Initial results of clinical application of mesenchymal stromal stem cell-derived extracellular microvesicles after abdominal delivery
Aim. To investigate the tolerability to the injection of extracellular microvesicles (EMV) of placenta-derived mesenchymal stromal cells (MSC) during abdominal delivery and assess the postoperative course. Materials and methods. The study included two groups of puerperal women after cesarean delivery. The study group consisted of 23 patients who received 500 μl EMV MSC during abdominal delivery and continuous closure of the low transverse uterine incision with vicryl suture. The comparison group (control group) included 30 patients with traditional continuous single-layer closure of uterine incision with vicryl suture. Results. All puerperal women in the study group had an uneventful postoperative course and were discharged home earlier (postoperative stay 4.26+0.09 vs. 5.33+0.38, p <0.05) than patients in the control group. All patients of the study group planned to become pregnant in the next 2–3 years and were included in the registry for subsequent vaginal delivery in the presence of a post-cesarean uterine scar. The patients of the study group did not have septic complications, while two (6.7%) and one (3.3%) patients in the control group had postpartum metroendometritis and lochiometra, respectively Conclusion. The study findings suggest a good tolerability profile and higher effectiveness of intraoperative EMV administration than traditional postpartum management. Initial results of the clinical application of exosomes open up new horizons for their use. It may help improve myometrium repair and predict future spontaneous delivery for patients with post-cesarean uterine scars.Sukhikh G.T., Pekarev O.G., Pekareva E.O., Maiborodin I.V., Silachev D.N., Baranov I.I., Pozdnyakov I.M., Bushueva N.S., Novikov A.V.
