Несмотря на доступность большого количества методов контрацепции, частота незапланированных беременностей во всем мире остается высокой [1]. 37% российских женщин, которые в период с января 2006 по 2011 гг. имели одну и более беременностей, отметили, что их последняя беременность была незапланированной, в равных пропорциях встречались случаи несвоевременной (18%) и нежелательной (18%) беременности. 92% абортов выполнены по причине несвоевременной (30%) или нежелательной (62%) беременности [2]. В России наибольшая частота абортов (до 37 на 1 тыс. женщин) приходится на женщин в возрасте 20–29 лет, а возраст рождения первенца увеличился до 26 лет [3]. Наиболее часто незапланированные беременности регистрируют у женщин в возрасте 20–29 лет [4, 5]. В большинстве случаев это связано с неправильным или нерегулярным использованием комбинированных оральных контрацептивов (КОК), которые являются предпочтительным методом контрацепции в этой популяции [6].
Несоблюдение режима приема снижает эффективность КОК, что подтверждается разницей в неэффективной контрацепции в течение первого года между «типичным» и «идеальным» использованием: 9 и 0,3% соответственно [7]. Обратимая контрацепция длительного действия (long-acting reversible contraception, LARC) является эффективным методом контроля рождаемости в течение продолжительного периода времени без необходимости ежедневного внимания со стороны женщины. Применение методов LARC, включая левоноргестрел-содержащие внутриматочные системы (ЛНГ ВМС), может снизить частоту незапланированной беременности. Ведущие международные организации выступают за использование ВМС (включая ЛНГ ВМС) у широкого круга женщин, независимо от их возраста или наличия родов в анамнезе [8–13]. Тем не менее в настоящее время типичным пользователем ВМС являются рожавшие женщины 35 лет и старше [14–16].
В исследовании III фазы [17] показана высокая эффективность низкодозированной ЛНГ ВМС, содержащей 19,5 мг ЛНГ, у рожавших и нерожавших женщин (n=1453) в возрасте 18–35 лет, где 5-летний индекс Перля составил 0,29 [17]. Использование данного контрацептивного метода в этой возрастной группе ассоциировалось с низкой частотой эктопических беременностей (5-летний индекс Перля – 0,18), воспалительных заболеваний органов малого таза (0,6%), перфораций (0,2%) и экспульсий ВМС (3,7%) [17].
В работе Hall A.M. et al. (2014) проводилась оценка удовлетворенности ЛНГ ВМС у молодых нерожавших женщин 18–30 лет при использовании в течение 13,4 месяца. Общая удовлетворенность была высокой – 83% «довольны» или «очень довольны» методом контрацепции. В течение года 89% пациенток продолжали использовать ЛНГ ВМС. Причинами выбывания были: экспульсии ВМС (3%), побочные эффекты (6%), отсутствие ожидаемой пользы (1%) и беременность (1%) [18].
Согласно Канадскому консенсусу о контрацепции, принятому в 2016 г., ВМС не увеличивают риск бесплодия. Женщины, которые прекращают использование ВМС с целью наступления беременности, способны зачать сопоставимо с женщинами, никогда не использовавшими ВМС. Использование ВМС не связано с увеличением бесплодия трубного фактора у первородящих женщин [19].
Цель исследования: оценить удовлетворенность молодых нерожавших и рожавших женщин использованием низкодозированной ЛНГ ВМС или приемом КОК, содержащего 30 мкг этинилэстрадиола и 3 мг дроспиренона, в течение 12 месяцев.
Материалы и методы
Дизайн. Многоцентровое проспективное открытое клиническое исследование серии случаев (ClinicalTrials.gov: NCT03074045).
Исследование проходило в условиях реальной клинической практики с марта 2017 г. по сентябрь 2018 г. в 9 исследовательских центрах России (Москва, Смоленск, Барнаул, Иркутск, Санкт-Петербург, Новосибирск (2), Ярославль, Красноярск).
При проведении исследования руководствовались Хельсинкской декларацией Всемирной Медицинской Ассоциации [20] и Руководством по надлежащей клинической практике Международной конференции по гармонизации (ICH GCP E6 R2) [21]. Все участницы подписали добровольное информированное согласие.
Объекты и исследуемые группы. 161 женщина прошла этап скрининга, 147 – были включены в исследование: 74 – в группу ЛНГ ВМС и 73 – в группу КОК.
Критерии включения: здоровые нерожавшие и рожавшие женщины в возрасте 18–29 лет (включительно), нуждающиеся в контрацепции, с регулярным менструальным циклом (интервалы 21–35 дней) и нормальным результатом цервикального мазка по Папаниколау.
