Pathomorphological features of placenta in patients with different clinical phenotypes of spontaneous preterm births
Aim. Investigation of morphological features of the placenta in patients with spontaneous preterm birth depending on the clinical phenotype of labor.Remneva O.B., Kolyado O.V., Pesotskaya A.V., Starodubtsev E.G., Gumenyuk I.S.
Materials and methods. Histological and immunohistochemical examination of placenta of 93 patients with spontaneous preterm labor was performed. The expression of antibodies to type I collagen, and antibodies to the products of collagen metabolism (carboxy-terminal telopeptide of type I and propeptide of type I procollagen) was assessed. The data of the group of patients with premature rupture of membranes (PROM) (n=71) and of the group of patients with spontaneous onset of preterm labor with unruptured fetal membranes (n=22).
Results. The signs of infection predominated in the placentas of patients with PPROM compared to patients with spontaneous onset of preterm labor – 55/71(77.5%) and 11/22(50%), respectively (p=0.02). The median duration of the latency period in patients with funiculitis was 350.0±51.1 hours (95% CI: 309.8–540.9 hours). Immunohistochemistry data showed a significant decrease in the expression of propeptide of type I procollagen in fetal membranes of patients with PPROM.
Conclusion. Infection of the placentas in women with PPROM is a result of a long latency period. Prolongation of latency period for more than 14 days should be avoided because of high risk of fetal infection. Impaired processes of collagen synthesis play a significant role in the pathogenesis of PPROM.
Keywords
Preterm labour (PL) is an outcome of failed interaction in the mother-placenta-fetus system, resulting in structural and functional changes at tissue and molecular levels. Despite great attention to the problem of PL, the morphological aspect of premature birth has been understudied. The scientific search is complicated by the various clinical phenotypes of PL, among which there are preterm premature rupture of the membranes (PPROM), and spontaneous onset of labor.
Previous studies indicate a significant role of infection in the pathogenesis of PL, which is confirmed by the results of pathomorphological studies of placentas, and clinical outcomes in preterm infants [1, 2]. The placenta in case of preterm labour does not always have signs of infection, and this indicates the need of search for other causes of PL [3]. Studies devoted to qualitative changes in the structure of fetal membranes are extremely limited, and review only patients with a normal pregnancy [4, 5]. Further search for the pathomorphological causes of premature birth is extremely important for the prevention of PL. Research in this field should be performed considering clinical variants of the onset of labor.
The aim of the study was to examine the morphological features of the placenta, including the parameters of collagen metabolism in membranes, depending on the clinical phenotype of spontaneous PL (spontaneous onset of labor, and PPROM).
Materials and methods
A prospective study conducted at the Altai State Clinical Perinatal Centre from 2018 to 2020 included 93 patients with spontaneous PL at 28–33.6 weeks. Depending on the onset of labor, two groups were formed: group I – 71 patients with PPROM and delayed onset of labor; group II – 22 women with spontaneous onset of PL and intact membranes. The exclusion criteria were: multiple pregnancies, fetal malformations, somatic diseases, and severe obstetric complications. A mandatory inclusion criterion was timely registration of pregnancy (in order to get the full information about the course of pregnancy). Macro- and microscopic examination of the placenta was performed in all postpartum women. Determination of collagen metabolism in membranes was performed by immunohistochemistry (IHC). The collagen network of membranes is represented mainly by type I collagen and type III collagen. There are no synthesis and degradation markers for type III collagen. In this regard, procollagen type I carboxy-terminal propeptide (PICP) was used as a marker of collagen synthesis, and type I collagen carboxy-terminal telopeptide (CITP) as a marker of degradation. These molecules have been widely used in diagnostics of connective tissue diseases [6]. Six pieces were taken for pathomorphological examination: umbilical cord; membranes – from the rupture site, a cylinder cut at the edge of the placental disc; a fragment of the decidual plate with villi; and a fragment of the chorionic plate with villi. After standard histological manipulation in automatic mode on the semi-automatic microtome Thermo Scientific Excelsior AS (USA) the sections with thickness of 5–7 µm were obtained. The sections were stained using standard H&E method. For IHC examination by the peroxidase method, the sections were simultaneously dewaxed with antigen unmasking solution using the Autostainer IHC staining device (Thermo Fisher Scientific Inc. (USA), followed by processing with primary antibodies. Rabbit polyclonal antibodies to type I collagen and mouse polyclonal antibodies to PICP and CITP from CLOUD-CLONE CORP (USA) were used. UltraVision Quanto Detection System HRP DAB was used for imaging. Microscopy was used to assess chorionic villous maturation, compliance with gestational age, state of intervillous space, severity of compensatory-adaptive reactions, stromal and vascular lesions (thrombosis, chorangiomatosis, fetal and maternal vascular malperfusion), immune and inflammatory processes with determination of infection pathway. Microscopic photos were taken using a microscopic image fixation system: AxioLab microscope (Carl Zeiss Jena, Germany) with a digital camera (Carl Zeiss Jena, Germany), at a magnification of 400×, 100×, at least 10 fields were analyzed). In the subsequent analysis of images, random fields of view were selected (at least 12 fields for each sample without defects or artifacts), which were processed in Rawtherapee software to normalize white balance and exposure. These images were used to quantify type I collagen proteins, PICP, and CITP content using Morphostain software for morphometric analysis of spots based on the color deconvolution method. The stained area with DAB chromagen was assessed using standard IHC protocols. After analysis, the difference in protein content in the tested samples was measured.