Keywords
The last decade has been marked by a radical change in priorities for primary cesarean births because of the utmost importance of a post-cesarean uterine scar. In this context, there is a need for new methods to improve myometrium repair after surgery so that 2–3 years after cesarean birth, a woman could give birth naturally. Over the recent 15 years, cell technologies have been successfully used in clinical medicine to improve tissue regeneration [1–3]. Nevertheless, there are specific difficulties for their widespread implementation [4–7]. One of the interesting mechanisms of stem cells' action can be their ability to communicate and exchange functional proteins and genetic material through the secretion of microvesicles, which can improve tissue repair, since they can transfer intercellular information and work as a modulator [8–13].
Based on the above, we assume that extracellular microvesicles (EMV) will improve the postoperative course and allow patients with a post-cesarean uterine scar to give birth naturally, thus reducing the cesarean birth rate. Moreover, the experimental studies on the cesarean birth model using an inbred line of Wag rats showed that both in intact animals and after a cesarean section, exosomes remain in the myometrium for at least eight days (Fig. 1–3) [14, 15 ].
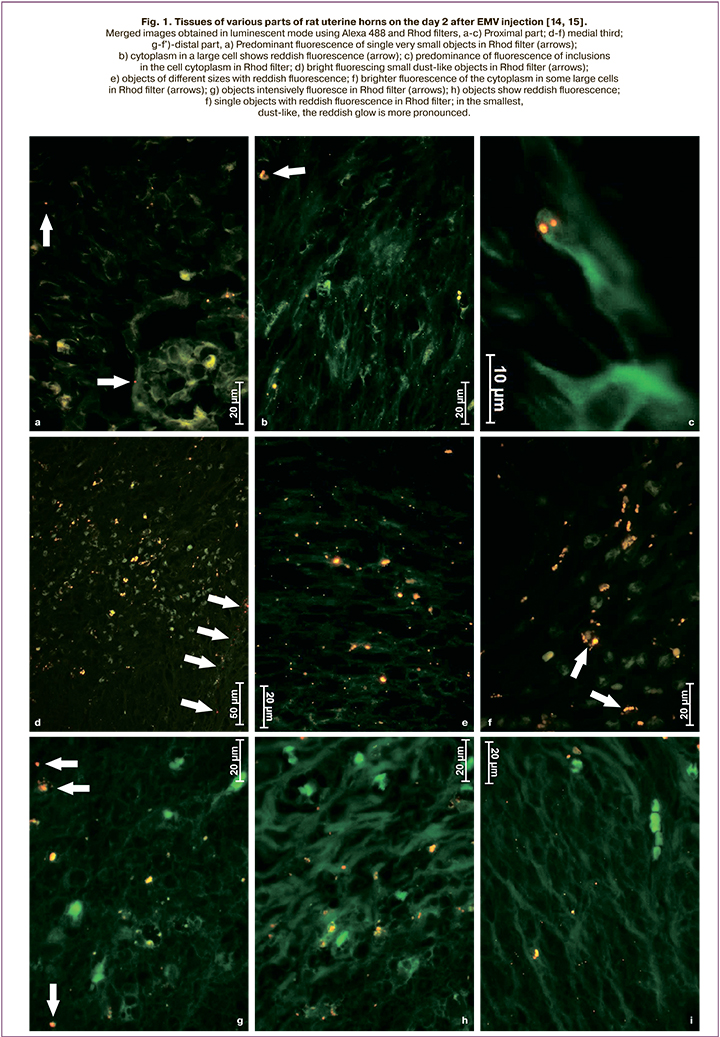
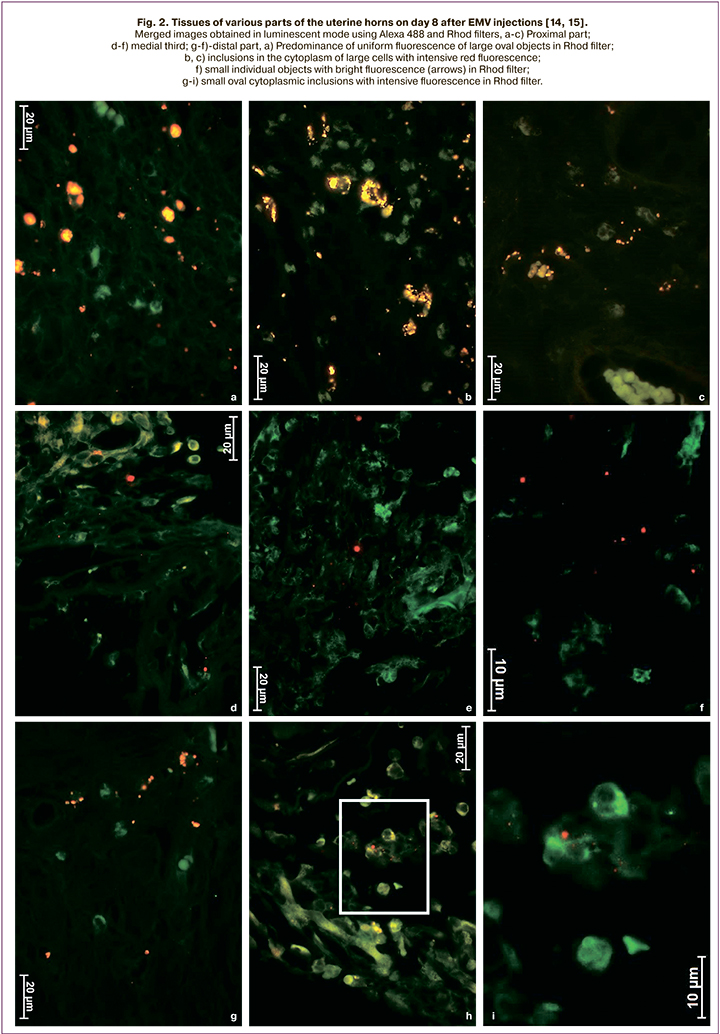
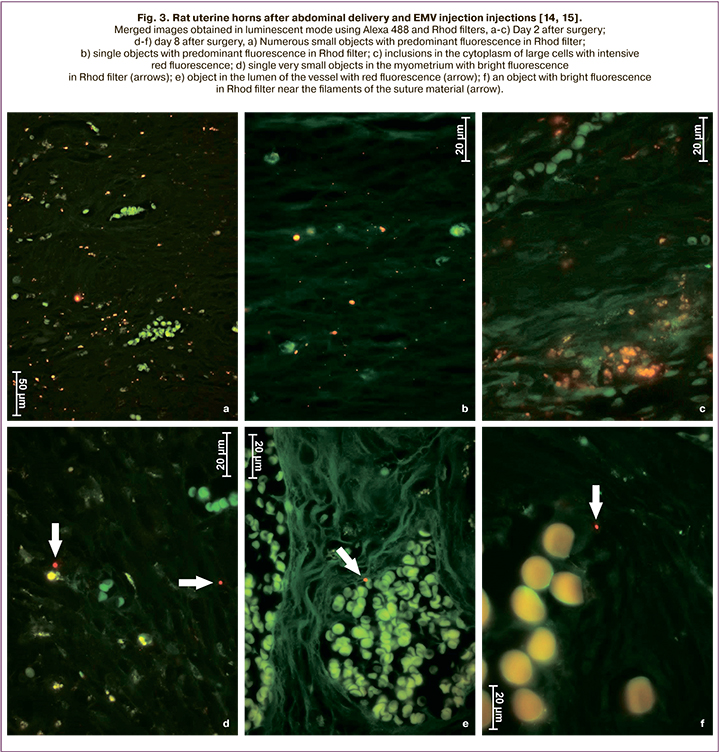
The study aimed to investigate the tolerability of the injection of EMV of placenta-derived mesenchymal stromal cells (MSC) during abdominal delivery and assess the postoperative course.
