The course of pregnancy and childbirth outcomes in different fetal growth restriction phenotypes
Objective: To investigate the clinical value of a phenotypic approach for the analysis of perinatal outcomes in fetal growth restriction (FGR).Yakubova D.I., Ignatko I.V., Megrabyan A.D., Bogomazova I.M.
Materials and methods: This retrospective analysis included 230 pregnancy cards and delivery notes. The patients were divided into Group I including 200/230 (87%) pregnant women with adverse FGR outcome; Group II consisted of 30/230 (13%) pregnant women with uncomplicated pregnancy and normal fetal growth trajectory. The phenotypic approach consisted of classifying FGR cases according to maternal, placental, and fetal risk factors for this pathology and then analyzing the contribution of each phenotype to the probability of an adverse fetal outcome.
Results: In step 1, all analyzed observations were divided into 7 clinical FGR phenotypes. In step 2, the FGR clinical phenotypes were divided into three risk groups according to adverse perinatal outcomes: high, intermediate and low risk of perinatal loss. High-risk according to the study results corresponds to the FGR clinical phenotypes "preterm delivery" (59.6%), "infections" (45.1%), "hypertensive disorders during pregnancy" (37.3%), and "bleeding in the second and third trimesters" (24.8%). The intermediate risk was "chronic maternal disease" (9.9%), "no baseline risk factors" (11.4%), and the lowest risk model included the remaining clinical phenotypes. Among stillborns with FGR (n=64), the following FGR phenotypes were observed: "infections" (34.4%), "preterm delivery" (20.3%), "hypertensive disorders during pregnancy" (14.1%), and "bleeding in the second and third trimesters" (14.1%). Early neonatal mortality was highest in the clinical phenotypes "preterm delivery" (39.3%) and "hypertensive disorders during pregnancy" (23.2%).
Conclusion: Seven clinical phenotypes of FGR associated with different risk of adverse perinatal outcomes were analyzed. The highest risk of stillbirth was noted in FGR concurrent with severe prematurity (the "premature delivery" phenotype), infectious affection of the fetal placental unit (the "infection" phenotype), and in II–III trimester hemorrhages caused by placental abruption, recurrent retrochorial hematomas, and placental abnormalities.
Keywords
Fetal growth restriction (FGR) is defined by the Russian Ministry of Health as "inadequate fetal growth that requires maternal care" (2022). This term describes a small for gestation fetus that has not reached its growth potential and has a high risk of perinatal complications including slow growth, estimated fetal weight and/or abdominal circumference <10th percentile, combined with abnormal changes in fetal Doppler indices [1–3]. There is a consensus among researchers and clinicians in different countries that FGR is a multifactorial disorder that affects the rate of fetal development and often results in multiple adverse perinatal and postnatal morbidities [2]. The role of various etiological factors of growth retardation in its realization remains poorly understood, but their study is important to develop more effective prognostic scales and algorithms, methods of prevention, curation and management tactics of patients [4–7]. Fetuses with growth retardation, especially severe and early growth retardation, have a higher risk of adverse perinatal outcomes [1, 8]. Early detection of small for gestation fetuses is associated with a decrease in adverse perinatal outcomes. After excluding FGR with malformations and chromosomal abnormalities, the basis for identifying FGR and determining its clinical variants, as well as management and prognosis includes ultrasound evaluation of amniotic fluid volume and fetal activity, and Doppler ultrasound of the fetal arterial and venous circulations [2, 3, 9]. Therefore, ultrasound and Doppler are the main diagnostic and prognostic tools in the treatment of high-risk patients [2, 3]. However, clinical and research attention is currently drawn to the relatively difficult to predict stillbirth among fetuses with a late form of growth restriction detected at the end of the third trimester, which dictates the need for new approaches in predicting adverse perinatal outcomes [3].