Критерии исключения: патология матки, которая может представлять проблемы для установки, удержания или удаления ВМС (аномалии, миома матки); наличие противопоказаний к использованию ВМС или КОК; роды или аборт в течение 6 недель до включения в исследование; беременность или лактация; инфекции, передаваемые половым путем; неспецифические инфекции нижних половых и мочевыводящих путей (до успешного излечения).
Не соответствовали критериям включения 14 пациенток, двоим женщинам, включенным в группу I, ЛНГ ВМС не вводилась, таким образом, популяцию для анализа составили 145 участниц: 72 и 73 в группах I и II соответственно. Завершили исследование 132 (91%) женщины (65 и 67 в группах I и II соответственно). Количество выбывших и завершивших исследование представлено на рисунке.

Исследуемые методы контрацепции. 1) ЛНГ ВМС LCS16, содержащая 19,5 мг ЛНГ, с первоначальной скоростью высвобождения in vitro 16 мкг ЛНГ/сут (группа I); 2) КОК в таблетках, содержащих 30 мкг этинилэстрадиола и 3 мг дроспиренона (30ЭЭ/ДРСП), с режимом приема с 1-го по 21-й дни менструального цикла включительно, с последующим 7-дневным перерывом (группа II).
Исследуемые переменные. Первичным критерием оценки была общая удовлетворенность пользователей методом контрацепции на момент окончания исследования или при досрочном завершении (до истечения 12 месяцев). Общая удовлетворенность пользователей оценивалась с помощью шкалы Лайкерта. Показатель общей удовлетворенности определялся как суммарный процент женщин, выбравших ответы «очень довольна» или «довольна».
Вторичные критерии эффективности: удовлетворенность пользователей по профилю менструальных кровотечений и боли (оценивалась с помощью специального вопросника через 6 и 12 месяцев), предпочтения при выборе метода контрацепции после завершения исследования, частота нежелательных явлений во время лечения (НЯВЛ). Дополнительно оценивали частоту случаев выбывания из исследования по причине НЯВЛ или по причине желания забеременеть.
Безопасность оценивалась по частоте НЯВЛ (экспульсия ЛНГ ВМС, перфорация матки, воспалительные заболевания, профиль кровотечения) и лабораторным показателям (общий анализ крови, глюкоза, липиды, ферменты печени).
Статистический анализ
Для обработки цифровых данных использовали программу Statistica 11.0 (StatSoft Inc., США) для Windows. Анализ выполнялся с помощью методов описательной статистики. Анализируемые признаки имели нормальное распределение (проверка проводилась с помощью W-критерия Шапиро–Уилка), значения приведены в виде среднего (M) и стандартного отклонения (SD). Для показателей, характеризующих качественные признаки, указывали абсолютное число (n) и относительную величину в процентах.
Результаты
Общая характеристика популяции. Средний возраст женщин, включенных в группы ЛНГ ВМС и КОК, составил 25,7 (2,7) и 25,0 (3,2) года соответственно. Исходные характеристики популяции, включая данные анамнеза, представлены в таблице 1.
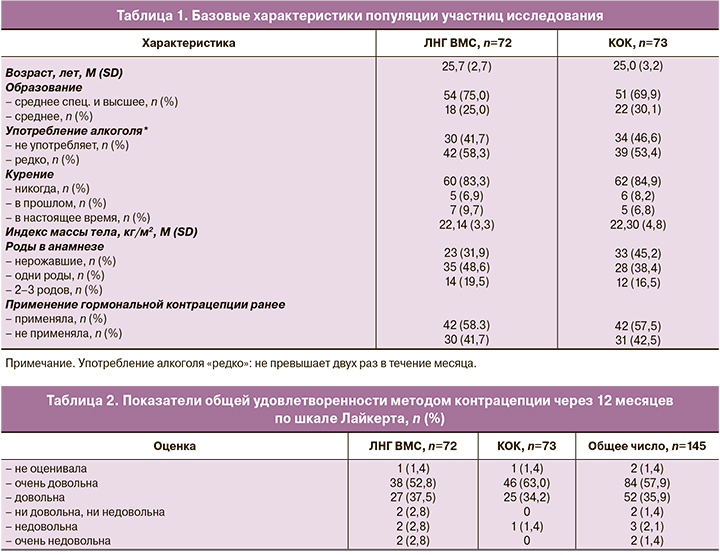
Общая удовлетворенность пользователей. После окончания исследования показатель удовлетворенности методом контрацепции в обеих группах был высоким: большинство участниц исследования в общей популяции исследования (n=145) были «очень довольны» (57,9%) и «довольны» (35,9%) используемым методом контрацепции. Через 12 месяцев в группе ЛНГ ВМС процент ответов «очень довольна» и «довольна» составил 90,3% (65/72), в группе КОК – 97,3% (71/73) (табл. 2).
