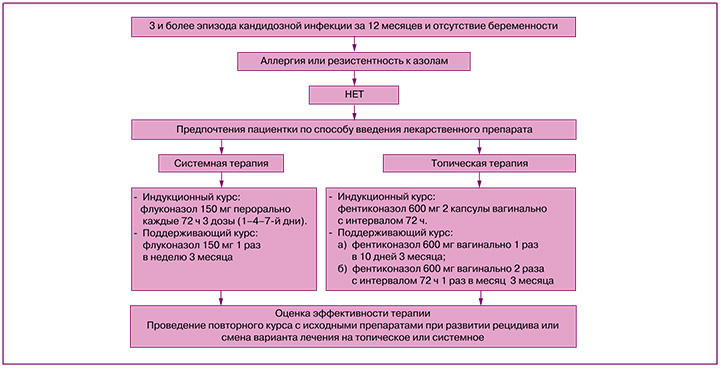
Рецидивирующий вульвовагинальный кандидоз (РВВК) представляет собой заболевание, вызываемое представителями грибов рода Candida (С. albicans и C. non-albicans) при условии наличия 3 и более эпизодов симптомной инфекции в течение одного года [1–3]. Чаще всего рецидив заболевания обусловлен реактивацией грибковой инфекции и самозаражением из резервуаров влагалища и кишечника.
В настоящее время отсутствует единый стандарт оказания медицинской помощи пациенткам с РВВК, однако большинство авторов рекомендуют применять индукционный и поддерживающий курсы лечения. Целью терапии является улучшение качества жизни женщины за счет купирования симптомов воспаления и предотвращения развития рецидивов [1, 4, 5]. Индукционная терапия включает применение препаратов системно или интравагинально, выбор определяется доступностью лекарственного средства и предпочтениями пациентки [6–8]. Необходимо учитывать, что эффективность индукционного курса очень высока, однако без поддерживающего лечения частота рецидивов может достигать 50–55% в течение последующих 6–12 месяцев [9]. Из системных препаратов используется флуконазол 150 мг каждые 72 ч (всего 3 дозы) с последующим переходом на поддерживающую терапию по 150 мг перорально 1 раз в неделю в течение 6 месяцев [9].
Индукционное лечение топическими препаратами включает клотримазол (вагинальная таблетка 100 мг 1 раз в сутки 7 дней), миконазол (суппозитории 100 мг 7 дней), бутоконазол (2% крем 5 г однократно) или итраконазол (вагинальная таблетка 200 мг 10 дней). Поддерживающая терапия проводится в течение 6 месяцев препаратами натамицина (суппозитории 100 мг 1 раз в неделю) или клотримазола (вагинальная таблетка 500 мг 1 раз в неделю) [10]. Симптоматика полностью купируется у 90% пациенток, но частота рецидива в течение последующих 6–12 месяцев достигает 50% [9, 11].
Постоянное развитие науки обусловливает появление на фармацевтическом рынке новых, более совершенных лекарственных средств. Практическая оценка их эффективности в терапии острых и хронических рецидивирующих заболеваний является крайне важной и актуальной. Одним из таких препаратов является фентиконазола нитрат, представляющий собой производное имидазола с широким спектром антимикотической (фунгистатической и фунгицидной) и антибактериальной (Streptococcus spp., Staphylococcus aureus, Trichomonas vaginalis, Malassezia furfur, Trichophyton spp., Microsporum spp., Epidermophyton floccosum, Bacillus subtilis и Gardnerella vaginalis) активности [12]. Фентиконазол нарушает структуру клеточной мембраны гриба и провоцирует его саморазрушение за счет ингибирования синтеза ферментов, отвечающих за их прикрепление к поверхности эпителиальных клеток. Фунгицидный механизм действия связан с ингибированием биосинтеза эргостерола, изменяющего проницаемость клеточной стенки грибов, приводя к ее разрушению [12].
На кафедре акушерства и гинекологии лечебного факультета РНИМУ им. Н.И. Пирогова под руководством профессора Доброхотовой Ю.Э. было проведено пилотное исследование по оценке эффективности применения фентиконазола у пациенток с РВВК [4].
