Практически сразу после выхода гормональных контрацептивов на рынок появились сообщения об их связи с повышенным риском развития венозной тромбоэмболии. Первый случай был зарегистрирован в 1961 г. у 40-летней женщины, которая получала первый зарегистрированный гормональный контрацептив «Эновид» для лечения эндометриоза. Оказалась, что эстрогены, входящие в состав комбинированных гормональных контрацептивов (КГК), вызывают изменения в свертывающей системе крови, которые могут способствовать тромбозу, причем изменения эти являются дозозависимыми. В частности, эстрогены повышают содержание фибриногена, фактора Виллебранда, ряда факторов свертывания крови (протромбина, факторов VII, IX, X, XII, XI, V), подавляют функцию антикоагулянтной системы, вызывая резистентность к активированному протеину С, снижают активность антитромбина III и протеина S [1–4].
Эновид содержал примерно в пять раз более высокую дозу эстрогенов по сравнению с используемыми в настоящее время препаратами. Современные КГК содержат в каждой таблетке от 15 до 35 мкг этинилэстрадиола (синтетического эстрогена) в комбинации с большим разнообразием гестагенов (синтетические аналоги прогестерона). Снижение дозы эстрогенов позволило существенно снизить риски для здоровья, в частности, венозных тромбозов, инфарктов и инсультов. Контрацептивный эффект КГК в основном обусловлен гестагенным компонентом, тогда как эстрогенный компонент в большей степени нужен для предотвращения нерегулярных кровянистых выделений в процессе приема контрацептивов. Дальнейшие попытки снизить дозу этинилэстрадиола до 10 мкг в таблетке и менее привели к недостаточно хорошему контролю цикла.
Влияние гестагена в составе гормональных контрацептивов на риск тромбозов
В последующем стало понятно, что риск тромбозов при применении гормональных контрацептивов связан не только с эстрогенным компонентом, но и с типом второго компонента в составе препарата – гестагена. Было отмечено, что более высокий риск тромбоза связан с гестагенами так называемого третьего поколения (дезогестрел, гестоден) по сравнению с гормональными контрацептивами с гестагенами второго поколения в составе (левоноргестрел). Помимо влияния на рецепторы прогестерона, гестагены могут обладать стимулирующим или тормозящим эффектом на рецепторы других стероидных гомонов – андрогенов, глюкокортикоидов и минералкортикоидов. Чтобы преодолеть нежелательные андрогенные эффекты левоноргестрела, начался поиск новых молекул с целью синтеза новых гестагенов, обладающих метаболической нейтральностью и не вызывающих такие нежелательные эффекты, как отечность, повышение массы тела, акне, гирсутизм. В результате этой работы появились гестагены третьего поколения, лишенные антиандрогенной и глюкокортикоидной активности. Однако метаболические преимущества новых гестагенов дались ценой повышения риска венозных тромбозов по сравнению с гестагеном второго поколения левоноргестрелом [5].
Повышение риска тромбозов, по сравнению с левоноргестрелом, было также описано для ципротерона ацетата и дроспиренона – гестагенов с антиандрогенной активностью, которые способствуют подавлению синтеза андрогенов и помогают бороться с такими проблемами, как акне и избыточный рост волос. Ципротерона ацетат обладает наиболее мощным антиандрогенным эффектом по сравнению с другими гестагенами и является хорошим вариантом для лечения пациенток с тяжелой формой акне и гирсутизма. Однако одна из наиболее авторитетных профессиональных ассоциаций – Европейское сообщество специалистов по проблемам репродукции человека (ESHRE) рекомендовало не использовать ранее широко применявшийся гормональный контрацептив с ципротерона ацетатом в составе в качестве первой линии при лечении пациенток с синдромом поликистозных яичников, в частности, в связи с повышенным риском тромбозов, связанным с применением этого препарата [6].
