Indicators of umbilical arterial blood acid-base status during spontaneous labor and cesarean section
Objective. To comparatively analyze the data on the umbilical arterial blood acid-base status in healthy newborn infants according to the method of delivery. Subjects and methods. The prospective examination covered the results of umbilical arterial blood (UCAB) gas analysis in 280 healthy newborns: 152 babies after spontaneous labor, 62 after cesarean section (CS) before the beginning of delivery, and 66 after CS during labor for reasons unrelated to hypoxia. Results. The mean umbilical arterial blood pH value during spontaneous labor was 7.28 (0.09) and was significantly lower than that in patients undergoing CS before delivery (7.34 (0.06) (p <0.001)). The base deficiency level during spontaneous labor was 7.4 (3.1) versus 2.8 (1.7) mmol/L in those undergoing CS before labor and 5.9 (3.0) during labor (p = 0.007). The level of lactate during spontaneous labor was 5.4 (2.0) and was much higher than in CS before delivery (2.1 (0.8)) and during labor (2.8 (1.9)) (p = 0, 0007). The partial pressure of carbon dioxide and oxygen was not different. Conclusion. The findings showed significant differences in the UCAB acid-base status after labor and CS done before the beginning of delivery. These differences must be considered when evaluating the status of a newborn infant. Even the clinically healthy newborns have a wide range of fluctuations in pH values, base deficiency, and cord blood lactate after both labor and CS. In some observations, there is a mismatch between cardiotocographic data at the birth, the satisfactory status of newborn health, and changes in the UCAB acid-base status.Prikhodko A.M., Baev O.R., Evgrafova A.V., Romanov A.Yu., Degtyarev D.N.
Keywords
The assessment of the newborn in the delivery room is crucial for the choice of tactics and the volume of emergency care, as well as for the prognosis of neonatal complications. In newborns who have undergone intranatal hypoxia, along with a clinical assessment by the Apgar scale, the most common additional evaluation method is the analysis of the umbilical arterial blood acid-base state (ABS) [1-3].
According to the literature, during the first minutes after birth, the average arterial blood pH in healthy newborns is 7.18–7.38. A normal pH excludes possible relationship between the course of labor and the subsequent development of hypoxic brain damage. In 1999, the International Consensus on Causation of Cerebral Palsy determined metabolic acidosis criteria based on the results of the study of umbilical arterial blood immediately after birth, which are significant for the development of subsequent neurological pathology. These criteria are currently accepted by most national medical communities: pH < 7.00 and base deficiency (BD) ≥ 12 mmol/L [4–6]. At the same time, in some studies, 7.05 pH limit is used to diagnose metabolic acidosis [7–11].
The fetal blood ABS is influenced by various factors, such as an increase in the level of catecholamines, the administration of glucocorticoids and beta-mimetics before childbirth, maternal hyperventilation during childbirth, the duration of labor and the features of its course [6, 12–16]. However, it remains unclear whether the method of delivery affects the fetal blood ABS or not. The aim of the study was to conduct a comparative analysis of umbilical arterial blood ABS in healthy newborns, depending on the method of delivery.
Materials and Methods
The prospective observational study was conducted at the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow and the Department of Obstetrics, Gynecology, Perinatology, and Reproductive Medicine of I.M. Sechenov First Moscow State Medical University. We collected 280 umbilical cord artery blood samples. Inclusion criteria were full-term singleton pregnancy, 5th minute Apgar score 8 points or greater, the absence of clinical or laboratory signs of fetal suffering during childbirth and complications in the early neonatal period. Exclusion criteria in the study were fetal malformations, severe somatic pathology (drug-dependent chronic arterial hypertension, acute infectious diseases, renal and liver failure, autoimmune diseases, severe preeclampsia (PE), premature placental abruption), as well as the suspicious or pathological type of cardiotocogram (CTG) before or during the labor.