Цель: изучение эффективности продленного курса местной терапии фентиконазолом для профилактики рецидива кандидозного вульвовагинита у пациенток с его хроническим рецидивирующим течением и оценка безрецидивного течения болезни в течение 6 последующих месяцев.
Материалы и методы
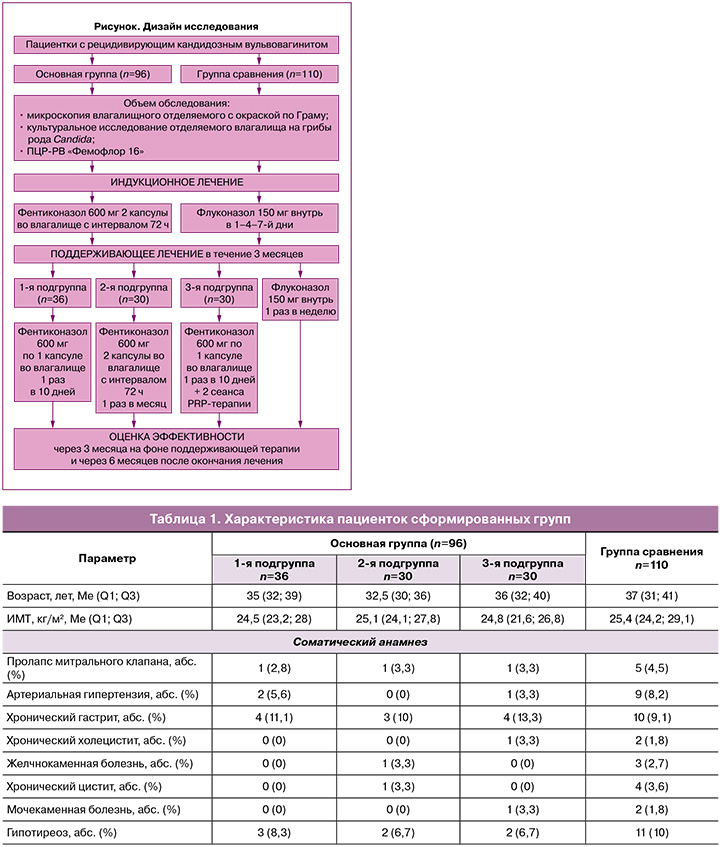
В исследование были включены 206 пациенток с РВВК, вызванным Candida albicans. В зависимости от проводимого лечения были сформированы основная группа (n=96) и группа сравнения (n=110). В основной группе женщины проходили индукционный и поддерживающий курсы с топическим введением фентиконазола. Индукционный курс был идентичен во всех случаях и включал применение двух капсул фентиконазола 600 мг интравагинально с интервалом 72 ч. Поддерживающее лечение было различным. В 1-й подгруппе основной группы (n=36) терапия проводилась фентиконазолом 600 мг интравагинально 1 раз в 10 дней в течение 3 месяцев. Во 2-й подгруппе основной группы (n=30) – по 600 мг 2 раза с интервалом 72 ч ежемесячно в течение 3 месяцев. Время для повторного введения фентиконазола определялось календарно (ровно через месяц после предыдущего введения) или при появлении дискомфорта и зуда во влагалище. Для уточнения причин дискомфорта пациентки использовали тест Frautest Candida, положительный результат которого свидетельствовал об обострении грибковой инфекции.
В 3-й подгруппе основной группы (n=30) лечение включало комбинацию фентиконазола 600 мг интравагинально 1 раз в 10 дней с курсом терапии плазмой, обогащенной тромбоцитами (Platelet Rich Plasma, PRP), во влагалище. Лечение PRP выполнялось 2 раза с интервалом 1,5 месяца, вне периода введения фентиконазола. Использовались синие пробирки «Реген Лаб СА» (Швейцария). Перед процедурой проводился забор крови из кубитальной вены в пробирки с антикоагулянтом и сепарирующим гелем с последующим центрифугированием. Полученная плазма методом линейно-ретроградной веерной техники с использованием иглы G30 вводилась в подслизистый слой стенок влагалища.
Группа сравнения включала 110 женщин с РВВК, получавших системное лечение флуконазолом (индукционный курс 150 мг перорально в 1–4–7-й дни, далее 150 мг 1 раз в неделю 3 месяца).