The increased perinatal morbidity caused by FGR leads to significant economic costs of treatment and rehabilitation of these children. In this regard, the main direction of solving this problem is to improve the effectiveness of prediction and prevention of this complication of pregnancy [10]. There is, therefore, an ongoing search for most promising biomarkers, which may help in identifying placental dysfunction and ischemic placental disease [7, 11, 12]. Biochemical markers include a fairly large number of indicators, of which the most studied and implemented to date in practical health care are pregnancy-associated plasma protein-A (PAPP-A), placental growth factor (PIGF), soluble fms-like tyrosine kinase-1 (sFlt-1).
The study by T.A. Yarygina et al. (2019) [5] confirmed the reliability of predicting low birth weight for gestational age fetuses with a decrease in RARP-A <0.5 MoM. A study by E.V. Timokhina et al. (2021) [6] confirmed the possibility of using PIGF markers and the sFlt-1/PIGF ratio to predict early FGR. However, this approach does not allow for timely detection of all FGRs, especially those with late manifestation.
Therefore, new strategies are needed to more accurately identify pregnant women at high risk of FGR. Fetal growth can be affected by various pathological conditions during pregnancy [4]. To provide more targeted prognostic, diagnostic, and preventive measures, we propose using a phenotypic classification of FGR to determine the highest risk of adverse outcomes, based not only on echographic and Doppler parameters, but also on available clinical data.
This study aimed to evaluate the risk of adverse perinatal outcomes in different phenotypic forms of FGR and classify FGR into 3 risk models for adverse perinatal outcomes based on stillbirth and perinatal mortality data.
Materials and methods
The study was conducted at the Perinatal Center of the S.S. Yudin City Clinical Hospital of the Moscow City Health Department. A total of 230 singleton pregnancy in 2021 were retrospectively analyzed. The patients were divided into Group I including 200/230 (87%) pregnant women with FGR and Group II consisting of 30/230 (13%) pregnant women with uncomplicated pregnancy and normal fetal growth trajectory. All patients signed an informed consent to participate in the study. The study was approved by the Research Ethics Committee of the I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University) (Protocol No. 03-20, dated February 19, 2020).
The baseline evaluation was based on data from antenatal care cards and delivery notes and included characteristics of the course of pregnancy, labor, postpartum period, and newborn condition. Women meeting inclusion criteria were included in the study.
The inclusion criteria for the study for the study group were reproductive age over 18 years, consent to participate in the study, gestational age of 22 weeks and pregnancy complicated by FGR.
Inclusion criteria for the control group were reproductive age over 18 years, consent to participate in the study, gestational age at 22 weeks, and absence of FGR.
Non-inclusion criteria: age less than 18 years and multiple pregnancies.
Exclusion criteria in both groups were the absence of outcome data, the refusal of further study, fetal malformations, and chromosomal abnormalities.
Gestational age was determined by the first prenatal screening, based on the measurement of the fetal crown-rump length.
Referring to previously published work on the application of clinical phenotypic classification of preterm birth and small for gestational age (FGR) [13, 14], we classified FGR cases according to the risk factors for this pathology as maternal, placental, and fetal (Table 1).
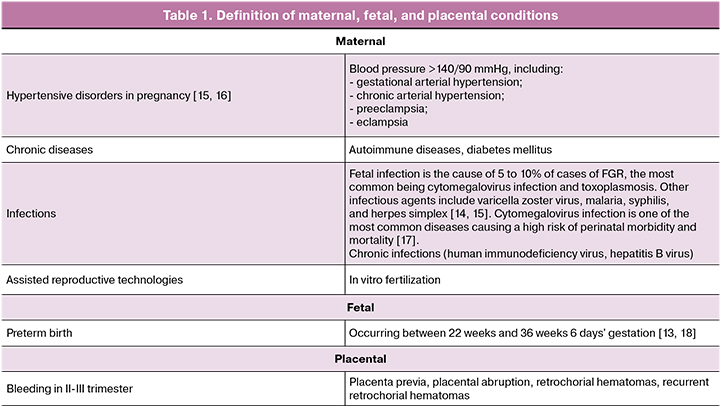
A comprehensive analysis resulted in seven clinical phenotypes of FGR. Among the clinical phenotypes related to maternal conditions, we identified 4 phenotypes including "hypertensive disorders during pregnancy," "infections," "chronic maternal diseases," and "assisted reproductive technology (ART). The clinical phenotype associated with fetal status was "preterm delivery". A clinical phenotype associated with placental status was "bleeding in the II–III trimester". Pregnant women with FGR without associated clinical and anamnestic manifestations were assigned to the clinical phenotype of "no baseline risk factors”.
