коагулопатии, в плаценте которой отложения периворсинкового фибрина и инфаркты отмечались уже при макроскопическом исследовании (от хориальной пластинки до децидуальной оболочки). При микроскопическом исследовании препаратов авторами дополнительно были выявлены признаки обширного интервиллузита в виде инфильтрации гистиоцитами (хронический компонент) и гранулоцитами (острый компонент). Иммуногистохимическими методами показана положительная реакция на SARS-CoV-2 в клетках трофобласта и стромы ворсин, а также зарегистрирован положительный результат на РНК SARS-CoV-2 при исследовании методом полимеразной цепной реакции мазка с плодной поверхности плаценты.
Наряду с материнской сосудистой недостаточностью в плаценте беременных с SARS-CoV-2-инфекцией отмечаются и сосудистые нарушения со стороны плода (нарушения кровоснабжения плода), частота которых отличается в различных исследованиях (табл. 2). При этом в исследовании R.N. Baergen et al. [32] частота плодных сосудистых нарушений (45% наблюдений) превысила частоту материнских сосудистых нарушений (25%). Чаще всего (в 15%) наблюдалась плодная тромботическая васкулопатия, реже (в 10%) – аваскулярные ворсины и еще реже (в 5%) – хорангиоз. В то же время наиболее частым поражением в исследовании M.C. Smithgal et al. [35] явился хорангиоз (в 15,7% наблюдений). E.D. Shanes et al. [34] отметили одинаковую частоту (в 26,7% наблюдений) встречаемости хорангиоза и аваскулярных ворсин. При этом общая частота материнских и плодных сосудистых нарушений была одинаковой.
Все вышеприведенные изменения отражают нарушения развития трех основных компонентов дистального ворсинкового дерева (трофобласта, капилляров и стромы) и свидетельствуют об изменениях перфузии крови в ворсинах плаценты. Основными причинами развития стромально-сосудистых поражений ворсин являются общая гипоксемия у беременной и хронические нарушения кровоснабжения. Соответственно, развитие плодной тромботической васкулопатии и аваскулярных ворсин у беременных с SARS-CoV-2-инфекцией может быть обусловлено как общими расстройствами кровообращения с развитием синдрома диссеминированного внутрисосудистого свертывания, так и прямым действием вируса SARS-CoV-2 на эндотелий.
В этой связи можно привести результаты исследования K. Stahl et al. [48], показавшими при помощи электронной микроскопии наличие вируса SARS-CoV-2 и везикул c частицами вириона в эндотелиоцитах интрамуральных сосудов толстой кишки у 43-летнего пациента c COVID-19, перенесшего гемиколэктомию по поводу некроза кишки. Важно, что данные вирусные частицы были выявлены в эндотелии сосудов кишечника примерно через 8 недель после заражения SARS-CoV-2, когда вирус уже не обнаруживался в дыхательных путях и образцах крови.
В свою очередь, хорангиоз, характеризующийся увеличением количества капилляров в терминальных ворсинах плаценты и, соответственно, их гиперваскуляризацией, можно расценивать в качестве компенсаторного процесса в ответ на хроническую гипоксию, в частности, у беременных, живущих на больших высотах [49], и при преэклампсии [50]. При этом степень развития гиперваскулярности терминальных ворсин является одним из основных факторов, определяющих, по нашему мнению, выраженность процессов секреции сосудисто-эндотелиального фактора роста и его рецепторов в синцитиотрофобласте ворсин плаценты при гипоксии, в частности, при преэклампсии [51].
Особого внимания заслуживают исследования, направленные на выявление воспалительных изменений в плаценте у беременных COVID. Действительно, организм беременной в целом и плацента в частности сохраняют способность к защите от чужеродных микроорганизмов, поддерживая при этом толерантность к аллогенному плоду.
Поражения плаценты при различных инфекционных заболеваниях, включая вирусные, описаны достаточно подробно. При этом, согласно международной классификации поражений плаценты, воспалительные изменения подразделяют на острые и хронические [38]. Острые воспалительные (клеточные) реакции развиваются при восходящем бактериальном инфицировании и отражают ответ двух различных иммунных систем [42]. Первый, острый хориоамнионит, характеризует материнский ответ и проявляется поступлением нейтрофилов из венул децидуальной оболочки в хориоамнион и из межворсинкового пространства в хориальную пластинку. Второй, фетальный и/или пуповинный васкулит, характеризующий реакцию плода, проявляется проникновением нейтрофилов через стенки крупных хориальных и пуповинных сосудов в хориальную пластинку и вартонов студень.
Хронические воспалительные клеточные реакции возникают в основном при гематогенной циркуляции вирусов или простейших и локализуются в основном в строме ворсин и межворсинковом пространстве, то есть проявляются в виде виллита (виллузита) и интервиллузита [52].
При анализе данных литературы о воспалительных поражениях плаценты при SARS-CoV-2 практически во всех работах имеются указания на подобные изменения. Так, при гистологическом изучении 51 плаценты родильниц, инфицированных SARS-CoV-2, M.C. Smithgall et al. [35] выявили признаки восходящей внутриутробной инфекции в 17 (33%) и 9 (17%) наблюдениях в виде материнского и плодного ответа соответственно. Однако выявленные изменения не имели значимых различий по сравнению с плацентами контрольной группы (беременные с отрицательными тестами на SARS-CoV-2). Аналогичные выводы об отсутствии значимых различий в частоте воспалительных изменений были сделаны и E.D. Shanes et al. [34] при изучении 19 плацент, а также M. Gulersen et al. [33], выявивших признаки хорионита, амнионита и виллита соответственно в 11 (22%), 9 (18%) и 2 (4%) наблюдениях в результате исследования 50 плацент родильниц, инфицированных SARS-CoV-2.