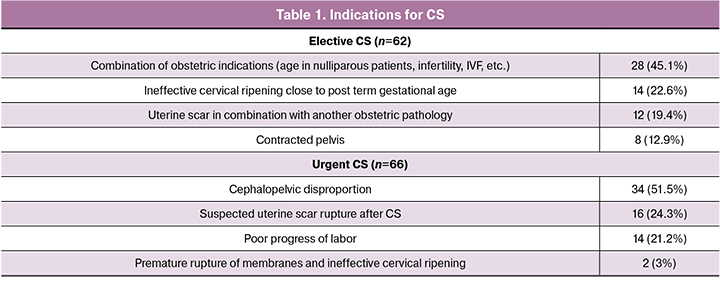
All newborns were divided into three groups depending on the delivery method: spontaneous vaginal delivery – 152 (54.3%) cases, cesarean section (CS) – 128 (45%) cases including 62 (22.1%) cases of planned CS before delivery (elective CS) and 66 (23.6%) cases of emergency CS (urgent CS) unrelated to fetal hypoxia (Table 1).
All operations were performed using a single surgical technique by transverse suprapubic laparotomy, a transverse incision in the lower segment of the uterus. Anesthesia was performed by the neuroaxial method (spinal, epidural or combined spinal-epidural anesthesia).
All women had manual and instrumental assessment of pregnancy course, labor and the fetus health, including sonography and CTG.
Blood samples were collected routinely immediately after delivery. Three clamps were placed to the umbilical cord. Between the 1st and 2nd clamps the umbilical cord was cut. Between the 2nd and 3rd clamps, blood sample was taken from the umbilical cord artery. Measurement of pH, base deficit (BD), lactate (lac), partial oxygen pressure (pO2) and partial carbon dioxide pressure (pCO2) was performed using an ABL800 FLEX gas analyzer (Radiometer Medical ApS, Denmark) no later than 10 min after sampling [17].
For statistical analysis and plotting we used the GraphPad Prism statistical package (GraphPad Software, USA) with t-test and Mann-Whitney test. Parametric quantitative data are presented as mean value and standard deviation, nonparametric quantitative data are presented as median and interquartile range, and quantitative data are presented as absolute value and percentage. Differences were considered statistically significant at p < 0.05. The study was approved by local institutional review board of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow.
Results
The mean age of the patients in the study was 31.1 (5.3) years, and the mean body mass index (BMI) was 25.3 (6.1) kg/m2. Most of the patients were nulliparous, 82.9%. None of the patients had a severe somatic pathology or a complicated course of this pregnancy. In all observations CTG type was normal according to the FIGO-2015 classification [18]. All patients delivered at term. The gestational age at the time of delivery did not differ in the groups of comparison and was 281.5 (6.8) days (40 weeks 1 day) in the spontaneous vaginal delivery group, 276.4 (4.5) days (39 weeks 2 days) in the elective CS group and 281.1 (6.6) days (40 weeks 2 days) in the urgent CS group (p = 0.47).
The body weight of newborns ranged from 2540 to 4330 g with mean of 3298 (350) g. The body length ranged from 46 to 56 cm, mean length was 51.4 (2.0) cm. The Apgar score was from 7 to 9 in all groups. The mean score in 1st and 5th minute was 8 (8 – 9) and 9 (9 – 9) points, respectively (p = 0.43).
In spontaneous vaginal delivery group, the duration of the 1st period of labor was 406.9 (143.2) minutes and the duration of the 2nd period was 71.5 (35.3) minutes. Epidural analgesia was used in 82 (53.9%) cases. Induction of labor by oxytocin was required in 15 (9.8%) cases.
The mean pH of the umbilical arterial blood in spontaneous vaginal delivery group was 7.28 (0.09), which was significantly lower than that in patients with elective CS – 7.33 (0.08), p < 0.001 (Table 2).
The mean BD level in spontaneous vaginal delivery group was 7.4 (3.1) mmol/L versus 2.8 (1.7) mmol/L in elective CS group, respectively, p = 0.007 (Table 3).