Because pregnant women with FGR could often be assigned to more than one clinical phenotype, we used a two-stage algorithm. In stage 1, we analyzed perinatal mortality rates for each of the seven clinical phenotypes. In stage II, pregnant women with FGR who belonged to more than one clinical phenotype were further assigned to only one clinical phenotype with the highest risk of adverse perinatal outcomes. Thus, ultimately, each FGR case was assigned to a single clinical phenotype. Of the 200 patients with FGR, 103/200 (51.1%) were women with preterm birth, but the adverse outcome was associated with delivery before 32 weeks or the presence of severe gestational complications (severe preeclampsia, placental abruption). Given the two-stage analysis, preterm birth was identified as a separate clinical phenotype in the case of spontaneous delivery or delivery according to fetal indications but in the absence of severe pre-eclampsia, placental abruption. This group included 93/200 (46.5%) pregnant women. Based on the rates of adverse perinatal outcomes, we evaluated the importance of each clinical phenotype and divided them into 3 risk levels.
Statistical analysis
Statistical analysis was performed using StatTech v. 2.5.9 (Stattech LLC, Russia). The distribution of continuous variables was tested for normality using the Shapiro–Wilk test (when the number of subjects was less than 50) or the Kolmogorov-Smirnov criterion (when the number of subjects was greater than 50). In the absence of a normal distribution, quantitative data were reported using median (Me) and lower and upper quartiles (Q1–Q3). Categorical variables were presented as counts and percentages. Continuous variables were compared with the non-parametric Mann–Whitney U test. Categorical variables were compared using Pearson's chi-square test (χ2) (for expected values greater than 10), Fisher's exact test (for expected frequencies >10), and Fisher's exact test (for expected frequencies <10). Differences at p<0.05 were considered statistically significant.
Results
We conducted a retrospective analysis of 230 women's delivery notes according to the above criteria. Patients were divided into 2 groups: Group 1 consisted of 200/230 (87%) pregnant women with FGR and Group 2 (control) consisted of 30/230 (13%) women with healthy pregnancies.
This study identified seven clinical phenotypes of FGR, depending on the underlying maternal, fetal, or placental conditions. They included "hypertensive disorders during pregnancy" in 21/200 (10.5%), "infections" in 27/200 (13.5%), "chronic maternal disease" in 19/200 (9.5%), "ART" in 13/200 (6.5%), "second and third trimester bleeding" in 5/200 (7.5%), "preterm delivery" in 93/200 (46.5%), "no baseline risk factors" in 12/200 (6.0%) pregnant women.
We found differences in baseline sociodemographic characteristics as well as in the course of pregnancy between the group with FGR and the control group. There were statistically significant differences in body mass index [p<0.001] and smoking [29.6% in the group with FGR versus 10.0% in controls; p=0.024] between groups.
Cesarean section rate in the FGR group was statistically significantly higher than in control group [36.7% versus 10.0%; p=0.004]. As expected, the FGR group had worse perinatal outcomes with higher perinatal mortality rates (Table 2).