В то же время E. Ozer et al. [53] приводят описание 37-летней родильницы с клинической картиной острого респираторного заболевания и положительными мазками на SARS-CoV-2. При гистологическом и иммуногистохимическом изучении плаценты авторы отметили наличие воспалительной инфильтрации ворсин и децидуальной оболочки, представленной преимущественно макрофагами и CD4- и CD8-позитивными Т-лимфоцитами. Учитывая отсутствие данных о наличии SARS-CoV-2 в ткани плаценты, авторы сделали заключение о развитии виллита неустановленной (неизвестной) этиологии. Последний отражает развитие неинфекционного иммунного ответа. При этом преобладание в инфильтрате CD4-позитивных лимфоцитов по сравнению с CD8-позитивными лимфоцитами указывает на развитие Th-1 типа иммунного ответа. По мнению авторов исследований [52], развитие подобного виллита могло быть обусловлено воздействием цитокинов, увеличение уровня которых характерно для SARS-CoV-2-инфицирования. В качестве подтверждения авторы приводят данные о том, что во всех случаях COVID-19 в ткани легких определяются CD4-позитивные Т-лимфоциты и антитела к ним, а CD8-позитивные Т-лимфоциты и антитела выявлялись не всегда.
В литературе также имеются указания о прокрашивании меконием плодных оболочек и самой плаценты у беременных, инфицированных SARS-CoV-2, в 30% [33] и 18% изученных наблюдений [32]. При этом у беременных с клиническими проявлениями COVID частота прокрашивания меконием была выше по сравнению с бессимптомными беременными (p=0,44) [33].
В этой связи следует добавить сведения о некоторых отличиях в степени поражения плаценты у беременных с клиническими проявлениями COVID и пациенток с бессимптомным течением. По данным M. Gulersen et al. [33], при наличии симптомов заболевания чаще встречались инфаркты и хорангиоз ворсин, отложения периворсинкового фибриноида и тромбы в межворсинковом пространстве; при бессимптомном течении инфицированных SARS-CoV-2 – чаще дистальная ворсинковая гипоплазия, плодные сосудистые нарушения, хорит, амнионит и виллит, хотя все вышеприведенные показатели не имели значимых различий. M.C. Smithgall et al. [35] также, указывая на отличия в частоте выявления поражений плаценты, отметили отсутствие значимых различий в зависимости от характера течения заболевания (с симптомами или без).
Говоря о поражениях плаценты у беременных, инфицированных SARS-CoV-2, необходимо остановиться на проблеме трансплацентарной (вертикальной) передачи инфекции от матери к плоду. На основании данных литературы, использованных при написании данного обзора, следует отметить неоднозначность имеющихся фактов. Одни авторы отрицают трансплацентарную передачу COVID-19 [54, 55], другие, наоборот, указывают на ее существование [56, 57]. Данный вопрос, по нашему мнению, заслуживает отдельного анализа. Однако следует указать, что внутриутробная передача вируса от матери к плоду потенциально может осуществляться посредством нескольких путей:
- через эндотелий материнских сосудов, трофобласт ворсин и эндотелий капилляров ворсин;
- через инфицированные макрофаги материнской крови и трофобласт ворсин;
- путем внеклеточного проникновения из крови матери в капилляры ворсин;
- путем восходящей внутриутробной инфекции.
Возвращаясь же к повреждениям плаценты и их патогенезу, можно констатировать следующие основные механизмы. Первый – это прямое цитопатическое действие циркулирующего SARS-CoV-2 на клетки плаценты и в первую очередь на синцитиотрофобласт, граничащий с материнской кровью и содержащий рецепторы к SARS-CoV-2. Второй – обусловленное вирусами и эндотелиальными клетками взаимодействие, вызывающее гиперкоагуляционное состояние с образованием микротромбов. Последние способствуют нарушениям материнского кровообращения в плаценте в виде развития межворсинковых тромбов, инфарктов ворсин, а также их агглютинации и ускоренного созревания. Страдает и плодная сосудистая сеть плаценты в виде тромбоза и облитерации сосудов ворсин и кариорексиса в эндотелиальных и стромальных клетках ворсин. При этом пневмония и острый респираторный дистресс-синдром, как типичные проявления COVID-19, приводят к прогрессированию гипоксемии и гипоксии структур плаценты. Важным звеном патогенеза поражений плаценты следует считать воздействие цитокинов, повышение уровня которых отмечено у больных, страдающих COVID-19. Соответственно, существенным компонентом поражения плаценты при COVID-19 является развитие воспаления в материнском и плодном компартменте плаценты.
Заключение
Таким образом, можно заключить, что SARS-CoV-2-инфекция, а тем более COVID-19, приводят к развитию нарушений материнского и плодного кровотока, а также воспалительно-иммунных процессов в ткани плаценты. В свою очередь, поражения плаценты закономерно могут явиться причиной развития осложнений беременности, плода и матери. Выявление таких поражений, включая определение специфических для SARS-CoV-2-инфекции, а также определение прогноза развития новорожденного возможно при комплексном морфологическом изучении структур плаценты. При этом макроскопическое исследование плаценты должно проводиться с обязательным соблюдением мер защиты, в том числе после предварительной формалиновой ее фиксации в течение 48–72 ч.