The study was approved by the Ethics Committee of the Altai State Medical University of the Ministry of Health of the Russian Federation. All patients included in the study had signed informed consent.
Statistical analysis
The statistical software IBM SPSS Statistics 23.0 was used for data analysis. The distribution of quantitative indicators was assessed using the Shapiro–Wilk test, based on the number of participants included in group II (n=22) and on the indicators of kurtosis and asymmetry. The distribution of quantitative indicators differed from normal. Their values are presented as median, and interquartile range: Me (Q1; Q3). The Mann–Whitney U-test was used to compare quantitative variables. Qualitative variables were presented as absolute and relative values as n (%). For their comparison the Pearson's chi-square test (χ2) was used. The dependence of binary indicators (probability of outcome) on quantitative and qualitative indicators was assessed using binary logistic regression. The Kaplan–Meier method was used to assess the dependence of the time factor on the outcome. Deviations were assumed to be statistically significant at p<0.05.
 Results
Results
The median age of postpartum women of group I was 29.0 (27.5; 30.2) years, of group II – 27.0 (24.9; 30.3) years (p 0.05). The labour onset time was comparable: 32.1 (29.6; 31.5) weeks in patients with PPROM and 32.5 (30.5; 32.4) weeks in women with spontaneous onset of labor (p>0.05). The duration of latency period in group I was 107.9 (79.9; 135.9) hours.
Macroscopic examination of the placenta found that the mean placental mass and fetal/placental weight ratio (FPWR) in the comparison groups did not differ and corresponded to the norms for gestational age. The placental mass in patients with PPROM was 360 g (348.9; 383.6), FPWR was 0.25 (0.23; 0.27), in group II – 380 g (344.6; 459.9), with a similar FPWR indicator – 0.25 (0.21; 0.25). A dispersed type of placental blood supply was observed in 48/71 (67.6%) patients in group I, and in 11/22 (50%) patients in group II (p>0.05). Anomalies of umbilical cord attachment (marginal, velamentous) occurred in patients of groups I and II with the same frequency: 5/71 (7%) and 1/22 (4.5%), respectively, similar tendency was seen in anomalies of placenta development (placenta circumvallata, placenta bipartite, hypoplastic placenta) in 9/71 (12.7%) and 4/22 (18.2%) patients, respectively (p>0.05). Placental microscopy data are presented in Table 1.