В группе ЛНГ ВМС дополнительно оценили показатель общей удовлетворенности у участниц с предшествующим опытом использования гормональной контрацепции и теми, кто ее ранее не использовал, при этом показатель составил 92,9% (39/42) у имевших такой опыт и 86,7% (26/30) – у не имевших.
Профиль кровотечений и боли. В группе ЛНГ ВМС уже через 6 месяцев 76,8% (53/69) женщин сообщили о снижении объема менструальных кровотечений; к окончанию 12-го месяца исследования эта цифра составила 80% (56/70). В группе КОК снижение объема менструальных кровотечений через 6 месяцев отметили 62,9% (44/70) пациенток, через 12 месяцев – 45,8% (33/72) (табл. 3).

Среднее количество дней кровотечений и кровомазаний снизилось в группе ЛНГ ВМС в среднем с 32,8 дня в 1-м 90-дневном периоде до 14 дней – в 4-м. В группе КОК количество дней кровотечений и кровомазаний оставалось относительно постоянным: в среднем 20,2 и 16,4 соответственно.
К окончанию 12-го месяца аменорея наблюдалась у 7,7% (5/65) участниц в группе ЛНГ ВМС и ни у кого в группе КОК; редкие кровотечения – у 27,7% (18/65) и 1,5% (1/67); частые – у 3,1% (2/65) и ни у кого; нерегулярные – у 15,4% (10/65) и 4,5% (3/67) соответственно; продолжительные кровотечения отмечались у 4,6% (3/65) женщин в группе ЛНГ ВМС и не были отмечены ни у одной пациентки в группе КОК. Спустя 12 месяцев в группе ЛНГ ВМС профилем кровотечений были «очень довольны» или «скорее довольны» 94,3% (66/72) участниц, в группе КОК – 95,8% (69/73).
Исходно около половины участниц не имели менструальной боли, около трети оценили свою боль как легкую. К концу исследования уменьшение интенсивности боли по сравнению с периодом до лечения отмечали 61,4% (43/72) женщин в группе I и 58,3% в группе II (42/73).
Доля участниц, удовлетворенных контрацепцией (отсутствие дискомфорта и неудобства метода), возросла в группе ЛНГ ВМС с 68,1 (47/72) до 77,1% (54/72) к концу исследования, но снизилась в группе КОК с 92,9% (65/73) до 79,2% (57/73).
Предпочтения участниц исследования. В конце 12-го месяца 81,4% (57/72) женщин в группе ЛНГ ВМС и 79,2% (57/73) в группе КОК высказались за продолжение использования тех же методов контрацепции, которые они использовали во время исследования.
Нежелательные явления и безопасность. НЯВЛ легкой и средней степени тяжести имели 50% (36/72) женщин в группе ЛНГ ВМС и 38,4% (28/73) в группе КОК. Во всех случаях эти явления были легкой или средней степени тяжести и включали в себя метроррагию, диарею, тошноту, увеличение веса и др., ни одно из них не было расценено как серьезное нежелательное явление. НЯВЛ были связаны с контрацептивом (13,9% (10/72) в группе ЛНГ ВМС и 5,5% (4/73) в группе КОК), с процедурами по протоколу (6,9% (5/72) и 2,7% (2/73) соответственно) и изредка требовали отмены исследуемого метода контрацепции (5,6% (4/72) в группе ЛНГ ВМС и 1,4% (1/73) в группе КОК).
Не было зарегистрировано ни одного случая серьезных НЯВЛ, смертельных исходов и беременностей. Частота межменструальных кровотечений составила 9,7% (7/72) в группе ЛНГ ВМС и 2,7% (2/73) в группе КОК. НЯВЛ, представляющие повышенный интерес с точки зрения безопасности (общая или частичная экспульсия ВМС, перфорация матки, эктопическая беременность и воспалительные заболевания органов малого таза), в ходе исследования не отмечались.
Лабораторные показатели безопасности (общий и биохимический анализ крови) были в пределах нормы у большинства женщин в обеих группах в момент включения в исследование и через 12 месяцев, ни в одной из групп не было клинически значимых отклонений.