Тромбогенность дроспиренон-содержащих гормональных контрацептивов: противоречия
Гестаген четвертого поколения дроспиренон отличается по своей структуре от других гестагенов тем, что является производным спиронолактона и обладает антиминералкортикоидной активностью. Благодаря этим свойствам дроспиренон предотвращает задержку жидкости, которую вызывают эстрогены, и не вызывает прибавку массы тела, которой опасаются большинство женщин, задумывающихся о применении гормональных контрацептивов. Такой благоприятный профиль метаболических эффектов обеспечил широкую популярность контрацептивам с дроспиреноном и успешный опыт их использования с начала 2000-х гг.
Однако в 2011 г. FDA (Управление по контролю качества пищевых продуктов и лекарственных препаратов) опубликовало предупреждение о возможном повышении риска тромбозов при использовании дроспиренонсодержащих КГК [7]. Дело в том, что в двух крупных исследованиях – в национальном когортном исследовании, проведенном в Дании, и в исследовании по типу случай-контроль в Голландии было выявлено повышение риска венозных тромбоэмболических осложнений (ВТЭО) при применении дроспиренонсодержащих КГК по сравнению с содержащими левоноргестрел [8, 9] Однако методология этих исследований вызвала критику, а их результаты шли в разрез с данными проспективных наблюдательных исследований EURAS (European Active Surveillance Study) и LASS (Long-term Active Surveiilance Study for Oral Contraceptives) с участием более 59 000 женщин, использовавших различные КГК. По данным этих исследований, риски ВТЭО были сходными для дроспиренон- и левоноргестрелсодержащих КГК [10]. В то же время результаты недавнего систематического обзора и метаанализа, опубликованного в 2018 г., также показали, что по сравнению с левоноргестрелом риск ВТЭО повышается примерно в 1,5–2 раза для гестагенов третьего поколения (дезогестрел, гестоден) и дроспиренона [11]. Несмотря на неутихающие споры вокруг тромбогенности КГК с разными гестагенами в составе, дроспиренонсодержащие КГК вот уже два десятилетия очень успешно применяются в разных странах мира и остаются одними из наиболее популярных контрацептивов. Более того, ведущие профессиональные сообщества, в частности American Society for Reproductive Medicine и American College of Obstetricians and Gynecologists, ставят под сомнение различия в тромбогенности КГК с разными гестагенами в составе и рекомендуют при выборе метода контрацепции учитывать лишь то, насколько КГК в целом приемлемы для пациентки с учетом ее персональных особенностей и возможных противопоказаний [12, 13].
Важно понимать, что с точки зрения тромботического риска беременность и роды представляют значительно большую опасность по сравнению с приемом КГК. Так, риск тромбозов, связанный с применением КГК, содержащих гестагены третьего поколения (гестоден, дезогестрел) и дроспиренон, составляет 9–12 случаев на 10 000 женщин в год (5–7 случаев на 10 000 для левоноргестрелсодержащих КГК), что меньше по сравнению с риском тромбоза связанным с беременностью (20 случаев на 10 000 женщин в год) и родами (40–65 случаев на 10 000 в год) [11, 14–17]. Так как частота тромбозов в общей популяции молодых женщин невелика и составляет около 1–5 случаев на 10 000 женщин в год, абсолютное число тромбозов, связанных с КГК, остается очень низким [18].
Поиски оптимального состава и пути введения для гормональных контрацептивов. Выбор эстрогенного компонента
Тем не менее поиски оптимального состава и формы гормональных контрацептивов продолжались. Были созданы комбинированные контрацептивы в виде пластыря и вагинального кольца; однако исследования показали, что другая форма доставки эстрогенов и гестагенов в организм не снижает тромботические риски по сравнению с таблетированными формами [12].