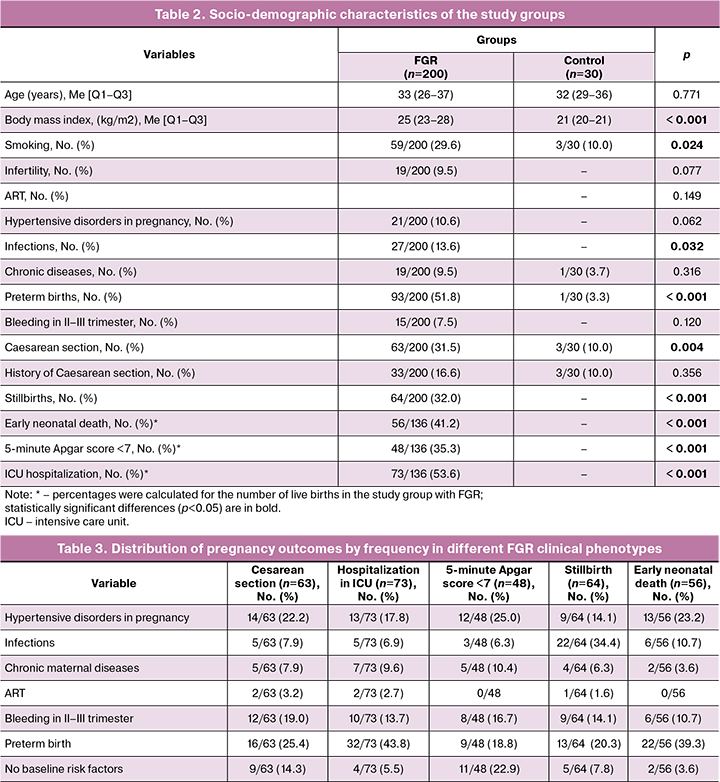
It should be noted that cases with the clinical phenotype "preterm birth" accounted for 51.8% of all observations, indicating the high significance of the combination of FGR and prematurity (especially extremely preterm, with a birth weight of 500–1499 g) with respect to multiplication of the risk of adverse perinatal outcomes. The remaining fetuses with FGR were evenly distributed across the remaining five clinical phenotypes. After reclassifying each case by clinical phenotype, we analyzed obstetric and perinatal outcomes (Table 3).
As can be seen from the study findings, cesarean sections (n=63) were performed for following indications: "preterm delivery" 16/63 (25.4%), "hypertensive disorders during pregnancy" 14/63 (22.2%), and "bleeding in the second to third trimesters of pregnancy" 12/63 (19.0%). It should be noted that the FGR phenotype "no baseline risk factors" accounted for 14.3% (9/63) of cesarean sections, indicating the difficulty in determining perinatal risk based only on clinical and anamnestic data. The lowest proportion of cesarean sections was in the "ART" phenotype (2/63, 3.2%). The distribution of newborns with FGR hospitalized in the ICU by phenotype was as follows: "preterm delivery" – 32/73 (43.8%), "hypertensive disorders during pregnancy" – 13/73 (17.8%), "bleeding in the II-III trimesters of pregnancy" – 10/73 (13.7%). The "no baseline risk factors" phenotype accounted for 4/73 (5.5%), and the "ART" phenotype accounted for the least, 2/73 (2.7%). The low Apgar score (less than 7 points) at 5 minutes was distributed by phenotype as follows: "hypertensive disorders during pregnancy" – 12/48 (25.0%), "no baseline risk factors" – 11/48 (22.9%) (which certainly suggests the need for special delivery approaches in FGR), "preterm delivery" – 9/48 (18.8%), "bleeding in II–III trimesters of pregnancy" – 8/48 (16.7%). Perinatal losses in different clinical phenotypes of FGR deserve special attention. Thus, the cases of stillbirth were distributed as follows: "infections" 22/64 (34.4%), "preterm delivery" 13/64 (20.3%), "hypertensive disorders during pregnancy" and "bleeding in the II–III trimesters of pregnancy" 9/64 observations (14.1% each). Cases of early neonatal deaths were distributed by frequency among the phenotypes as follows: "preterm birth" 22/56 (39.3%), "hypertensive disorders during pregnancy" 13/56 (23.2%), "infections" and "bleeding in II–III trimesters of pregnancy" 6/56 observations (10.7% each).
The rate of adverse perinatal outcomes was higher in the clinical phenotypes of FGR: "preterm delivery" 59.6%, "infections" 45.1%, "hypertensive disorders during pregnancy" 37.3%, and "bleeding in II and III trimesters" 24.8%.
Taking into account the different numbers of cases in the groups, we also performed a reverse analysis to determine the frequency of one or another outcome specifically in this group (Table 4, Figure).