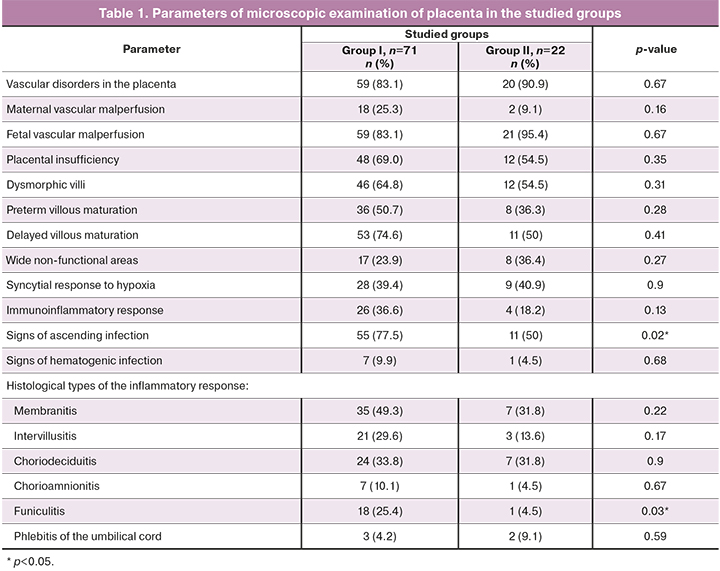
Morphological examination of the placenta revealed abnormalities in villous differentiation in more than a half of patients, manifesting with both preterm and delayed maturation. Signs of placental insufficiency (in mother and fetus) with the formation of non-functional areas were found. Assessment of involutive-dystrophic processes did not reveal significant differences between the volume of calcifications and areas of thrombosis of the intervillous space between the groups. The functional state of the placental complex was qualified as chronic subcompensated placental insufficiency in 63/71 (88.7%) patients of group I and in 19/22 (86.4%) patients of group II (p>0.05). In other cases, the signs of decompensated placental insufficiency were found. Adaptive-compensatory reactions in 57/71 (80.3%) patients of group I were estimated as weak, in 12/71 (16.9%) patients – as moderate. In group II, weak adaptive-compensatory reactions were found in 15/22 (68.1%) of patients, and moderate – in 6/22 (27,3%). There were no statistical differences in the degree of compensatory reactions between the compared groups. Signs of infection of the placenta, due to the ascending route of infection were observed significantly more often in women with PPROM than in women in group II: in 55/71 (77.5%) and in 11/22 (50%) cases, respectively. Funiculitis was found in the placenta of women with PPROM more often than in women in group II: in 18/71 (25.4%) and in 1/22 (4.5%), respectively (p=0.03); in all cases it was complicated by membranitis, confirming ascending infection. The inflammatory response of the fetus was classified as stage I in 3/19 (15.8%) patients, as stage II in 5/19 (26.3%) patients, and as stage III in 11/19 (57.9%) patients [7]. Inflammatory changes common for the hematogenic spread were equally frequent.
A binary logistic regression model was used to assess the probability of funiculitis depending on the presence of known infection risk factors, such as cervical insufficiency, chronic maternal infections (urinary and respiratory tract infections), vaginal microbiocenosis disorders (vaginitis, bacterial vaginosis), acute infections of other localizations (acute respiratory diseases, gestational pyelonephritis), and on the duration of latency period. The stepwise regression method was used to build the model. The results at the stage of inclusion of the studied factors in the analysis are presented in Table 2.
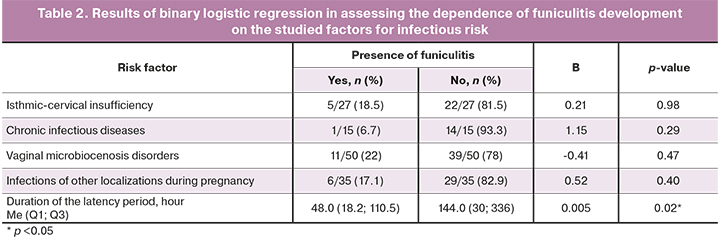
As a result of stepwise regression, only the factor of the duration of latency period was included in the logistic regression model. The dependence of funiculitis development on the duration of latency period was described by the equation p=1/(1+e-z)×100%, z=-2.05+0.005×Xl/p, where p is the probability of funiculitis development, and Xl/p is the duration of latency period (hour). The model is statistically significant (p=0.008), however based on the coefficient of determination (R=0.116), the model includes only 12% of factors that determine the probable outcome and has low sensitivity (11.6%), so it is not applicable in practice. Further search of prognostic criteria is required to improve the model.
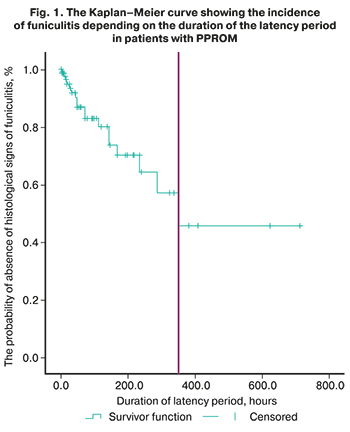
 The analysis performed by the Kaplan–Meier method among patients of group I with prolonged latency period showed that the median duration of the latency period during the subsequent verification of funiculitis was 350.0±51.1 hours (95% CI: 309.8–540.9 hours), which clinically corresponds to the time of accomplishing of antibiotic therapy in patients with PPROM. The course of antibiotic therapy, depending on the chosen treatment, was 7–10 days.