Эстрогенный компонент КГК оставался неизменным в течение длительного времени. Традиционно в составе оральных контрацептивов используется синтетический эстроген этинилэстрадиол, который обеспечивает хороший контроль менструального цикла и предотвращает нерегулярные кровянистые выделения, возникающие на фоне применения чистых гестагенов. Несколько исследований показали, что натуральный эстрадиол в меньшей степени влияет на синтез печеночных белков по сравнению с этинилэстрадиолом. Еще в 1970-х гг. были предприняты попытки заменить этинилэстрадиол натуральным эстрадиолом. Однако из-за низкой пероральной биодоступности эстрадиола создать гормональный контрацептив с натуральным эстрогеном в составе и при этом добиться хорошего контроля менструального цикла оказалось непростой задачей. Тем не менее разработать КГК, содержащие идентичные натуральным эстрогены, удалось (это четырехфазный контрацептив, содержащий эстрадиола валерат и диеногест, который появился в 2009 г., и монофазный контрацептив, содержащий микронизированный 17-бета-эстрадиол и номегестрола ацетат, зарегистрированный в 2011 г.). Исследования показали, что применение этих КГК с натуральным эстрогеном в составе может быть связано с несколько меньшим риском тромбозов по сравнению с КГК, содержащими левоноргестрел [19, 20]. Это означает, что не только левоноргестрелсодержащие КГК могут быть единственной опцией для снижения риска венозных тромбозов при применении гормональной контрацепции. Кроме того, КГК с одним и тем же гестагенным компонентом в составе, но с разными эстрогенами могут вести себя по-разному с точки зрения тромботического риска. Поэтому гипотеза о том, что замена эстрогенного компонента в составе дроспиренонсодержащих КГК позволит создать препарат с улучшенным профилем безопасности в отношении тромбозов, представляется весьма обнадеживающей.
Свойства эстетрола – натурального эстрогена с избирательным действием в тканях в составе нового КГК «Эстеретта». Влияние на систему гемостаза и тромботическая безопасность
Потребность в создании новых контрацептивов с оптимальными метаболическими свойствами и минимальным влиянием на систему гемостаза оставалась всегда, однако более десяти лет интересных новостей в области контрацепции не было. И вот наконец появился принципиально новый и уникальный по своему составу КГК. В 2021 г. Европейское медицинское агентство на основании результатов многоцентрового исследования III фазы FREEDOM с участием в общей сложности 3725 женщин из Европы, России и США зарегистрировало новый КГК, содержащий инновационный эстроген эстетрол и давно зарекомендовавший себя и широко применяемый во всем мире гестаген дроспиренон [21, 22]. В России этот препарат был зарегистрирован под торговым наименованием «Эстеретта». Эстеретта представляет собой монофазный комбинированный гормональный контрацептив, содержащий 15 мкг эстетрола и 3 мг дроспиренона, с удобным и давно зарекомендовавшим себя режимом применения (24 активные + 4 неактивные таблетки). В исследованиях III фазы были подтверждены контрацептивный эффект, хороший контроль цикла, благоприятный профиль безопасности и высокий уровень удовлетворенности пациенток. Кроме того, ряд исследований свидетельствует о благоприятном профиле эстетрола/дроспиренона с точки зрения влияния на метаболические показатели, включая уровень триглицеридов, и на параметры гемостаза [23–25].
В чем же уникальность нового контрацептива «Эстеретта» и за счет чего при создании этого препарата удалость добиться благоприятного профиля в отношении влияния на функцию печени и параметры гемостаза?
Эстетрол по своим фармакологическим свойствам принадлежит к принципиально новому классу эстрогенов. Это первый натуральный эстроген с избирательным действием в тканях (NEST). Эстетрол – это фетальный эстроген, который синтезируется только в печени плода, но который удалось получить синтетическим путем из растительных источников.
Все эстрогены связываются с двумя субпопуляциями альфа-рецептора эстрогена (ERα): ядерными ERα, которые индуцирует транскрипцию генов, и мембранными ERα, которые инициируют быструю передачу сигналов. Эстетрол активирует ядерные рецепторы эстрогенов, однако, в отличие от других эстрогенов, эстетрол противодействует активности мембранных рецепторов эстрогенов. Благодаря такому избирательному действию на ткани эстетрол способен подавлять овуляцию за счет блокады ERα в яичниках, связываться с мембранными ERα в молочной железе и ограничивать нежелательные процессы клеточного роста и деления в этом органе. Одновременно эстетрол за счет активации ядерных ERα предотвращает деминерализацию костей, оказывает нейропротективный и защитный эффекты в отношении кровеносных сосудов. Эстетрол обладает нейтральным эффектом в отношении печени, что крайне важно с точки зрения тромботической безопасности этого эстрогена (рис. 1).