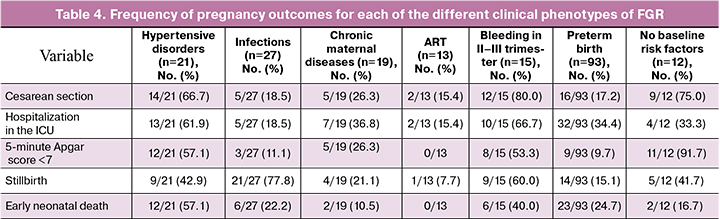
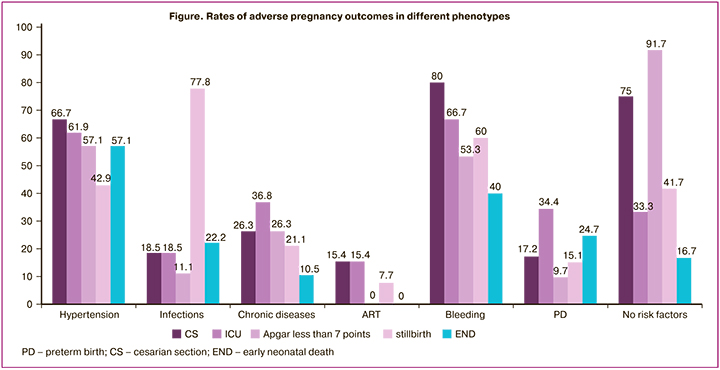
The phenotypes "bleeding in the second and third trimesters" and "hypertensive disorders during pregnancy" had the highest operative delivery (80.0%, 66.7%). However, the high rate of cesarean section in the phenotype of "no baseline risk factors” is noteworthy, with decompensation of placental insufficiency decompensation and fetal distress with delayed development being the most common indications, as this group also has the highest rate (11/12, 91.7%) of low Apgar score neonates. For this reason, more studies are required to stratify the risk of delivery in FGR, especially in the absence of an obvious background for fetal growth abnormalities (which are more often correlated with late manifestations of FGR). In the "infection" and "bleeding in the second and third trimesters" phenotypes, there was a high rate of stillbirth among adverse outcomes: 21/27 (77.8%) and 9/15 (60.0%), respectively. The phenotypes "hypertensive disorders in pregnancy," "bleeding in II and III trimesters," and "preterm birth" have the highest rates of early neonatal mortality: 12/21 (57.1%), 6/15 (40.0%), 23/93 (24.7%), respectively. The most favorable outcomes in FGR with lowest incidence of reproductive loss (only 1 case of stillbirth in severe FGR) and severe outcomes in our study were observed in the "ART" phenotype, most likely due to careful prenatal management at all stages of gestation.
Discussion
In our study, we identified seven clinical phenotypes of FGR associated with statistically significant different risk patterns for adverse perinatal outcomes. We proposed a practical phenotypic classification of FGR based on clinical data to improve early diagnosis and prevention of FGR. The combination of clinical data analysis with ultrasound findings will improve risk stratification and management strategy for pregnant women with FGR [19].
In a 2021 study, J. Villar et al. [20] identified 12 preterm birth phenotypes associated with different neonatal and neurodevelopmental outcomes before the age of 2 years. Based on data from 1274 patients, L.P. Chew et al. (2021) [15] identified 9 phenotypes of small for gestational age cases. The study group with small fetuses of gestational age had a 3-fold higher risk of perinatal mortality compared to the control group [1.4% vs. 0.4%; P<0.001]. 33.3% of fetuses with small for gestational age were classified as FGR and 13 of 18 (72%) perinatal deaths in this study occurred in this group. In the clinical phenotype "congenital anomalies," the rate of hospitalization in the ICU was [58.3%; OR=10.65, 95% CI 3.07–36.97] [15]. The authors suggested that outcomes such as preterm delivery [20] and fetus small for gestational age [15] could be better predicted based on the clinical state of the mother, fetus and placenta.