Materials and methods
The study included 53 puerperal women after cesarean birth, who were divided into two groups. Patients in the study group (n=23) during abdominal delivery received an injection of 500 μl EMV MSC obtained in the laboratory of cell technologies of V.I. Kulakov NMRC for OG&P into the incision area and had continuous closure of the low transverse uterine incision with vicryl suture. Patients in the control group (n=30) underwent traditional continuous single-layer closure of uterine incision with vicryl suture.
The age characteristics of the study participants are presented in the Table 1.
As shown in the table, the patients were young, and the age in the study groups did not differ significantly.
Comorbidities among the study participants are presented in Table 2. There were no significant differences between the study groups regarding the rates of non-obstetric diseases. Almost every second patient had chronic tonsillitis (from 50 to 56.5%). More than 40% were diagnosed with chronic inflammation of the kidneys and 17% of the bronchi.
The infectious index (the ratio of the total number of infectious diseases to the number of patients) in the study and control groups was 1.3 and 1.2, respectively (Table 2).
In terms of socioeconomic status, almost half of pregnant women who had a cesarean birth (46.6% to 47.8%0 did not work and were engaged in housekeeping (Table 3).
Outcomes of previous pregnancies of the control group patients are presented in Table 4.
It is noteworthy that 6 (26.1%) and 7 (23.3%) in the study and control group had a history of voluntary induced termination of pregnancy, which could subsequently provoke spontaneous termination of pregnancy in 4 (17.4%) and 6 (20%) women from groups I and II, respectively.
Many abortions were directly related to the low level of contraceptive education of the study participants. Only every tenth patient (8.7%), who received 500 μL of exosomes during cesarean birth, used contraceptive methods. In the control group, the indicator did not fundamentally differ – 3 (10%).
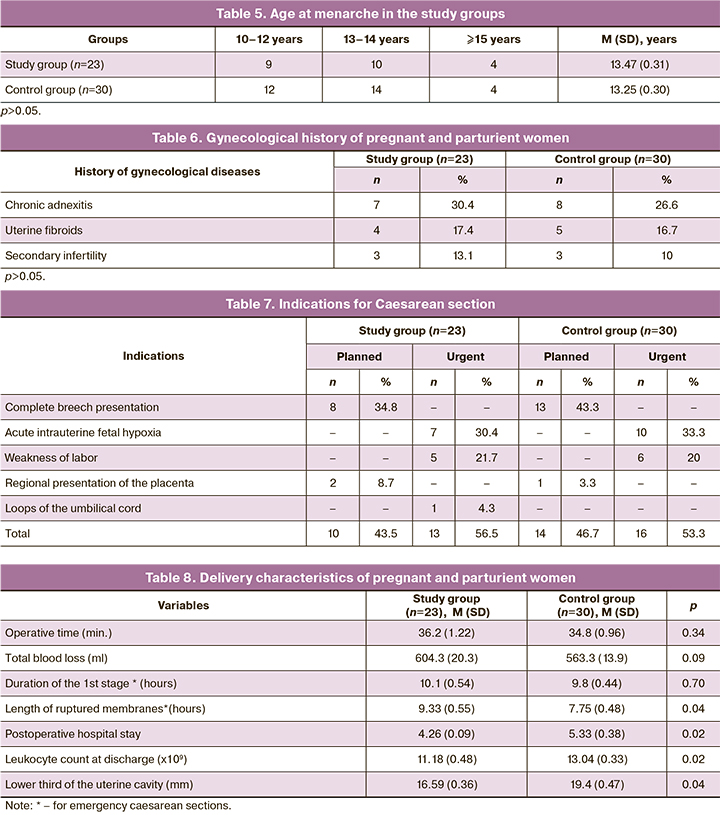
The age at menarche in the patients was standard for Russian women and averaged 13 years (Table 5), not differing significantly from the control group (p > 0.05). There was a high rate of inflammatory diseases (Table 6), which was reported by almost every eighth pregnant woman in the study [3 (13.1%)] and every tenth [3 (10%)] in the control group.