После окончания лечения наблюдение продолжалось в течение 6 месяцев с оценкой частоты рецидивов заболевания и качества жизни женщин (опросник SF-36).
Критерии включения в основную группу и группу сравнения: добровольное информированное согласие пациентки на участие в исследовании, возраст от 20 до 45 лет включительно, РВВК, вызванный Candida albicans (3 и более эпизодов в год), согласие на использование контрацепции в ходе всего исследования.
Критерии исключения: наличие других заболеваний вульвы или влагалища, которые могут затруднить интерпретацию результатов исследования, РВВК, вызванный Candida non-albicans (С. glabrata, С. crusei) или Candida albicans, резистентной к азолам, беременность, лактация или планируемая беременность в период исследования, гиперчувствительность к компонентам флуконазола и фентиконазола, необходимость в приеме антибиотиков во время участия в исследовании, прием иммунодепрессантов, системных и топических глюкокортикостероидов в процессе исследования, тяжелые заболевания эндокринной системы (сахарный диабет в стадии декомпенсации), печени (цирроз печени, хронический гепатит) и органов желудочно-кишечного тракта (болезнь Крона, язвенный колит).
Для проведения микроскопического исследования забор материала проводился из заднего свода влагалища стерильным ватным тампоном, образец наносился на сухое предметное стекло с последующим стандартным окрашиванием по Граму. Культуральное исследование для видовой идентификации грибов выполнялось с использованием угольной транспортной среды и посева на питательную среду Сабуро. Микроскопическое и культуральное исследования выполнялись однократно на этапе включения в исследование.
Изучение влагалищной микробиоты проводили с использованием молекулярно-биологического метода полимеразной цепной реакции с детекцией результатов в режиме реального времени (ПЦР-РВ) при помощи тест-системы «Фемофлор-16» (ООО «НПО ДНК-Технология») в лаборатории «Гемотест». Оценка биоценоза выполнялась на этапе включения в исследование, через 3 месяца на фоне поддерживающей терапии и через 6 месяцев после окончания поддерживающей терапии.
Оценка эффективности терапии проводилась на основании субъективных ощущений (заполнение опросника SF-36), лабораторных данных (ПЦР-РВ «Фемофлор-16») и частоты рецидивов на фоне и после окончания лечения. Длительность терапии составила 3 месяца, длительность последующего наблюдения – 6 месяцев.
Дизайн исследования представлен на рисунке.
Статистический анализ
Статистический анализ результатов исследования выполнен с использованием программы Statistica 10 (StatSoft, Russia). Данные с асимметричным распределением анализировались с помощью непараметрических тестов. При распределении параметров, отличных от нормального, результаты представлены в виде медианы (Ме), 25% и 75% процентилей (Q1 и Q3), для определения значимости различий применены непараметрические критерии. При межгрупповом сравнении использован критерий Краскела–Уоллиса с определением одного общего значения р для всех групп. Затем для сравнений с положительным результатом (р≤0,05) проводились попарные сравнения групп для уточнения, между какими именно группами имеются различия, с помощью критерия Манна–Уитни с поправкой Бонферрони. Сравнение количественных данных в двух несвязанных группах выполнено с применением U-критерия Манна–Уитни. Для показателей, характеризующих качественные признаки, указывали абсолютное значение и относительную величину в процентах, проверку статистических гипотез о совпадении наблюдаемых и ожидаемых частот осуществляли с использованием точного критерия Фишера. Различия считали статистически значимыми при уровне критерия менее 0,05.
Результаты
Возраст пациенток сформированных групп составил в среднем 35 лет в 1-й подгруппе, 32,5 и 36 лет – во 2-й и 3-й подгруппах, 37 лет – в группе сравнения. Межгрупповых различий по возрасту и индексу массы тела (ИМТ) выявлено не было (табл. 1).