Our findings suggest that it is reasonable to identify clinical phenotypes with significantly increased risk of adverse perinatal outcomes: "preterm delivery", "hypertensive disorders during pregnancy", "infections" and "bleeding in the second and third trimesters", "chronic maternal diseases", "ART", "no baseline risk factors". We classified the clinical phenotypes of FGR into 3 risk models for adverse perinatal outcomes based on stillbirth and perinatal mortality.
High-risk according to the study results corresponds to the FGR clinical phenotypes "preterm delivery" (59.6%), "infections" (45.1%), "hypertensive disorders during pregnancy" (37.3%), and "bleeding in the second and third trimesters" (24.8%). The average risk was "chronic maternal disease" (9.9%), "no baseline risk factors" (11.4%), and the lowest risk model included the remaining clinical phenotypes. When analyzing the results, it should be noted that the risk models for FGR differ from the low-risk models for gestational age of the fetus. The model of high risk small for gestational age fetus includes "congenital anomalies" and "bleeding in the second and third trimesters"; the medium risk model includes "gestational diabetes mellitus" and "preterm birth"; the lowest risk model includes "hypertensive disorders during pregnancy", "chronic maternal disease", "ART" [14].
Ch.P. Bhale et al. (2021) [21] confirmed that hypertensive disorders during pregnancy proved to be the most common maternal factor associated with perinatal mortality (24.24%). FGR has the same genesis as hypertensive disorders during pregnancy. FGR can be isolated or combined with other placental-associated complications. The most frequent hypertensive disorders during pregnancy occur in the early form of FGR [16].
Placenta-associated complications (FGR and preeclampsia) are classified into early and late forms due to their different pathophysiology, histopathology, and pregnancy outcomes.
N. Gonen et al. (2021) [8] reported an interesting application of the phenotypic approach. They classified placental abruption into early and late forms, as placental abruption refers to placenta-associated complications. The results of this study showed that severe neonatal morbidity was associated with the early form of placental abruption [OR=5.3, 95% CI 3.9–7.6] [8].
Conclusion
As a result of our study, we identified seven clinical phenotypes of FGR associated with statistically significant different risk patterns for adverse perinatal outcomes. We classified FGR into three risk patterns for adverse perinatal outcomes: high, medium and low risk.
Considering the study results, it should be noted that we assigned each pregnant woman with FGR to a single clinical phenotype, although there may be several risk factors in a number of cases, indicating the need to consider the most significant.
Further multicenter prospective studies are needed to develop specific practical and clinical protocols and recommendations.
References
- Министерство здравоохранения Российской Федерации. Клинические рекомендации «Недостаточный рост плода, требующий предоставления медицинской помощи матери (задержка роста плода)». 2022. [Ministry of Health of Russia. Clinical guidelines. Insufficient growth the fetus, reguiring the provision of medical attention to the mother; 2022. (in Russian)].
- Gordijn S.J., Beune I.M., Thilaganathan B., Papageorghiou A., Baschat A.A., Baker P.N. et al. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet. Gynecol. 2016; 48(3): 333-9. https://dx.doi.org/10.1002/uog.15884.
- McCowan L.M., Figueras F., Anderson N.H. Evidence-based national guidelines for the management of suspected fetal growth restriction: comparison, consensus, and controversy. Am. J. Obstet. Gynecol. 2018; 218(2, Suppl.): S855-68. https://dx.doi.org/10.1016/j.ajog.2017.12.004.
- Волочаева М.В., Баев О.Р. Современные представления о патогенезе задержки роста плода. Акушерство и гинекология. 2021; 8: 13-7. [Volochaeva M.V., Baev O.R. Current views on the pathogenesis of fetal growth restriction. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 8: 13-7 (in Russian)]. https://dx.doi.org/10.18565/aig.2021.8.13-17.
- Ярыгина Т.А., Батаева Р.С. Прогнозирование рождения маловесного для гестационного возраста ребенка: оценка эффективности алгоритма Фонда медицины плода (Fetal Medicine Foundation) в первом триместре беременности. Ультразвуковая и функциональная диагностика. 2019; 2: 16-32. [Yarygina T.A., Bataeva R.S. Prediction of the birth of a child: evalution of the effectiveness of the algorithm Fetal Medicine Foundation (Fetal Medicine Foundation) in the first trimester of pregnancy. Ultrasound and Functional Diagnostics. 2019; 2: 16-32. (in Russian)].