The analysis performed by the Kaplan–Meier method among patients of group I with prolonged latency period showed that the median duration of the latency period during the subsequent verification of funiculitis was 350.0±51.1 hours (95% CI: 309.8–540.9 hours), which clinically corresponds to the time of accomplishing of antibiotic therapy in patients with PPROM. The course of antibiotic therapy, depending on the chosen treatment, was 7–10 days.
In patients with latency period up to 168 hours, funiculitis was found in 9/44 women (20.2%); in patients with a latency period of 168–336 days – in 4/18 women (22.2%); in patients with a duration of latency period over 336 hours, funiculitis was found in 5/9 women (55.5%) (p=0.001). This suggests that the higher incidence of inflammatory changes in the placenta in patients of the main group is associated with ascending infection in the presence of a prolonged latency period, rather than with the initial infectious of membranes leading to PPROM.
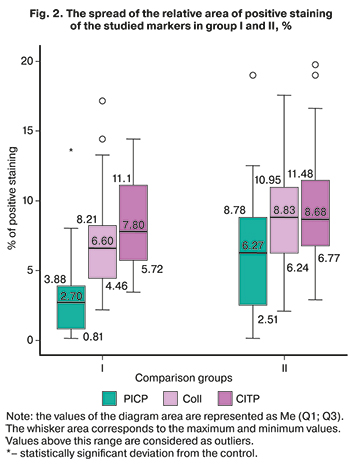
The search for qualitative changes in the structure of membranes, particularly collagen metabolism, showed that a significant decrease in the content of PICP, a propeptide involved in collagen synthesis, was found in the membranes of the women in the main group, and was worsened by a decrease in the quantity of type I collagen. The significance of the differences in type I collagen expression was close to critical (p=0.054). There were no differences in the content of the collagen degradation marker, CITP, in patients with spontaneous onset of labor, and in pregnant women with PPROM. The spread of the staining area with antibodies to PICP, type I collagen, and CITP is shown in the diagrams of Figure 2.
Microscopical shot of ruptured membranes stained with antibody to PICP (Fig. 3) show weaker staining in a patient of group I compared to a patient of group II.
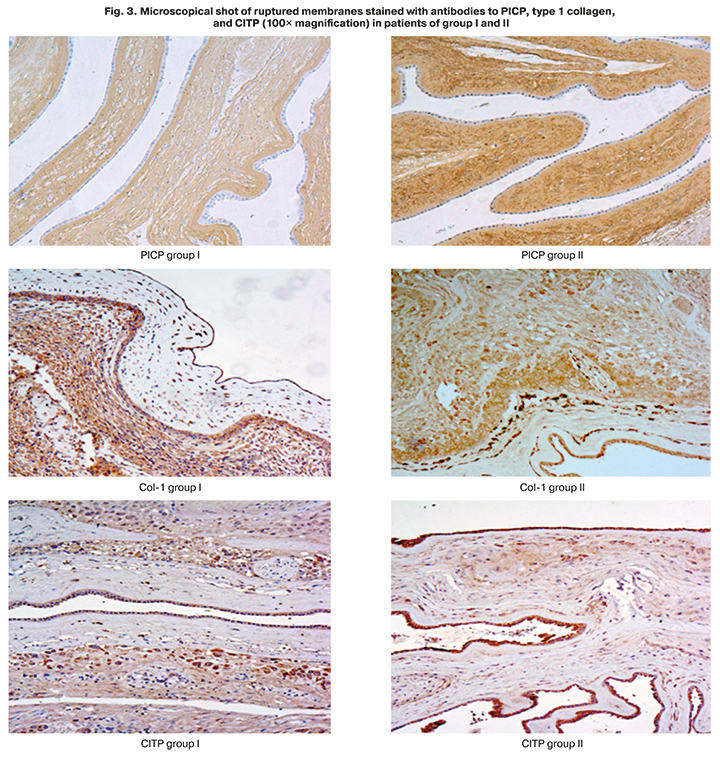
The findings demonstrate the role of pathology of the membrane structure at the level of collagen formation processes as a possible pathogenetic cause of PPROM.