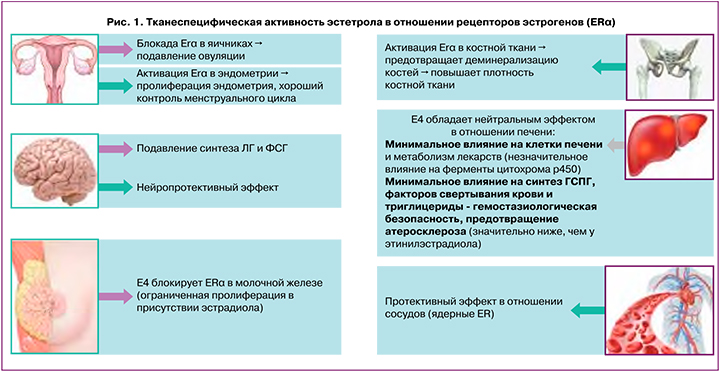
Эстрогены стимулируют синтез в печени различных белков, в том числе глобулина, связывающего половые гормоны (ГСПГ), некоторые факторы свертывания крови и ингибиторы коагуляции. Изменения маркеров гемостаза могут способствовать дисбалансу между прокоагуляционными и антикоагуляционными факторами, это может повышать риск тромбозов. Так как чистые гестагены практически не влияют на синтез прокоагулянтных белков, была выдвинута гипотеза о том, что разный риск тромбоза при применении различных КГК может быть обусловлен различным модулирующим влиянием гестагенов на протромботические эффекты этинилэстрадиола. То есть эффекты гестагенов на гемостаз реализуются не самостоятельно, а через различное влияние на эффекты этинилэстрадиола. Выделяют такое понятие, как эстрогенность КГК, что означает суммарное влияние как эстрогена, так и гестагена на риск тромбоза и подразумевает модулирующее влияние гестагенов на эффекты эстрогена. Чрезмерная эстрогенность увеличивает риск тромбозов.
Биомаркером, который наилучшим образом отражает эстрогенность, является ГСПГ. Это белок-носитель эстрогена и тестостерона, вырабатываемый печенью, синтез которого очень чувствителен к эстрогену. Пероральный прием только этинилэстрадиола приводит к значительному дозозависимому увеличению ГСПГ, тогда как прогестагены вызывают снижение ГСПГ, причем степень этого снижения зависит от андрогенной активности гестагена. Гестагены с мощной андрогенной активностью вызывают более выраженное снижение уровня ГСПГ, чем менее андрогенные или антиандрогенные [26]. Наблюдалась взаимосвязь между уровнями ГСПГ и повышенным риском венозных тромбозов, связанным с КГК [27]. Однако ГСПГ не является белком системы гемостаза, а лишь маркером, отражающим влияние КГК на гемостаз.
При разработке новых контрацептивов Европейское медицинское агентство (EMA), наряду с другими показателями, которые могут характеризовать разные фармакологические свойства КГК (включая фрагмент протромбина 1+2, D-димер, факторы VII, VIII, II, антитромбин III, протеин S), рекомендует оценивать ГСПГ и резистентность к активированному протеину С [28]. Эти показатели являются суррогатными маркерами риска ВТЭО на фоне КГК. Повышение резистентности к активированному протеину С – это один из возможных факторов негативного влияния КГК с высокой эстрогенностью на риск ВТЭО. Именно резистентность к активированному протеину С может определять различия в тромбогенности КГК, содержащих гестагены второго и третьего поколения [29].
Активированный протеин С – это физиологический антикоагулянт, который разрушает активные факторы свертывания крови V и VIII и блокирует дальнейшее образование тромбина и образование тромбов. Врожденный функциональный фенотип плазмы, называемый резистентностью к активированному протеину С, был впервые описан Dahlbäck в 1993 г. Наследственная резистентность к активированному белку С в основном вызвана точечной мутацией в гене фактора V, которая делает фактор V нечувствительным к активированному протеину С. Это состояние было названо мутацией Лейдена (FV Leiden) в честь голландского города Лейден, где этот вариант гена фактора V впервые был идентифицирован в 1994 г. Роже Марией Бертиной. Мутация Лейдена относится к наиболее часто встречающимся тромбофилиям и в европейской популяции обнаруживается примерно у 5% населения [30]. Наиболее частыми причинами приобретенной резистентности к активированному протеину С являются беременность и применение КГК.