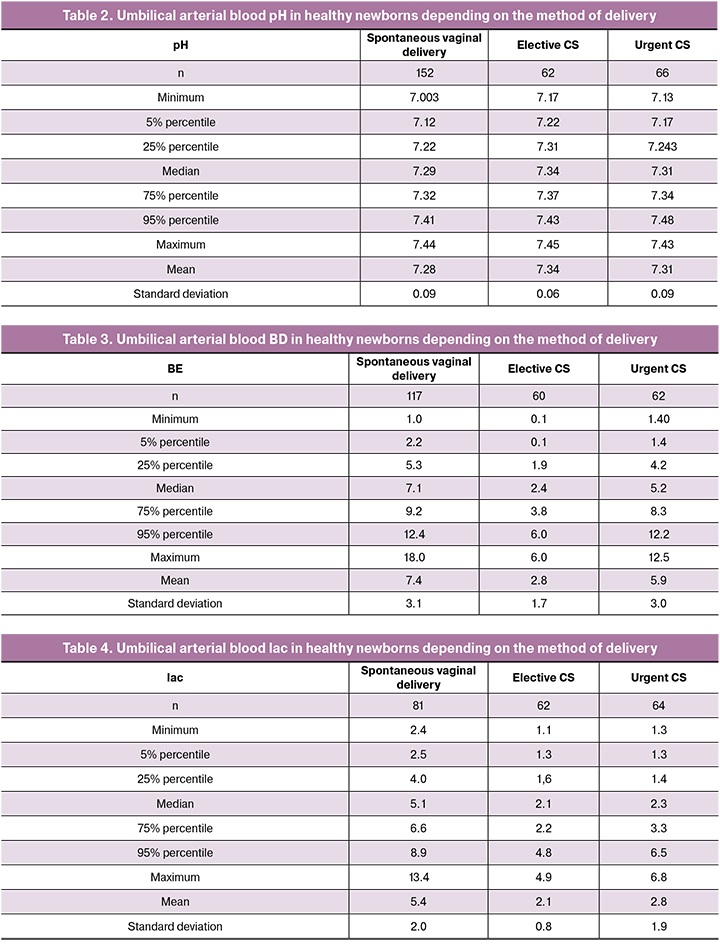
The mean lac level in spontaneous vaginal delivery was 5.4 (2.0) mmol/L and was significantly higher than that in patients with elective CS – 2.1 (0.8) mmol/L and in patients with urgent CS 2.8 (1.9) mmol/L (p < 0.001) (Table 4).
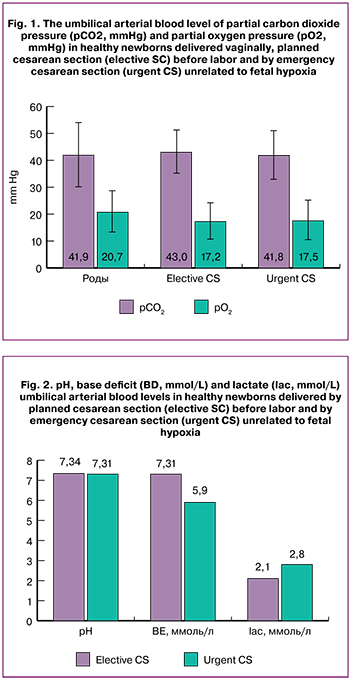 The levels of pCO2 and pO2 did not significantly differ in the groups of comparison (Fig. 1).
The levels of pCO2 and pO2 did not significantly differ in the groups of comparison (Fig. 1).
The 5% percentile pH was 7.12 in spontaneous vaginal delivery, which was lower than that in elective CS – 7.22 (Table 2). The 95% percentile BD was 12.4 mmol/L in spontaneous vaginal delivery and 6.0 mmol/L in elective CS (Table 3). The 95% percentile lac was 8.9 mmol/L in spontaneous vaginal delivery and 4.8 in elective CS (Table 4).
There were no statistically significant differences in pH, BD, and lac levels between elective CS and urgent CS groups (Fig. 2).
Discussion
Assessment of ABS in umbilical arterial blood is a standard for neonatal acidosis diagnosis [7]. In this work we conducted the analysis of ABS, aimed to find normal values depending on the method of delivery.
The pH level in spontaneous uncomplicated delivery of a healthy newborn was significantly lower than in planned CS performed before the onset of labor, and BD and lac levels were higher. The levels of pCO2 and pO2 did not differ significantly. Revealed features of ABS indicate that there are metabolic changes in fetus experiencing labor stress due to the anaerobic metabolism. It was caused by transient hypoxemia due to reduced utero-placental circulation during uterine contractions [19]. At the same time, a decrease in pH and an increase in BD are characteristic of all newborns who have experienced labor stress, regardless of whether the labor ended vaginally or by CS.