Therefore, therewerenosignificantdifferencesbetween the study groups regarding clinical characteristics.
Statistical analysis
Statistical analysis was performed using Microsoft Excel spreadsheets and the GraphPad Prism 6 software (GraphPad Software, USA). Quantitative variables showing normal distribution were expressed as means (M) and standard deviation (SD). Statistical comparisons were performed using the paired Student's t-test (for paired samples). Differences were considered significant at p <0.05.
Results and discussion
The vast majority of patients in the study group [21 (91.3%)] administered intraoperatively with 500 μl of EMV were primiparous without a complicated obstetric history, wishing vaginal birth after cesarean within 2–3 years. Ten women (43.5%) underwent a planned cesarean section due to mixed breech presentation. In two cases (8.7%), the operation was performed for the marginal placenta previa.
An emergency cesarean section was performed in 13 (56.5%) women in labor due to acute intrauterine fetal hypoxia (30.4%), labor dystocia not amenable to drug correction (21.7%), and in one case due to the loss of umbilical cord loops during prelabor rupture of membranes (Table 7).
In all women, the cesarean section was performed under epidural anesthesia with intraoperative antibiotic prophylaxis.
Among patients undergoing emergency abdominal delivery, there were no between-group differences in operative time, total intraoperative blood loss, and duration of the first stage of labor (Table 8).
The present comparative study revealed significant differences between puerperal women in the study and control groups in duration of ruptured membranes (Table 8), which in the patients of the control group was 7.75 (0.48) hours and was shorter than in patients who received 500 μl EMV (p=0.041). Significant differences were also observed in the duration of the postoperative hospital stay [(4.26 (0.09) in group I vs. 5.33 (0.38) in group II (p=0.02)]. A similar dynamics were observed in leukocyte counts at discharge, which in the group with the intraoperative injection of exosomes was 11.18 (0.48) x 109/l, which was was significantly lower than in the control group [13.04 (0.33) x 109/l (p=0.02)]. Patients of the study group had no postpartum inflammatory complications, while in the control group, there was two endometritis (6.7%) and one lochiometra (3.3%).
Conclusion
The study findings suggest a good tolerability profile and higher effectiveness of intraoperative EMV administration than traditional postpartum management. Initial results of the clinical application of exosomes open up new horizons for their use. It may help improve myometrium repair and predict future spontaneous delivery for patients with post-cesarean uterine scars.
References
- Майбородин И.В., Якимова Н.В., Матвеева В.А., Пекарев О.Г., Майбородина Е.И., Пекарева Е.О. Ангиогенез в рубце матки крыс после введения аутологичных мезенхимальных стволовых клеток костномозгового происхождения. Бюллетень экспериментальной биологии и медицины. 2010; 150(12): 705-11. [Mayborodin I.V., Yakimova N.V., Matveeva V.A., Pekarev OG, Mayborodina E.I., Pekareva E.O. Angiogenesis in the uterine rumen of rats after administration of autologous mesenchymal stem cells of bone marrow origin. Bulletin of Experimental Biology and Medicine. 2010; 150(12): 705-11. (in Russian)].
- Майбородин И.В., Якимова Н.В., Матвеева В.А., Пекарев О.Г., Майбородина Е.И., Пекарева Е.О., Ткачук О.К. Морфологический анализ результатов введения аутологичных стволовых стромальных клеток костномозгового происхождения в рубец матки крыс. Морфология. 2010; 138(6): 47-55. [Mayborodin I.V., Yakimova N.V., Matveeva V.A., Pekarev O.G., Maiborodina E.I., Pekareva E.O., Tkachuk O.K. Morphological analysis of the results of the introduction of autologous stromal stem cells of bone marrow origin into the rat uterine scar. Morphology. 2010; 138(6): 47-55. (in Russian)].