Discussion
The review of the literature showed that the infectious factor is considered as the main cause of premature birth. The frequency of infectious and inflammatory changes in the placenta of studied women coincides with most of the previous studies [8–10]. A key issue in practical obstetrics is the duration of the latency period before the onset of chorionamnionitis. This knowledge will optimize the expectant management in case of preterm labour. In a study by Park et al., 156 patients with preterm labour and PPROM were examined. At the time of membrane rupture, the patients had no clinical, laboratory or bacteriological signs of intraamniotic infection, as confirmed by examination of amniotic fluid sampled during amniocentesis [11]. In patients with PPROM and histological verification of chorionamnionitis, the median latency period was 131.3 hours, which was less than in our study (350+51.1 hours); however, the study by Park et al did not indicate whether the patients had received antibiotic therapy during expectant management or not. We did not find any other studies of histological verification of the optimal latency period. The data on the structural changes of membranes during premature rupture in a full-term pregnancy were obtained by Bolotskikh N.M. [5]. According to this study conclusions, activation of membrane type 1 matrix metalloproteinase and reduction of its inhibitor activity play a significant role in the pathogenesis of PPROM in full-term pregnancy, which leads to collagen destruction and rupture of the membrane. The study of Sukhikh G.T. et al. proved that in addition to membrane type 1 matrix metalloproteinase, matrix metalloproteinase-9 also acts as an inducer of collagenolysis in full-term pregnancy [4]. Therefore, the majority of scientific literature reports a predominance of collagen degradation processes in patients with premature rupture of membranes. However, all studies have considered only full-term pregnancy. In our research, we studied the PPROM in preterm labour, where other mechanisms may be involved, and initially damaged membrane is assumed.
Conclusion
The study demonstrates that different variants of spontaneous PL have different pathogenetic mechanisms. The key cause of premature birth in all PL variants is chronic placental dysfunction, which is associated with infection in 50% of cases. In case of PPROM, the initial structural pathology of membrane plays a crucial role, contributing to preterm rupture of membranes. This problem requires more detailed studies for prevention of PL.
References
- Torchin H., Ancel P.Y. Epidemiology and risk factors of preterm birth. J. Gynecol. Obstet. Biol. Reprod. 2016; 45(10): 1213-30. https://dx.doi.org/10.1016/j.jgyn.2016.09.013.
- Nijman T.A., van Vliet E.O., Benders M.J., Mol B.W., Franx A., Nikkels P.G., Oudijk M.A. Placental histology in spontaneous and indicated preterm birth: A case control study. Placenta. 2016; 48: 56-62. https://dx.doi.org/10.1016/j.placenta.2016.10.006.
- Morgan T.K. Role of the placenta in preterm birth: a review. Am. J. Perinatol. 2016; 33(3): 258-66. https://dx.doi.org/10.1055/s-0035-1570379.
- Sukhikh G.T., Kan N.E., Tyutyunnik V.L., Sannikova M.V., Dubova E.A., Pavlov K.A., Amiraslanov E.Y., Dolgushina N.V. The role of extracellular inducer of matrix metalloproteinases in premature rupture of membranes. J. Matern. Fetal Neonatal Med. 2016;29(4):656-9. https://dx.doi.org/10.3109/14767058.2015.1015416.
- Болотских В.М. Современные представления об этиологии и патогенезе преждевременного излития околоплодных вод. Журнал акушерства и женских болезней. 2011; 60(2): 3-13. [Bolotskikh V.M. Current concept of the etiology and pathogenesis of premature rupture of membranes. Journal of obstetrics and women’s diseases. 2011; 2. (in Russian)]. URL: https://cyberleninka.ru/article/n/sovremennye-predstavleniya-ob-etiologii-i-patogeneze-prezhdevremennogo-izlitiya-okoloplodnyh-vod.
- Щеголев А.И., Серов В.Н. Клиническая значимость поражений плаценты. Акушерство и гинекология. 2019; 3: 54-62. [Shchegolev A.I., Serov V.N. Clinical significance of placental lesions. Akusherstvo i Gynekologiya/ Obstetrics and Gynecology. 2019; 3: 54-62. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.3.54-62.