Частота выбывания из исследования составила 9,7% (7/72) в группе ЛНГ ВМС и 8,2% (6/73) в группе КОК. НЯВЛ были причинами выбывания в группе ЛНГ ВМС в 4 наблюдениях, в группе КОК – в одном.
Обсуждение
В качестве метода контрацепции ЛНГ ВМС LCS16 присутствует на мировом рынке с 2016 г. и успешно применяется в ряде стран (США, Канада, Австрия, Швейцария). Препарат зарегистрирован в Российской Федерации в 2021 г. Данная работа является первой по изучению этого контрацептива в российской популяции.
Исследование подтверждает, что женщины в возрасте 18–29 лет, примерно треть из которых были нерожавшими, демонстрируют высокий уровень удовлетворенности при использовании ЛНГ ВМС и КОК. В конце исследования 81,4% женщин в группе ЛНГ ВМС высказались за продолжение применения назначенного им контрацептива, что совпадает с данными европейского исследования III фазы (82%) [22].
В группе ЛНГ ВМС нежелательные явления имели 50% женщин, в группе КОК – 38,4%. Все явления были легкой или средней степени тяжести. Профиль безопасности ЛНГ ВМС и КОК соответствует ранее полученным данным из других исследований с этими препаратами [23, 24]. Таким образом, можно заключить, что ЛНГ ВМС и КОК хорошо переносились.
Прекращение участия в исследовании по причине изменений характера маточных кровотечений было низким – в группе ЛНГ ВМС (2,8%), в группе КОК такие случаи отсутствовали, что, вероятно, свидетельствует о хорошем консультировании в плане ожидаемых изменений профиля кровотечений до начала использования контрацепции. Среднее количество дней кровотечений или мажущих выделений в течение 90-дневных интервалов в группе ЛНГ ВМС снижалось, в то время как в группе КОК оставалось относительно постоянным. К окончанию исследования в группе женщин, использовавших ЛНГ ВМС, большее число пациенток имели тенденцию к уменьшению количества дней кровотечений и их объема, что соответствует ожидаемым изменениям профиля кровотечений при применении ВМС [23–26].
Показатель общей удовлетворенности через 12 месяцев использования ЛНГ ВМС составил 90,3%, что несколько ниже аналогичного показателя через 3 года по данным исследования III фазы (96%) [22]. Возможно, это связано с тем, что в исследовании [22] вопросник удовлетворенности пользователя начали применять в середине исследования, поэтому возможные оценки женщин, которые выбыли до начала использования вопросника, не были учтены. Кроме того, в исследовании III фазы участницы не знали, какой из двух низкодозированных ЛНГ ВМС (содержащих 13,5 или 19,5 мг ЛНГ) они будут получать, в то время как в настоящем исследовании женщины получали два контрацептива, отличающихся путями введения [26].
Сравнение результатов разных исследований КОК затруднительно ввиду различий в дизайне и изучаемых популяциях; удовлетворенность пользователей в исследованиях варьирует от высокой (94%) [27] до низкой (36%) [28].
Данное исследование призвано привлечь внимание к особенностям использования ЛНГ ВМС у молодых женщин, а также помочь врачам-гинекологам в их повседневной практике для формирования персонализированного подхода в подборе контрацептивных средств.
Заключение
Исследование показало, что оба метода контрацепции – ЛНГ ВМС и КОК – характеризуются высокими показателями удовлетворенности пользователей, составившими 90,3% (65/72) и 97,3% (71/73) соответственно. Через 12 месяцев при использовании обоих методов наблюдались уменьшение объема кровотечений и болевого синдрома и снижение количества дней кровопотери в группе ЛНГ ВМС. Развитие аменореи наблюдалось у 7,7% (5/72) участниц в группе ЛНГ ВМС и ни у кого в группе КОК. Профилем кровотечений были «очень довольны» или «скорее довольны» 94,3% (66/72) и 95,8% (69/73) участниц соответственно. В группе ЛНГ ВМС НЯВЛ легкой и средней степени имели 50% женщин (36/72), в группе КОК – 38,4% (28/73). Эти явления редко требовали отмены исследуемого метода контрацепции: 5,6% (4/72) и 1,4% (1/73) соответственно. Доля участниц, готовых продолжить использование ЛНГ ВМС после завершения исследования, составила 81,4% (57/72), КОК – 79,2% (57/73), что свидетельствует о привлекательности применения обоих методов среди молодых женщин, в том числе нерожавших.