- Тимохина Е.В., Стрижаков А.Н., Зафириди Н.В., Федюнина И.А., Асланов А.Г. Ранняя задержка роста плода: новый подход к выбору тактики ведения. Акушерство и гинекология. 2021; 9: 42-9. [Timokhina E.V., Strizhakov A.N., Zafiridi N.V., Fedyunina L.A., Aslanov A.G. Early fetal growth restriction: a new approach to guide the choice of management strategy. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 9: 42-9. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.9.42-49.
- Стрижаков А.Н., Мирющенко М.М., Игнатко И.В., Попова Н.Г., Флорова В.С., Кузнецов А.С. Прогнозирование синдрома задержки роста плода у беременных высокого риска. Акушерство и гинекология. 2017; 7: 34-44. [Strizhakov A.N., Miryushchenko M.M., Ignatko I.V., Popova N.G., Florova V.S., Kuznetsov A.S. Prediction of fetal growth restriction in high-risk pregnant women. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2017; (7): 34-44. (in Russian)]. https://dx.doi.org/10.18565/aig.2017.7.34-44.
- Gonen N., Levy M., Kovo M., Schreiber L., Noy L.K., Volpert E. et al. Placental histopathology and pregnancy outcomes in "early" vs. "late" placental abruption. Reprod. Sci. 2021; 28(2): 351-60. https://dx.doi.org/10.1007/s43032-020-00287-3.
- Alfirevic Z., Stampalija T., Dowswell T. Fetal and umbilical Doppler ultrasound in high-risk pregnancies. Cochrane Database Syst. Rev. 2017; 6(6): CD007529. https://dx.doi.org/10.1002/14651858.CD007529.pub4.
- Flores M., Glusman G., Brogaard K., Price N.D., Hood L. P4 medicine: how systems medicine will transform the healthcare sector and society. Per. Med. 2013; 10(6): 565-76. https://dx.doi.org/10.2217/pme.13.57.
- Paules C., Youssef L., Miranda J., Crovetto F., Estanyol J.M., Fernandez G. et al. Maternal proteomic profiling reveals alterations in lipid metabolism in late-onset fetal growth restriction. Sci. Rep. 2020; 10(1): 21033. https://dx.doi.org/10.1038/s41598-020-78207-3.
- Whitehead C.L., McNamara H., Walker S.P., Alexiadis M., Fuller P.J., Vickers D.K. et al. Identifying late-onset fetal growth restriction by measuring circulating placental RNA in the maternal blood at 28 weeks' gestation. Am. J. Obstet. Gynecol. 2016; 214(4): 521. https://dx.doi.org/10.1016/j.ajog.2016.01.191.
- Villar J., Papageorghiou A.T., Knight H.E., Gravett M.G., Iams J., Waller S.A. et al. The preterm birth syndrome: a prototype phenotypic classification. Am. J. Obstet. Gynecol. 2012; 206(2): 119-23. https://dx.doi.org/10.1016/j.ajog.2011.10.866.
- Ruiz-Martinez S., Delgado J.L., Paules C., Cavallaro A., De Paco C., Villar J. et al. Clinical phenotypes for risk stratification in small-for-gestational-age fetuses. Ultrasound Obstet. Gynecol. 2022; 59(4): 490-6. https://dx.doi.org/10.1002/uog.23765.
- Chew L.C., Verma R.P. Fetal growth restriction. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan.
- Хлестова Г.В., Карапетян А.О., Шакая М.Н., Романов А.Ю., Баев О.Р. Материнские и перинатальные исходы при ранней и поздней преэклампсии. Акушерство и гинекология. 2017; 6: 41-7. [Khlestova G.V., Karapetyan A.O., Shakaya M.N., Romanov A.Yu., Baev O.R. Maternal and perinatal outcomes in early and late preeclampsia. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2017; 6: 41-7. (in Russian)]. https://dx.doi.org/10.18565/aig.2017.6.41-7.