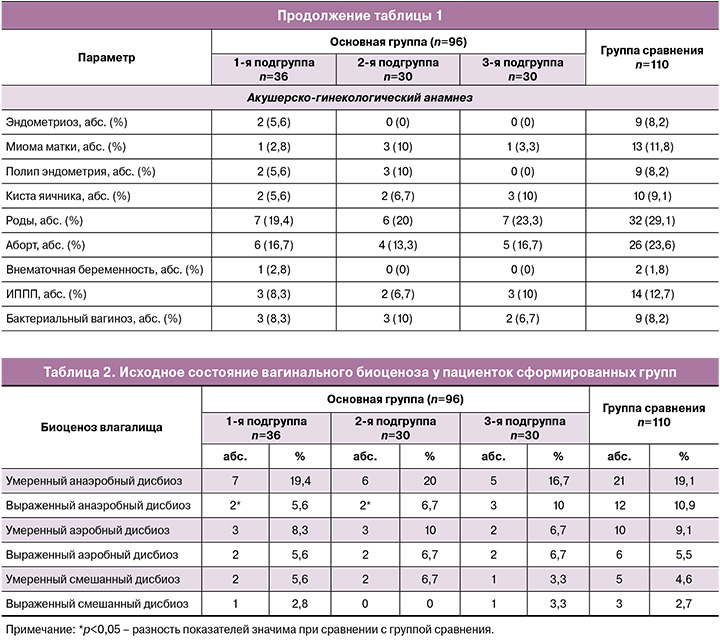
В структуре экстрагенитальных заболеваний в сравниваемых группах значимых различий выявлено не было. Наиболее распространенными патологиями были хронический гастрит (11,1% (4/36), 10% (3/30), 13,3% (4/30) и 9,1% (10/110) соответственно) и гипотиреоз (8,3% (3/36), 6,7% (2/30), 6,7% (2/30) и 10% (11/110) соответственно), а в структуре гинекологических заболеваний в анамнезе инфекции, передающиеся половым путем (ИППП), выявлялись у 8,8% (3/36), 6,7% (2/30), 10% (3/30) и 12,7% (14/110) пациенток соответственно. Значимых различий по гинекологическим заболеваниям выявлено не было.
Исследование вагинального микробиоценоза показало, что РВВК часто сопровождается дисбиозом влагалища (табл. 2). У 47,2% (17/36) пациенток 1-й подгруппы, 50% (15/30) – 2-й, 46,7% (14/30) – 3-й подгруппы и у 51,8% (57/110) женщин группы сравнения имелся сопутствующий дисбиоз влагалища. При межгрупповом сравнении значимо чаще он диагностировался у пациенток группы сравнения относительно 1-й и 2-й подгрупп основной группы (р=0,04).
Условный нормоценоз с доминированием лактобацилл (более 80%) и наличием грибов Candida spp. ≥104 ГЭ/мл выявлялся у 19/36 (52,8%), 15/30 (50%) и 16/30 (53,3%) пациенток подгрупп основной группы и у 53/110 (48,2%) женщин группы сравнения (р≥0,05).
По частоте встречаемости типы нарушений влагалищного биоценоза не имели значимых различий между группами. Наиболее часто выявлялся умеренный анаэробный дисбиоз – у 19,4% (7/36), 20% (6/30), 16,7% (5/30) и 19,1% (21/110) пациенток соответственно (р=0,69).
Выраженный анаэробный дисбиоз значимо чаще встречался у пациенток группы сравнения, чем в 1-й и 2-й подгруппах основной группы (р=0,04). Его наличие потребовало проведения в группе сравнения дополнительного лечения с применением метронидазола (0,75% гель 5 г во влагалище 5 дней). В 1-й подгруппе дополнительные препараты не назначались, так как спектр активности фентиконазола перекрывал чувствительность выявленных бактерий.
Умеренный аэробный дисбиоз был диагностирован у 8,3% (3/36), 10% (3/30), 6,7% (2/30) и 9,1% (10/110) пациенток соответственно. Выраженный аэробный дисбиоз (доля лактобацилл в составе биоценоза менее 20%) суммарно выявлялся у 5–6% пациенток в каждой группе и по частоте не имел значимых различий. При диагностике аэробного вагинита пациенткам группы сравнения дополнительно назначался клиндамицин (вагинальные суппозитории 100 мг в течение 5 дней). В основной группе дополнительная терапия не проводилась в связи с эффективностью местного применения фентиконазола в отношении выявленных микроорганизмов.