The lac level after vaginal delivery is higher than that after planned and urgent CS. This confirms an increased lac production in the second stage of labor due to a higher intensity of labor and an increase in episodes of transient hypoxemia during contractions [20].
Some researchers indicate that the lower limit of umbilical arterial blood pH in healthy newborns is 7.15 or 7.20 [7, 21]. In our study, the 5th percentile of the pH after uncomplicated spontaneous labor was 7.12, and after planned and urgent CS it was 7.17 and 7.22, respectively.
The mean lac level during spontaneous labor in our study was 5.4 mmol/L, the mean BD level was 7.4 mmol/L, which basically coincides with the data of Allanson E. et al. (2016), who determined normal lac values – up to 6 mmol/L, and normal BD values – up to 8 mmol/L [22].
However, in three cases, low pH was observed in newborns after uncomplicated labor (pH = 7.003; BD = 12 mmol/L; lac = 7 mmol/L; pCO2 = 74.6 mmHg; pO2 = 58 mmHg), with higher values of BD (pH = 7.087; BD = 18 mmol/L; lac = 12.7 mmol/L; pCO2 = 42.7 mmHg; pO2 = 25.8 mmHg) and lac (pH = 7.051; BD = 15 mmol/L; lac = 13.4 mmol/L). At the same time, no significant features were revealed during the early neonatal period, in comparison with other neonates whose ABS parameters fell within the range of 5–95 percentile. These results are indicative of great compensatory capabilities of full-term newborns, even with metabolic acidosis in labor. However, observations of other newborns who were not included in this study due to the pregnancy and labor complications confirm that in most cases high concentrations of lac and BD are associated with early neonatal disadaptation and a high incidence of neurological complications.
Conclusion
Thus, we showed significant differences in umbilical arterial blood ABS after spontaneous vaginal delivery and planned CS. These differences must be taken into account for more accurate assessment of the newborn’s condition.
It should also be noticed that even in healthy newborn there can be a wide range in blood pH, BD and lac both after childbirth and CS. In some observations, there can be a mismatch between normal CTG, normal newborn’s condition and changes in ABS, which is indicative of metabolic acidosis, caused by the rapid development of acute hypoxia and rapid elimination of its cause after delivery in case of not compromised compensatory reserve of the child.
References
- MacLennan A. A template for defining a causal relation between acute intrapartum events and cerebral palsy: international consensus statement. BMJ. 1999; 319(7216): 1054–9. doi: 10.1136/bmj.319.7216.1054
- Hankins G.D. V, Speer M. Defining the pathogenesis and pathophysiology of neonatal encephalopathy and cerebral palsy. Obstet Gynecol. 2003; 102(3): 628–36. doi: 10.1038/sj.jp.7210912
- Приходько А.М., Романов А.Ю., Шуклина Д.А., Баев О.Р. Показатели кислотно-основного равновесия и газовый состав артериальной и венозной пуповинной крови в норме и при гипоксии плода. Акушерство и гинекология. 2019; (2): 93–7. [Prikhodko A.M., Romanov A.Yu., Shuklina D.A., Baev O.R. The indicators of acid-base balance and the gas composition of umbilical cord arterial and venous blood in health and fetal hypoxia. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; (2): 93–7. (in Russian)]. http://dx.doi.org/10.18565/aig.2019.2.93-97
- Chauhan S.P., Cowan B.D., Meydrech E.F., Magann E.F., Morrison J.C., Martin J.N. Determination of fetal acidemia at birth from a remote umbilical arterial blood gas analysis. Am J Obstet Gynecol. 1994; 170(6): 1705–9; discussion 1709-12. doi:10.1016/S0002-9378(12)91839-6
- Goodwin T.M., Belai I., Hernandez P., Durand M., Paul R.H. Asphyxial complications in the term newborn with severe umbilical acidemia. Am J Obstet Gynecol. 1992; 167(6): 1506–12. doi: 10.1016/0002-9378(92)91728-s
- Ferreira C.S., Melo Â., Fachada A.H., Solheiro H., Nogueira Martins N. Umbilical Cord Blood Gas Analysis, Obstetric Performance and Perinatal Outcome. Rev Bras Ginecol Obstet. 2018; 40(12):740–8. doi: 10.1055/s-0038-1675187.