- Rawlinson W.D., Boppana S.B., Fowler K.B., Kimberlin D.W., Lazzarotto T., Alain S. et al. Congenital cytomegalovirus infection in pregnancy and the neonate: consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect. Dis. 2017; 17(6): e177-e188. https://dx.doi.org/10.1016/S1473-3099(17)30143-3.
- Министерство здравоохранения Российской Федерации. Клинические рекомендации «Преждевременные роды». 2020. [Ministry of Health of Russia. Clinical guidelines. Preterm birth. 2020. (in Russian)].
- Lees C.C., Stampalija T., Baschat A.A., da Silva Costa F., Ferrazzi E., Figueras F. et al. ISUOG Practice Guidelines: diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet. Gynecol. 2020; 56(2): 298-312. https://dx.doi.org/10.1002/uog.22134.
- Villar J., Restrepo-Méndez M.C., McGready R., Barros F.C., Victora C.G., Munim S. et al. Association between preterm-birth phenotypes and differential morbidity, growth, and neurodevelopment at age 2 years: results from the INTERBIO-21st Newborn Study. JAMA Pediatr. 2021; 175(5): 483-93. https://dx.doi.org/10.1001/jamapediatrics.2020.6087.
- Bhale Ch.P., Vare A., Gupta A. Fetal autopsy-categories and causes of death at a tertiary care center. Am. J. Forensic Med. Pathol. 2021; 42(1): 12-5. https://dx.doi.org/10.1097/PAF.0000000000000608.
Received 01.04.2022
Accepted 08.08.2022
About the Authors
Diana I. Yakubova, Post-Graduate Student, Department of Obstetrics, Gynecology and Perinatology of Institute of Clinical Medicine, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia (Sechenov University), +7(969)282-82-85, diana_28_03_94@mail.ru, https://orcid.org/0000-0002-7561-0706,8/2 Trubetskaya str., Moscow, 119991, Russian Federation.
Irina V. Ignatko, Corresponding Member of RAS, Dr. Med. Sci., Professor at the Department of Obstetrics, Gynaecology and Perinatology of Institute of Clinical Medicine,
I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia (Sechenov University), +7(910)461-73-02, ignatko_i_v@staff.sechenov.ru,
https://orcid.org/0000-0002-9945-3848, 8/2 Trubetskaya str., Moscow, 119991, Russian Federation.
Aren D. Megrabyan, Post-Graduate Student, Department of Obstetrics, Gynecology and Perinatology of Institute of Clinical Medicine, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia (Sechenov University), +7(910)499-99-98, arentek@mail.ru, https://orcid.org/0000-0002-8542-7630,
8/2 Trubetskaya str., Moscow, 119991, Russian Federation.
Irina M. Bogomazova, PhD, Associate Professor, Department of Obstetrics, Gynecology and Perinatology of Institute of Clinical Medicine, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia (Sechenov University), +7(926)305-04-03, bogomazova_i_m@staff.sechenov.ru, https://orcid.org/0000-0003-1156-7726,
8/2 Trubetskaya str., Moscow, 119991, Russian Federation.
Corresponding author: Irina V. Ignatko, ignatko_i_v@staff.sechenov.ru
Authors' contributions: Ignatko I.V., Yakubova D.I. – conception and design of the study; Yakubova D.I., Megrabyan A.D. – data collection, statistical analysis and visualization, manuscript drafting; Ignatko I.V., Yakubova D.I., Bogomazova I.M. – manuscript reviewing and editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was approved by the Research Ethics Committee of the I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University) (Protocol No. 03-20, dated February 19, 2020).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Yakubova D.I., Ignatko I.V., Megrabyan A.D., Bogomazova I.M. The course of pregnancy and childbirth outcomes in different fetal growth restriction phenotypes.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 8: 54-62 (in Russian)
https://dx.doi.org/10.18565/aig.2022.8.54-62