Эстетрол/дроспиренон обладает меньшей эстрогенностью (влиянием на синтез ГСПГ) и меньшим эффектом на систему гемостаза по сравнению с КГК c этинилэстрадиолом.

В исследованиях было показано минимальное изменение резистентности к активированному протеину С при применении нового контрацептива, содержащего эстетрол и дроспиренон 3 мг, по сравнению с низкодозированным КГК, содержащим 20 мкг этилэстрадиола и 3 мг дроспиренона, и по сравнению с КГК, содержащим 30 мкг этинилэстрадиола и левоноргестрел (эталонный гестаген, с которым сравнивают все другие гестагены, когда хотят оценить их влияние на систему гемостаза) [25, 31] (рис. 2). Эстетрол/дроспиренон оказывал меньшее или сравнимое влияние на маркеры гемостаза по сравнению с группой этинилэстрадиола/левоноргестрела и меньшее влияние на маркеры гемостаза по сравнению с этинилэстрадиолом/дроспиреноном. Изменения уровня ГСПГ под влиянием эстетрола/дроспиренона сравнимы с эффектом этинилэстрадиола 30 мкг/левоноргестрела, при этом уровень ГСПГ увеличивался достоверно в меньшей степени по сравнению с этинилэстрадиолом 20 мкг/дроспиреноном (рис. 3). По данным еще одного исследования на небольшой группе пациенток, в отличие от препарата, содержащего этинилэстрадиол и дроспиренон, эстетрол/дроспиренон не оказывал влияния на многочисленные изученные параметры гемостаза, включая важнейшие параметры антикоагулянтной системы – активность антитромбина III, протеина С, протеина S, резистентность к активированному протеину С, и даже снижал уровень D-димера [24]. Эти данные свидетельствуют в пользу того, что влияние КГК на гемостаз может быть обусловлено преимущественно эстрогенным компонентом, а включение в состав ГКГ «правильного» эстрогена может иметь ключевое значение с точки зрения тромботической безопасности.

На сегодняшний день у пользователей КГК, содержащих эстетрол, недостаточно клинического опыта для оценки риска венозных тромбозов, связанного с этим эстрогеном нового типа. В исследовании III фазы тромбоз глубоких вен развился у одной 32-летней женщины с индексом массы тела 21,5 кг/м2 на 4-м месяце приема препарата [22]. В течение 3 лет пациентка принимала эсциталопрам, для которого имеются сообщения о случаях тромбоза [32]. Других факторов риска тромбозов у пациентки выявлено не было. Тромбоз у участницы исследования разрешился без последствий на фоне антикоагулянтной терапии.
Эффекты эстетрола/дроспиренона на маркеры гемостаза и ГСПГ (маркер эстрогенности) указывают на потенциально меньший риск тромбоэмболических осложнений, по сравнению с КГК, содержащими этинилэстрадиол и дроспиренон. Таким образом, выбор эстрогена в КГК имеет важное значение с точки зрения влияния на гемостаз и риска тромбоза, а замена этинилэстрадиола на эстетрол представляется верной стратегией для снижения риска тромбозов.