У пациенток сформированных групп смешанный дисбиоз встречался редко и по частоте не имел значимых различий. При смешанном дисбиозе в группе сравнения дополнительно назначался клиндамицин (суппозитории 100 мг в течение 5 дней). В основной группе дополнительная терапия не требовалась в связи с эффективностью фентиконазола.
После окончания 3-месячного курса поддерживающей терапии исследование вагинального микробиоценоза выявило, что абсолютный нормоценоз, характеризующийся доминированием лактобацилл (более 80% общей бактериальной массы) и минимальным (менее 104 ГЭ/мл) количеством или полным отсутствием ассоциантов микробиоценоза (Ureaplasma spp., Mycoplasma hominis, Candida spp.), присутствовал у 26/36 (72,2%), 21/30 (70%) и 23/30 (76,7%) пациенток основной группы и у 77/110 (70%) женщин группы сравнения (при межгрупповом сравнении р≥0,05).
У 27,8% (10/36) пациенток 1-й подгруппы, 30% (9/30) – 2-й, 23,3% (7/30) – 3-й подгруппы и у 30% (33/110) женщин группы сравнения сохранялись изменения во влагалищной флоре (табл. 3). Все изменения носили умеренный характер, выраженного анаэробного, аэробного и смешанного дисбиоза обнаружено не было.
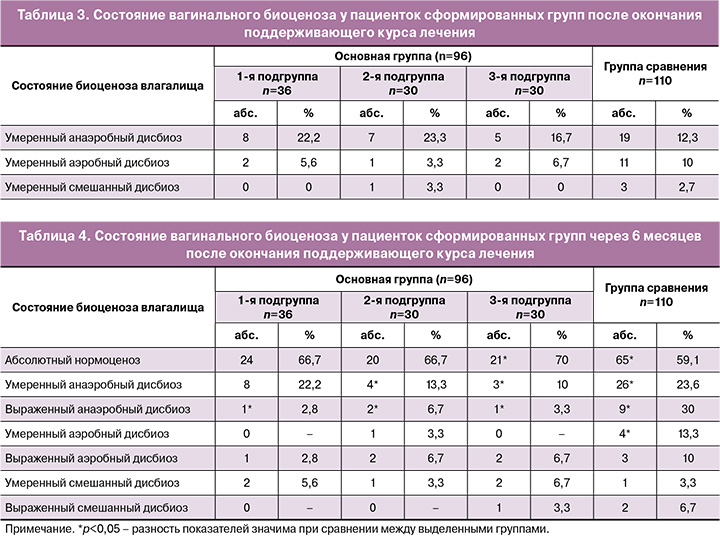
Межгрупповых различий по частоте дисбиотических состояний после окончания терапии не выявлено (p=0,59). Всем пациенткам с сохраняющимися изменениями микрофлоры влагалища был назначен курс эубиотиков (лиофилизированная культура лактобактерий L. casei rhamnosus Doderleini) по 1 капсуле вагинально в течение 14 дней.
Через 6 месяцев после окончания терапии было выполнено финальное исследование вагинального микробиоценоза. Абсолютный нормоценоз отмечался у 24/36 (66,7%), 20/30 (66,7%) и 21/30 (70%) пациенток основной группы и у 65/110 (59,1%) женщин группы сравнения (при сравнении 3-й подгруппы и группы сравнения р=0,038). Было выявлено, что в группе сравнения, где терапия проводилась изолированно антимикотиком, в последующем чаще выявлялись нарушения микробиоценоза влагалища.
У 33,3% (12/36) пациенток 1-й подгруппы, 33,3% (10/30) – 2-й, 30% (9/30) – 3-й подгруппы и у 40,9% (45/110) женщин группы сравнения сохранялись изменения во влагалищной флоре (табл. 4).
Через 6 месяцев после окончания терапии примерно у 40% пациенток выявлялись нарушения влагалищного биоценоза. Чаще всего нарушения носили умеренный характер с преобладанием анаэробной флоры и значимо чаще отмечались в группе сравнения (p=0,039). Выраженный анаэробный дисбиоз был диагностирован у 30% женщин группы сравнения (p=0,041).
Аэробный и смешанный дисбиоз встречались крайне редко, по их частоте не имелось значимых различий между группами.