- Maisonneuve E., Audibert F., Guilbaud L., Lathelize J., Jousse M., Pierre F., et al. Risk factors for severe neonatal acidosis. Obstet Gynecol. 2011; 118(4): 818–23. doi: 10.1097/AOG.0b013e31822c9198.
- Belfort M.A., Saade G.R., Thom E., Blackwell S.C., Reddy U.M., Thorp J.M., et al. A Randomized Trial of Intrapartum Fetal ECG ST-Segment Analysis. N Engl J Med. 2015; 373(7): 632–41. doi: 10.1056/NEJMoa1500600.
- Bernardes T.P., Broekhuijsen K., Koopmans C.M., Boers K.E., van Wyk L., Tajik P., et al. Caesarean section rates and adverse neonatal outcomes after induction of labour versus expectant management in women with an unripe cervix: a secondary analysis of the HYPITAT and DIGITAT trials. BJOG. 2016; 123(9): 1501–8. doi: 10.1111/1471-0528.14028.
- Bullens L.M., Smith J.S., Truijens S.E.M., van der Hout-van der Jagt M.B., van Runnard Heimel P.J., Oei S.G. Maternal hemoglobin level and its relation to fetal distress, mode of delivery, and short-term neonatal outcome: a retrospective cohort study. J Matern Fetal Neonatal Med. 2019; 1–7. doi: 10.1080/14767058.2019.1573221.
- Hulsenboom A.D.J., Verdurmen K.M.J., Vullings R., van der Hout–van der Jagt M.B., Kwee A., van Laar J.O.E.H., et al. Relative versus absolute rises in T/QRS ratio by ST analysis of fetal electrocardiograms in labour: A case-control pilot study. Pueyo E, editor. PLoS One. 2019; 14(3): e0214357. doi: 10.1371/journal.pone.0214357.
- Tomimatsu T., Kakigano A., Mimura K., Kanayama T., Koyama S., Fujita S., et al. Maternal hyperventilation during labor revisited: its effects on fetal oxygenation. Reprod Sci. 2012; 19(11): 1169–74. doi: 10.1177/1933719112443881.
- Elbay A., Celik U., Celik B., Ozer O.F., Kilic G., Akkan J.C.U., et al. Intraocular pressure in infants and its association with hormonal changes with vaginal birth versus cesarean section. Int Ophthalmol. 2016; 36(6): 855–60. doi: 10.1007/s10792-016-0215-6.
- Limesand S.W., Rozance P.J. Fetal adaptations in insulin secretion result from high catecholamines during placental insufficiency. J Physiol. 2017; 595(15): 5103–13. doi: 10.1113/JP273324.
- Landau R., Liu S.-K., Blouin J.-L., Smiley R.M., Ngan Kee W.D. The effect of maternal and fetal β2-adrenoceptor and nitric oxide synthase genotype on vasopressor requirement and fetal acid-base status during spinal anesthesia for cesarean delivery. Anesth Analg. 2011; 112(6): 1432–7. doi: 10.1213/ANE.0b013e3182179424.
- Приходько А.М., Киртбая А.Р., Романов А.Ю., Баев О.Р. Биомаркеры повреждения головного мозга у новорожденных. Неонатология. 2018; 7(1): 70–6. [Prikhod’ko A.M., Kirtbaya A.R., Romanov A.Yu., Baev O.R. Biomarkers of brain damage in newborns. Neonatologija. 2018; 7(1): 70–6.(in Russian)]. doi: 10.24411/2308-2402-2018-00009
- Приходько А.М., Баев О.Р. Определение кислотно-основного состояния пуповинной крови. Показания и техника. Акушерство и гинекология. 2018; (5): 127–31. [Prikhodko A.M., Baev O.R. Determination of umbilical cord blood acid-base status. Indications and techniques. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2018; (5): 127–31. (in Russian)]. doi: 10.18565/aig.2018.5.127-131
- Ayres-de-Campos D., Spong C.Y., Chandraharan E., FIGO Intrapartum Fetal Monitoring Expert Consensus Panel. FIGO consensus guidelines on intrapartum fetal monitoring: Cardiotocography. Int J Gynaecol Obstet. 2015; 131(1): 13–24. doi: 10.1016/j.ijgo.2015.06.020.