Подбор гормональной контрацепции у пациенток из группы высокого риска по развитию тромбоэмболических осложнений
Важно понимать, что ни один из доступных на сегодня КГК не является полностью нейтральным с точки зрения тромбических рисков. Около 1% тромбозов, развившихся на фоне приема гормональных контрацептивов, приводит к смертельному исходу, а сам по себе тромбоз – тяжелое осложнение, которое требует длительного приема антикоагулянтов и может приводить к инвалидизации [33]. У большинства женщин прием КГК безопасен и связан с меньшим тромботическим риском, чем беременность и роды. Однако существует ряд женщин, у которых прием КГК может сопровождаться неприемлемо высоким риском тромбозов, например, при артериальных или венозных тромбозах в настоящее время или в анамнезе или при наличии тромбофилий. Наиболее часто в европейской популяции встречается мутация FV Leiden (5%), на втором месте находится мутация протромбина G20210A с частотой встречаемости 2%. Риск первого тромбоза у гетерозиготных носителей этих мутаций повышен в 3–7 раз, а у гомозигот – в 30–80 раз по сравнению с общей популяцией. Риск тромбоэмболии у пациентов с такой мутацией увеличивается в 7–30 раз при использовании оральных контрацептивов и особенно возрастает при наличии тромбозов у близких родственников, а у гомозигот с отягощенной семейной тромботический историей повышается до 70 раз [2, 4, 8, 34, 35]. При сочетании тромбофилии с другими факторами риска (например, курение, ожирение) риски тромбозов не просто суммируются, а возрастают многократно, так, риск тромбозов на фоне приема КГК у таких пациентов может возрастать в 35–70 раз [2, 32].
Гораздо реже встречаются дефициты белков антикоагулянтной системы (антитромбина III, протеина С, протеина S) (менее 0,5%), однако риск тромбозов при этих состояниях повышается значительно – в 10–50 раз [2, 4, 36].
Особое внимание необходимо уделять семейной истории тромбозов и помнить о том, что тромбофилические состояния выявляются только у 30% пациентов с наследственными тромбозами [37]. Если тромбофилия не выявляется, это не означает отсутствие риска тромбоза при применении КГК. В случае отягощенного семейного тромботического анамнеза риск тромбоза при приеме КГК возрастает более чем в два раза, а если тромбоз был у двух и более родственников или в семье был гормон-ассоциированный тромбоз, риск повышается в 4 раза [38]. При этом семейная история тромбоза может быть важным индикатором риска развития первого тромбоза и иметь большее значение с клинической точки зрения, чем лабораторное обследование на тромбофилию [3, 38, 39].
По мнению рабочей группы ВОЗ, рутинный скрининг при подборе контрацепции на наличие тромбофилических состояний нецелесообразен в связи с высокой его стоимостью и высокой вероятностью получения ложноположительных результатов. Так, было установлено: чтобы предотвратить 2 летальных исхода, связанных с тромбоэмболией на фоне КГК, надо обследовать на тромбофилию перед началом приема препарата 1 млн женщин [33].
В настоящее время риск тромбозов перед назначением КГК определяют только на основании клинических характеристик. Не существует алгоритма, который бы оценивал параметры гемостаза или включал какие-то лабораторные тесты, на которые мы бы могли опереться при прогнозировании тромботического риска перед назначением КГК. Обследование на тромбофилии рекомендовано только при наличии отягощенной семейной истории по тромбозам и в случае подтвержденной тяжелой тромбофилии у близких родственников (дефицит протеина С, протеина S, антитромбина III) [40]. Что касается гипергомоцистеинемии, ранее считалось, что она связана с умеренным повышением риска ВТЭО [41]; однако более свежие данные говорят о том, что повышенный уровень гомоцистеина является слабым фактором риска венозных тромбозов [42], а мутация MTHFR сама по себе при отсутствии гипергомоцистеинемии не связана с повышением тромботического риска [43].
Тромбоз – многофакторное заболевание, спровоцировать которое могут различные факторы, включая травмы, операции, иммобилизации (в том числе путешествия на автомобиле на длительные расстояния, авиаперелеты, госпитализации более 3 дней), инфекции, онкологические заболевания вне зависимости от наличия или отсутствия тромбофилии. Таким образом, анализ приобретенных факторов риска имеет большее значение по сравнению с генетическим исследованием.