У пациенток основной группы, которым была проведена местная терапия фентиконазолом, до лечения частота дисбиотических расстройств была 47,9% (46/96), через 3 месяца от начала лечения она составила 27,1% (26/96), а через 6 месяцев без лечения – 32,3% (31/96). При сравнении в динамике было выявлено значимое снижение частоты развития влагалищного дисбиоза после продленного курса применения фентиконазола (р=0,032).
В группе сравнения исходно нарушение микробиоценоза было диагностировано у 51,8% (57/110) пациенток, через 3 месяца поддерживающей терапии флуконазолом – у 30% (33/110), а через 6 месяцев без лечения – уже у 40,9% (45/110).
Ожидаемо, что при сочетании условно-патогенной бактериальной флоры с грибами рода Candida изолированная антимикотическая терапия может быть менее эффективной.
На фоне проводимой в течение 3 месяцев терапии частота рецидивов кандидозного вульвовагинита не имела значимых различий между группами и составила 9/96 (9,4%) при топическом и 6/110 (5,5%) – при пероральном лечении (табл. 5).
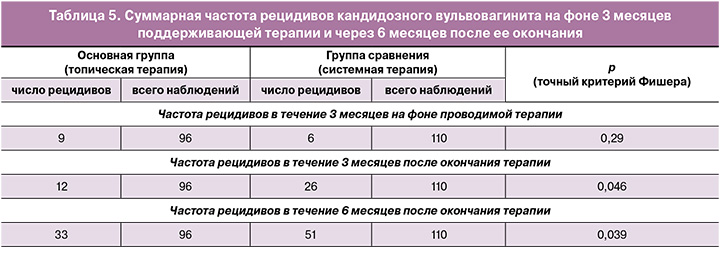
После индукционного курса к концу поддерживающей терапии (первые 3 месяца лечения) показатели микологического излечения составили 90,6% (87/96) и 94,5% (104/110) в группе фентиконазола и в группе флуконазола соответственно (p>0,05).
На фоне поддерживающей терапии рецидив в группе топического лечения развивался в 11,1% (4/36), 10% (3/30) и 6,7% (2/30) случаев соответственно подгруппам и суммарно составил 9,4% (9/96). В группе перорального применения флуконазола частота рецидивов на фоне лечения составила 5,5% (6/110) и не имела значимых отличий по сравнению с основной группой (p≥0,05). В случае рецидива ВВК пациентки возобновляли курс исходно назначенного лечения.
При динамическом наблюдении выявлено, что частота рецидивов была значимо выше в группе сравнения как через 3, так и через 6 месяцев после окончания поддерживающего курса лечения.
После 3 месяцев поддерживающей терапии частота рецидивов в группе с топическим применением фентиконазола составила 12,5% (12/96), а в группе с системной терапией флуконазолом – 23,6% (26/110) (p=0,046).
При анализе частоты рецидивов в основной группе не было отмечено различий между подгруппами, то есть эффективность продленного курса применения фентиконазола, вне зависимости от кратности введения препарата, оказалась одинаковой. Также дополнительная терапия PRP не улучшила показатели частоты рецидивов как на фоне лечения, так и в течение последующих 3 месяцев наблюдения.
Через 6 месяцев после окончания поддерживающего курса частота рецидивов также была выше в группе сравнения. В основной группе рецидив ВВК отмечался у 34,3% (33/96) пациенток, а в группе сравнения – почти в каждом втором случае, у 46,4% (51/110) соответственно (p=0,039).
После окончания индукционного курса, на фоне поддерживающей терапии пациентки заполняли опросник самооценки качества жизни SF-36. Сравнительный анализ полученных результатов представлен в таблице 6.