- Майбородин И.В., Оноприенко Н.В., Частикин Г.А. Морфологические изменения тканей матки крыс и возможность самопроизвольных родов в результате введения мультипотентных мезенхимных стромальных клеток на фоне гидрометры. Бюллетень экспериментальной биологии и медицины. 2015; 159(4): 511-6. [Mayborodin I.V., Onoprienko N.V., Chastikin G.A. Morphological changes in the tissues of the uterus of rats and the possibility of spontaneous delivery as a result of the introduction of multipotent mesenchymal stromal cells against the background of a hydrometer. Bulletin of Experimental Biology and Medicine. 2015; 159(4): 511-6. (in Russian)].
- Rodrigues M., Yates C.C., Nuschke A., Griffith L., Wells A. The matrikine tenascin-C protects multipotential stromal cells/mesenchymal stem cells from death cytokines such as FasL. Tissue Eng. Part A. 2013; 19(17-18): 1972-83. https://dx.doi.org/10.1089/ten.TEA.2012.0568.
- Майбородин И.В., Матвеева В.А., Маслов Р.В., Оноприенко Н.В., Кузнецова И.В., Частикин Г.А., Аникеев А.А. Некоторые реакции регионарных лимфатических узлов крыс после имплантации в дефект костной ткани мультипотентных стромальных клеток, адсорбированных на полигидроксиалканоате. Морфология. 2016; 149(2): 21-6. [Mayborodin I.V.,Matveeva V.A., Maslov R.V., Onoprienko N.V., Kuznetsova I.V., Chastikin G.A., Anikeev A.A. Some reactions of rat regional lymph nodes after implantation of multipotent stromal cells adsorbed on a polyhydroxy alkanoate into a bone defect. Morphology. 2016; 149(2): 21-6. (in Russian)].
- Майбородин И.В., Морозов В.В., Аникеев А.А., Фигуренко Н.Ф., Маслов Р.В., Частикин Г.А., Матвеева В.А., Майбородина В.И. Макрофагальный ответ у крыс на введение мультипотентных мезенхимальных стромальных клеток в регион хирургической травмы. Новости хирургии. 2017; 25(3): 233-41. [Mayborodin I.V., Morozov V.V., Anikeev A.A., Figurenko N.F., Maslov R.V., Chastikin G.A., Matveeva V.A., Mayborodina V.I. Macrophage response in rats to the introduction of multipotent mesenchymal stromal cells into the region of surgical injury. Surgery news. 2017; 25 (3): 233-41. (in Russian)].
- Yates C.C., Nuschke A., Rodrigues M., Whaley D., Dechant J.J., Taylor D.P., Wells A. Improved transplanted stem cell survival in a polymer gel supplemented with Tenascin C accelerates healing and reduces scarring of murine skin wounds. Cell Transplant. 2017; 26(1): 103-13. https://dx.doi.org/10.3727/096368916X692249.
- Takeda Y.S., Xu Q. Neuronal differentiation of human mesenchymal stem cells using exosomes derived from differentiating neuronal cells. PLoS One. 2015; 10(8): e0135111. https://dx.doi.org/10.1371journal.pone.0135111.
- Furuta T., Miyaki S., Ishitobi H., Ogura T., Kato Y., Kamei N. et al. Mesenchymal stem cell-derived exosomes promote fracture healing in a mouse model. Stem Cells Transl. Med. 2016; 5(12): 1620-30. https://dx.doi.org/10.5966/sctm.2015-0285.
- Narayanan R., Huang C.C., Ravindran S. Hijacking the cellular mail: exosome mediated differentiation of mesenchymal stem cells. Stem Cells Int. 2016; 2016: 3808674. https://dx.doi.org/10.1155/2016/3808674.
- van der Pol E., Böing A.N., Harrison P., Sturk A., Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 2012; 64(3): 676-705. https://dx.doi.org/10.1124/pr.112.005983.
- Akers J.C., Gonda D., Kim R., Carter B.S., Chen C.C. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neurooncol. 2013; 113(1): 1-11. https://dx.doi.org/10.1007/s11060-013-1084-8.
- Février B., Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr. Opin. Cell Biol. 2004; 16(4): 415-21. https://dx.doi.org/10.1016/j.ceb.2004.06.003.