Применение КГК также сопровождается очень небольшим повышением риска инфаркта и инсульта по сравнению с женщинами того же возраста, не принимающими КГК. Абсолютный риск остается очень низким (2,1 инсульта и 1,0 инфаркта на 10 000 человеко-лет) [44]. Конечно, такие случаи крайне редки у молодых женщин. Однако КГК рекомендуется избегать женщинам с множественными факторами риска, включая артериальную гипертензию, курение, нарушения липидного обмена. Хотя у здоровых женщин КГК могут применяться до 50-летнего возраста [44], у женщин старше 35, курящих более 15 сигарет в день, прием КГК становится недопустимым, так как значительно повышает сердечно-сосудистые риски. КГК также противопоказаны при мигрени с аурой, при артериальной гипертензии, особенно при неконтролируемой, и при цифрах артериального давления более 160/100 мм рт. ст., антифосфолипидном синдроме [44, 45].
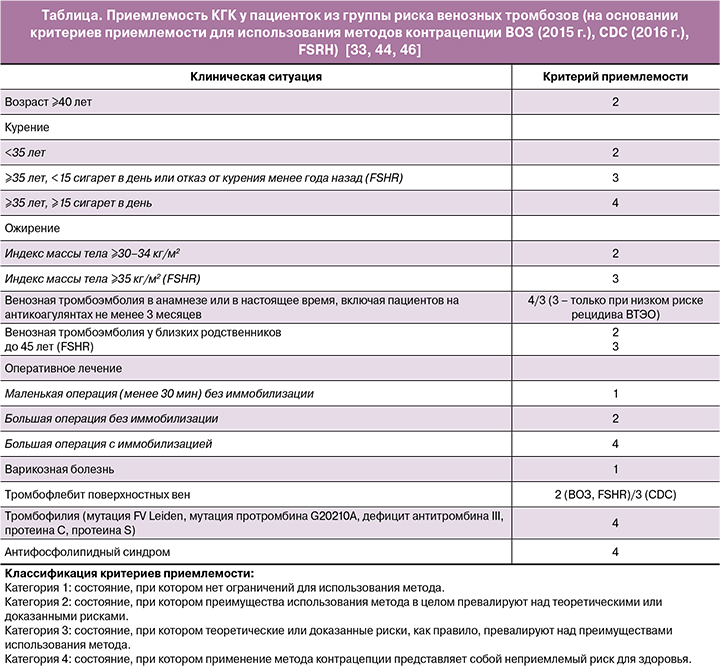
Различные международные организации (ВОЗ; Centers for Disease Control and Prevention, СDC; Faculty of Sexual & Reproductive Healthcare, FSRH) разработали критерии приемлемости для различных методов контрацепции, которые позволяют оценить безопасность применения контрацептивов в каждом конкретном случае [33, 44, 46]. Любой гормональный контрацептив, вне зависимости от типа и дозировки эстрогена или гестагена в своем составе, должен назначаться с учетом критериев приемлемости. В таблице представлены данные по безопасности применения КГК при наличии различных факторов риска венозных и артериальных тромбозов на основании международных критериев приемлемости.
Заключение
Эстетрол – первый селективный эстроген, который относится к принципиально новому классу эстрогенов с избирательным действием в тканях и показал себя многообещающим кандидатом в области контрацепции. На сегодняшний день у пользователей контрацептивов, содержащих эстетрол, недостаточно клинического опыта для оценки риска ВТЭО. Однако минимальное влияние Эстеретта на такие суррогатные маркеры, как параметры системы гемостаза, включая резистентность к активированному протеину С, и ГСПГ (маркер эстрогенности), указывает на потенциально меньший риск тромбоэмболических осложнений по сравнению с КГК, содержащими этинилэстрадиол и дроспиренон.
В отсутствие факторов риска необходимости в дополнительном обследовании перед началом применения Эстеретта или в мониторировании параметров гемостаза на фоне приема этого препарата нет, но перед назначением этого контрацептива необходимо исключить все исходные риски венозных и артериальных тромбозов. Любой гормональный контрацептив, вне зависимости от типа и дозировки эстрогена или гестагена в своем составе, должен назначаться с учетом критериев приемлемости КГК. Несмотря на более благоприятный профиль безопасности в отношении влияния на систему гемостаза, противопоказания к использованию этого контрацептива такие же, как у других КГК, а тромботическую безопасность этого препарата необходимо будет проанализировать на основании результатов исследований с большим количеством пациентов и с учетом реальной клинической практики.