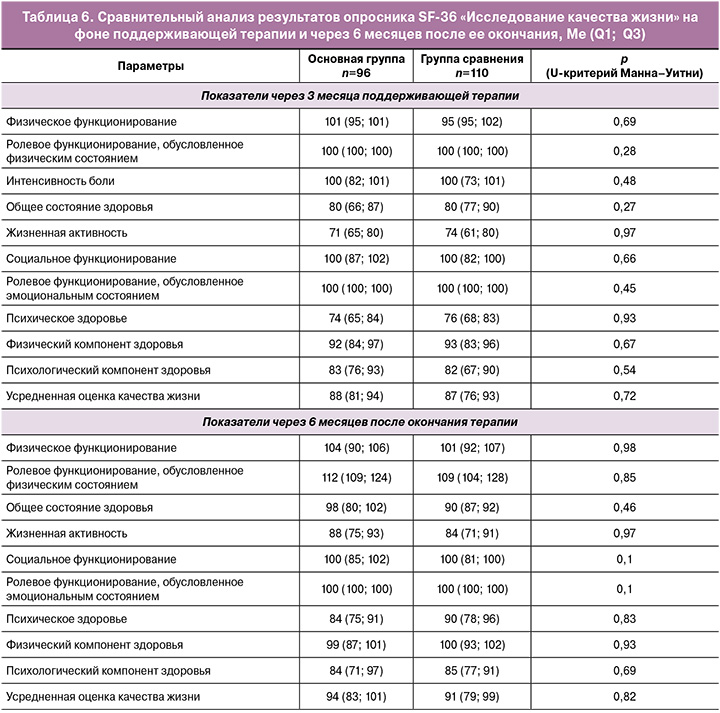
Показатели интенсивности боли и ее влияния на способность заниматься повседневной деятельностью, включая работу по дому и вне дома, свидетельствовали о том, что боль не ограничивала активность пациенток исследуемых групп. В основной группе и группе сравнения соответствующий показатель равнялся 100 баллам (p=0,48). При оценке общего состояния здоровья также не было выявлено значимых различий (p=0,27). Оценка жизненной активности у пациенток в основной группе была несколько ниже и составила 71 балл, в группе сравнения – 74 балла (p=0,97).
Показатели социального функционирования, определяемого степенью, в которой физическое или эмоциональное состояние ограничивало бы социальную активность, и ролевого функционирования, обусловленного эмоциональным состоянием, в которой эмоциональное состояние мешало бы выполнению работы или другой повседневной деятельности, в исследуемых группах составили 100 баллов на фоне лечения и порядка 110 баллов через 6 месяцев без терапии.
Полученные результаты свидетельствуют об отсутствии ограничения социальных контактов, уровня общения, выполнения повседневной работы, связанного с физическим и эмоциональным состоянием как на фоне лечения (индукционного и поддерживающего курсов), так и в течение последующих 6 месяцев наблюдения. Показатель психического здоровья, характеризующий настроение, отсутствие депрессивных, тревожных переживаний и психического неблагополучия, не различался в обеих группах как на фоне лечения, так и через 6 месяцев после его окончания.
Физический компонент здоровья, включающий шкалы физического функционирования, ролевого функционирования, обусловленного физическим состоянием, интенсивности боли, общее состояние здоровья, в основной группе составил 92 балла, в группе сравнения – 93 балла (р=0,67). Через 6 месяцев после окончания терапии он возрос до 99 и 100 баллов соответственно (р=0,93).
Психологический компонент здоровья исследуемых групп, включающий психическое здоровье, ролевое функционирование, обусловленное эмоциональным состоянием, социальное функционирование и жизненную активность, составил 83 и 82 балла соответственно (p=0,54). Через 6 месяцев он возрос до 84 и 85 баллов (p=0,69). Усредненная оценка качества жизни в группах не имела отличий и составила 88 и 87 баллов (p=0,72), через 6 месяцев показатель увеличился до 94 и 91 баллов соответственно (p=0,82).
Обсуждение
Суммарная частота РВВК достигает 5% [5]. В глобальном масштабе ежегодно он выявляется у 138 млн женщин (от 103 до 172 млн), с распространенностью примерно 3871 случай на 100 000 женщин [13].
Рецидивирующее течение заболевания значительно снижает качество жизни женщин, приводя к невозможности осуществления обычной повседневной деятельности [14]. Эффективность терапии грибковой инфекции достаточно высока, однако без применения поддерживающего лечения частота рецидивов в течение 6–12 последующих месяцев может достигать 50% [9, 11].