- Сухих Г.Т., Пекарев О.Г., Майбородин И.В., Силачев Д.Н., Шевцова Ю.А., Горюнов К.В., Оноприенко Н.В., Майбородина В.И., Галенок Р.В., Новиков А.М., Пекарева Е.О. К вопросу о сохранности экстрацеллюлярных микровезикул мезенхимных стромальных клеток после абдоминального родоразрешения в эксперименте. Клеточные технологии в биологии и медицине. 2020; 1: 3-11. [Sukhikh G.T., Pekarev O.G., Mayborodin I.V., Silachev D.N., Shevtsova Yu.A., Goryunov K.V., Onoprienko N.V., Mayborodina V.I., Galenok R.V., Novikov A.M., Pekareva E.O. On the question of the preservation of extracellular microvesicles of mesenchymal stromal cells after abdominal delivery in the experiment. Cell technologies in biology and medicine. 2020; 1: 3-11. (in Russian)].
- Sukhikh G.T., Pekarev О.G., Maiborodin I.V., Silachev D.N., Shevtsova Y.А., Gоrуunоv K.V., Onoprienko N.V., Maiborodina V.I., Galenok R.V., Novikov A.M., Pekareva Е.О. Preservation of mesenchymal stem cell-derived extracellular vesicles after abdominal delivery in the experiment. Bull. Exp. Biol. Med. 2020; 169(1): 122-9. https://dx.doi.org/10.1007/s10517-020-04838-1.
Received 22.10.2020
Accepted 24.11.2020
About the Authors
Gennady T. Sukhikh, Dr. Med. Sci., Professor, Academician of the RAS, Director of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Tel.: +7(495)438-18-00. E-mail: g_sukhikh@oparina4.ru. 4, Oparina str., Moscow, 117997, Russia.Oleg G. Pekarev, Dr. Med. Sci., Professor, Deputy Director of the Institute of Obstetrics, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
E-mail: o_pekarev@oparina4.ru. ORCID: 0000-0001-7122-6830. 4, Oparina str., Moscow, 117997, Russia.
Evgenia O. Pekareva, Ph.D., Obstetrician-Gynecologist at the Novosibirsk City Clinical Perinatal Center. Tel.: +7(383)267-89-55. E-mail: pekareva_e@mail.ru.
ORCID: 0000-0002-6335-2121. 32, Lezhena str., Novosibirsk, 630090, Russia.
Igor V. Maiborodin, Dr. Med. Sci., Professor, Head Researcher of the Stem Cell Laboratory, Institute of Chemical Biology and Fundamental Medicine, SB of the RAS.
Tel.: +7(383)363-51-50. E-mail: imai@mail.ru. ORCID: 0000-0002-8182-5084. 8, Ac. Lavrentyeva str., Novosibirsk, 630090, Russia.
Denis N. Silachev, Ph.D., Head of the Cell Technologies Laboratory, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. E-mail: silachevdn@genebee.msu.ru.
ORCID: 0000-0003-0581-9755. 4, Oparina str., Moscow, 117997, Russia.
Igor I. Baranov, Dr. Med. Sci., Professor, Head of the Department of Scientific and Educational Programs, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. E-mail: i_baranov@oparina4.ru. ORCID: 0000-0002-9813-2823. 4, Oparina str., Moscow, 117997, Russia.
Ivan M. Pozdnyakov, Dr. Med. Sci., Professor, Chief Physician of Novosibirsk City Clinical Perinatal Center. Tel.: +7(383)267-93-11. ORCID: 0000-0003-0600-3053.
32, Lezhena str., Novosibirsk, 630090, Russia.
Natalya S. Bushueva, M.D., Head of the Maternity Department, Novosibirsk City Clinical Perinatal Center. ORCID: 0000-0002-1036-6925.
32, Lezhena str., Novosibirsk, 630090, Russia.
Aleksey M. Novikov, M.D., Junior Researcher at the Stem Cell Laboratory, Institute of Chemical Biology and Fundamental Medicine, SB of the RAS.
ORCID: 0000-0003-1371-7492. 8, Ac. Lavrentyeva str., Novosibirsk, 630090, Russia.
For citation: Sukhikh G.T., Pekarev O.G., Pekareva E.O., Maiborodin I.V., Silachev D.N., Baranov I.I., Pozdnyakov I.M., Bushueva N.S., Novikov A.V. Initial results of clinical application of mesenchymal stromal stem cell-derived extracellular microvesicles after abdominal delivery.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 1: 52-60 (in Russian)
https://dx.doi.org/10.18565/aig.2021.1.52-60