- Lofsjogard J., Kahan T., Diez J., Lopez B., Gonzalez A., Ravassa S. et al. Usefulness of collagen carboxy-terminal propeptide and telopeptide to predict disturbances of long-term mortality in patients ≥60 years with heart failure and reduced ejection fraction. Am. J. Cardiol. 2017; 119(12): 2042-8. https://dx.doi.org/10.1016/j.amjcard.2017.03.036.
- Курносенко И.В., Долгушина В.Ф., Пастернак А.Е. Воспалительные изменения в последе у женщин с преждевременными и своевременными родами. Современные проблемы науки и образования. 2016; 3: 172. [Kurnosenko I.V., Dolgushina V.F., Pasternak A.E. Inflammatory changes in the placenta in women with preterm and timely delivery. Modern problems of science and education. 2016; 3. (in Russian)]. URL: http://science-education.ru/ru/article/view?id=24802.
- Малышкина А.И., Назарова А.О., Кулида Л.В., Козырина А.А., Жолобов Ю.Н., Назаров С.Б. Патоморфологические особенности плацент у женщин с преждевременными родами в зависимости от срока гестации. Акушерство, гинекология и репродукция. 2017; 11(4): 23-9. [Malyshkina A.I., Nazarova A.O., Kulida L.V., Kozyrina A.A., Zholobov Yu.N., Nazarov S.B. Pathomorpholy of placenta in women with preterm births at different age of gestation. Obstetrics, Gynecology and Reproduction. 2017; 11(4): 23-9. (in Russian)]. https://dx.doi.org/10.17749/2313-7347.2017.11.4.023-029.
- Chisholm K.M., Norton M.E., Penn A.A., Heerema-McKenney A. Classification of preterm birth with placental correlates. Pediatr. Dev. Pathol. 2018; 21(6): 548-60. https://dx.doi.org/10.1177/1093526618775958.
- Park C.W., Park J.S., Norwitz E.R., Moon K.C., Jun J.K., Yoon B.H. Timing of histologic progression from chorio-deciduitis to chorio-deciduo-amnionitis in the setting of preterm labor and preterm premature rupture of membranes with sterile amniotic fluid. PLoS One. 2015; 10(11): e0143023. https://dx.doi.org/10.1371/journal.pone.0143023.
Received 16.04.2021
Accepted 24.05.2021
About the Authors
Olga V. Remneva (Corresponding author), Dr. Med. Sci., Professor, Head of the Department of Obstetrics, Gynecology and Postgraduate education,Altai State Medical University, Ministry of Health of Russia, Barnaul, Russia. E-mail: rolmed@yandex.ru. ORCID: 0000-0002-5984-1109.
656038, Russia, Barnaul, Lenin Ave., 40.
Olga V. Kolyado, postgraduate student, Department of the Obstetrics, Gynecology and Postgraduate education, Altai State Medical University, Ministry of Health of Russia, Barnaul, Russia. E-mail: kolyado.ov@gmail.com. ORCID: 0000-0002-5812-4925. 656038, Russia, Barnaul, Lenin Ave., 40.
Anastasiya V. Pesotskaya, Head of the Pathological Laboratory with the Department of Immunohistochemistry, Altai State Medical University, Ministry of Health of Russia, Barnaul, Russia. E-mail: pesokpesotskaya@yandex.ru. ORCID: 0000-0002-4625-0916. 656045, Russia, Barnaul, Fomina str., 154.
Evgeniy G. Starodubtsev, PhD, Pathological Laboratory with the Department of Immunohistochemistry, Altai State Medical University, Ministry of Health of Russia,
Barnaul, Russia. E-mail: evstar35@mail.ru. ORCID: 0000-0002-1053-3834. 656045, Russia, Barnaul, Fomina str., 154.
Ivan S. Gumenyuk, assistant of the Department of Patologic Anatomy, Kuban State Medical University, Ministry of Health of Russia, Krasnodar, Russia.
E-mail: meklon@gmail.com. ORCID: 0000-0001-5289-2344. 350063, Russia, Krasnodar, M. Sedina str., 4.
For citation: Remneva O.B., Kolyado O.V., Pesotskaya A.V., Starodubtsev E.G., Gumenyuk I.S. Pathomorphological features of placenta in patients with different clinical phenotypes of spontaneous preterm births.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 8: 111-118 (in Russian)
https://dx.doi.org/10.18565/aig.2021.8.111-118