- Araujo O.R., Diegues A.R., Silva D.C.B. da, Albertoni A. de C.S., Louzada M.E.R., Cabral E.A.F., et al. Agreement and correlation of pH, bicarbonate, base excess and lactate measurements in venous and arterial blood of premature and term infants. Rev Bras Ter intensiva. 2007; 19(3): 322–6. doi:10.1590/S0103-507X2007000300009
- Nordström L., Achanna S., Naka K., Arulkumaran S. Fetal and maternal lactate increase during active second stage of labour. BJOG. 2001; 108(3): 263–8. doi: 10.1111/j.1471-0528.2001.00034.x
- Qian G., Xu X., Chen L., Xia S., Wang A., Chuai Y., et al. The effect of maternal low flow oxygen administration during the second stage of labour on umbilical cord artery pH: a randomised controlled trial. BJOG An Int J Obstet Gynaecol. 2017; 124(4): 678–85. doi: 10.1111/1471-0528.14418.
- Chou Y.H., Tsou Yau K.I., Wang P.J. Clinical application of the measurement of cord plasma lactate and pyruvate in the assessment of high-risk neonates. Acta Paediatr. 1998; 87(7):764–8. doi: 10.1080/080352598750013851
Received 17.06.2019
Accepted 21.06.2019
About the Authors
Andrey M. Prikhodko, PhD, physician of the maternity department, assistant of the Department Obstetrics and Gynecology, Researcher of the Innovative Technologies Department of Obstetrics Institute, National Medical Research Center for Obstetrics, Gynecology and Perinatology Ministry of Healthcare of Russian Federation.Tel.: +7 (495) 438-30-47. E-mail: a_prikhodko@oparina4.ru
117997, Russia, Moscow, Ac. Oparina str. 4
Oleg R. Baev, MD, Head of maternity department, National Medical Research Center for Obstetrics, Gynecology and Perinatology Ministry of Healthcare of the Russian Federation; professor of the Department of Obstetrics, Gynecology, Perinatology, and Reproductology of I.M. Sechenov First Moscow State Medical University
of Ministry of Healthcare of Russian Federation. Tel.: +7 (495) 438-11-88. E-mail: o_baev@oparina4.ru orcid.org/0000-0001-8572-1971
117997, Russia, Moscow, Ac. Oparina str. 4
Alexandra V. Evgrafova, postgraduate student, National Medical Research Center for Obstetrics, Gynecology and Perinatology Ministry of Healthcare of Russian Federation. Tel. +7 (977) 643-27-13.E-mail: a_evgrafova@oparina4.ru
117997, Russia, Moscow, Ac. Oparina str. 4
Andrey Yu. Romanov, clinical resident, National Medical Research Center for Obstetrics, Gynecology and Perinatology Ministry of Healthcare of Russian Federation.
Tel.: +7 (903) 158-94-00. E-mail: romanov1553@yandex.ru
ORCID: 0000-0003-1821-8684
117997, Russia, Moscow, Ac. Oparina str. 4
Dmitriy N. Degtyarev, MD, professor, head of the Department of Neonatology, I.M. Sechenov First Moscow State Medical University of Ministry of Healthcare of Russian Federation; Deputy Director of National Medical Research Center for Obstetrics, Gynecology and Perinatology Ministry of Healthcare of the Russian Federation.
E-mail: glav_neolog@yahoo.com, d_degtiarev@oparina4.ru
117997, Russia, Moscow, Ac. Oparina str. 4
For citation: Prikhodko A.M., Baev O.R., Evgrafova A.V., Romanov A.Yu., Degtyarev D.N. Indicators of umbilical arterial blood acid-base status during spontaneous labor and cesarean section.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2019; 12: 83-9.(In Russian).
https://dx.doi.org/10.18565/aig.2019.12.83-89