Систематические обзоры показали, что пероральное и интравагинальное лечение одинаково эффективно у женщин с РВВК [8, 15]. Рекомендуемые в настоящее время схемы терапии РВВК направлены на подавление симптомов и предупреждение развития рецидива инфекции [8, 16]. Однако необходимо учитывать частое сочетание грибковой инфекции с дисбиозом влагалища. Условно-патогенные микроорганизмы, формируя биопленки, способствуют поддержанию рецидивирующего характера течения вагинита и снижению эффективности системной и местной терапии.
В проведенном нами исследовании было выявлено, что практически у 50% женщин с РВВК присутствуют различные нарушения вагинального биоценоза. В исследовании Sanguinetti M. et al. (2019) была продемонстрирована высокая эффективность фентиконазола в отношении Staph. aureus, E. coli, S. agalactiae, C. albicans, C. non-albicans и G. vaginalis [17]. Показатель излечения составлял 85% при вагините бактериального происхождения и 97,5% – при вагините, вызванном Candida albicans [18]. Учитывая эти данные, в нашем исследовании пациенткам основной группы при выявлении дисбиозов мы не назначали дополнительных препаратов в связи с широким спектром антибактериальной активности фентиконазола. В то же время в группе пероральной терапии флуконазолом потребовалось дополнительное назначение метронидазола или клиндамицина.
В метаанализе, включившем 3 исследования и 106 пациенток с РВВК, не было выявлено различий в частоте клинически значимых рецидивов у пациенток, получавших системное и местное лечение. К концу 6-го месяца терапии пероральными или местными азолами рецидивы развивались у 21 и 12% (ОР 1,66; 95% ДИ 0,83–3,31), а через 12 месяцев – у 47 и 49% (ОР 0,95; 95% ДИ 0,71–1,27) пациенток соответственно [6].
В нашем исследовании в группе пациенток с системным применением флуконазола эффективность поддерживающей терапии составила 94,6%, а после окончания терапии эффект сохранялся в течение последующих 3 месяцев у 76,4%, 6 месяцев – у 53,6% пациенток. Суммарная частота рецидивов во время проведения работы составила 51,9%, при этом на фоне применения флуконазола она была 5,5%, а после прекращения лечения – 46,4%.
Местное лечение фентиконазолом было эффективным у 92,7% пациенток, и эффект сохранялся в течение последующих 3 месяцев у 87,5%, 6 месяцев – у 65,6%. В основной группе рецидив ВВК после прекращения лечения отмечался у 34,3% пациенток – значимо реже, чем в группе сравнения.
В исследовании Sobel J.D. после местной продленной терапии клотримазолом частота рецидивов достигала 38% (18/48) [16]. В нашем исследовании суммарная частота рецидивов в группе топического применения фентиконазола составила 19,8%. На фоне терапии препаратом рецидивы ВВК развивались у 7,3%, а в течение последующих 3 месяцев без лечения – у 12,5% пациенток.
Таким образом, в нашем исследовании были получены сопоставимые результаты, свидетельствующие о высокой эффективности локального применения фентиконазола при РВВК как на этапе индукционной, так и поддерживающей терапии.
В последнее время увеличилась частота выявления сочетанных инфекций, что в ряде случаев требует применения многокомпонентной терапии, снижает общие показатели эффективности лечения и повышает его стоимость. В нашем исследовании не было выявлено значимых различий в оценке качества жизни пациенток на фоне поддерживающей системной и местной терапии рецидивирующей кандидозной инфекции. Поэтому выбор схемы лечения может основываться на предпочтении пациентки и удобстве для нее.
По результатам исследования было показано, что поддерживающая терапия РВВК одинаково эффективна при использовании топических азолов (фентиконазола) и системных азолов (флуконазола).
Частота рецидивов в течение 6 месяцев после окончания поддерживающей терапии была значимо ниже после местного лечения фентиконазолом. Показатель микологического излечения РВВК, вызванного C. albicans, составил 65,6% (63/96) и 53,6% (59/110) в двух группах соответственно.
Заключение
Таким образом, назначение индукционного и продленного курсов лечения фентиконазолом может быть приемлемой альтернативой системной терапии, а дополнительные антибактериальные свойства препарата позволят избежать полипрагмазии и удорожания лечения (Приложение).
Приложение
Терапия пациенток с рецидивирующим вульвовагинальным кандидозом