Management of pregnancy and childbirth in women with peripartum cardiomyopathy
Objective: To investigate the clinical characteristics of patients with peripartum cardiomyopathy (PPCM) and the indications, timing, and mode of delivery.Zazerskaya I.E., Rudenko K.A., Osipova N.A., Karelkina E.V., Irtyuga O.B., Bautin A.E.
Materials and methods: This study retrospectively analyzed medical records of 16patients diagnosed with PPCM from 2012 to 2021, who were divided into those who developed PPCM before (n=10) and after (n=6) delivery. Results: PPCM manifested before delivery at 26-37 weeks in 62.5% of patients and 6 cases (37.5%) on days 1—9 postpartum. All patients with PPCM had left ventricular heart failure (HF) symptoms with an acute onset manifesting as NYHA functional class III/IV in 37.5% (n=6). Left ventricular ejection fraction (LVEF) at the onset of PPCM ranged from 19 to 45%, with a median of 38.5% (24.75%; 40%). Among the patients with PPCM onset before delivery (n=10), 100% were delivered by cesarean section. Of these, 70% had cardiovascular indications, and three patients (30%) underwent early delivery due to a significant decrease in LVEF with decompensated HF. Ten and 60% of the patients required emergency and urgent Cesarean section, respectively, and 30% had elective surgical delivery due to stable hemodynamics against the background of decreased LVEF. Two patients (12.5%) required mechanical circulatory support systems, and one (6.25%) required heart transplantation. In 87.5% of patients, LVEF was restored to > 45%; mortality was 6.25%.
Conclusion: Based on the analysis of PPCM clinical cases managed at the Almazov NMRC, indications for delivery were evaluated. Cardiovascular indications were identified in 70% of patients who had decompensated HF despite the ongoing therapy. Management of pregnancy and childbirth of women with PPCM is determined on a case-by-case basis; therefore, a generalization of the experience, based on the clinical cases of 16 patients, is of great importance for the further development of obstetric clinical protocols.
Keywords
Cardiovascular disease has become the leading cause of maternal mortality during pregnancy and the postpartum period, accounting for 2.24 deaths per 100 000 live births. More than half of deaths are due to ischemic heart disease, peripartum cardiomyopathy (PPCM), and dissecting aortic aneurysm [1]. PPCM is idiopathic cardiomyopathy manifesting as acute or slowly progressive heart failure (HF) due to left ventricular dysfunction with an ejection fraction < 45%, occurring late in pregnancy and within the first few months postpartum when any organic heart disease is excluded [2]. The diagnosis of PPCM is often delayed, as its symptoms initially mimic physiological changes in late pregnancy and the postpartum period, leading to significant maternal mortality [3]. The incidence of PPCM has recently increased partly to a greater awareness of this pathology [2]. The prevalence of PPCM varies by geographic region and its diagnostic criteria, with a prevalence of 1 per 1000–4000 pregnancies in the USA [4], 1:100 in South Africa, and 1:2000 in Japan [5].
The etiology and pathogenesis of PPCM are not yet fully understood. The most common risk factors are pre-eclampsia, age >35 years and adolescence, obesity, multiple pregnancies, and gestational diabetes mellitus [6, 7].
Even though recovery of left ventricular function occurs in most women in labor, adverse outcomes are not uncommon, including persistent left ventricular dysfunction, life-threatening atrial and ventricular arrhythmias, thromboembolic complications, and sudden cardiac death [8]. Maternal mortality due to PPCM, according to Kerpen et al. (2019), is 9%, with higher rates in developing countries (14%), compared with developed countries (4%) [9].
Due to high maternal mortality and cardiovascular complications rates, the European Society of Cardiology has established a register of patients with PPCM based on data from 49 countries, including 739 patients from 2012 to 2018 [10]. Currently, the Almazov NMRC is also developing a Russian registry of PPCM, which is quite difficult due to the lack of a unified medical database in our country.
The management of patients with PPCM, both prenatal and postpartum, remains an open issue due to the lack of accepted clinical guidelines for obstetricians and gynecologists [11], so it is particularly relevant to summarize the experience in the management of patients with this rare and diagnostically complex pathology.
This study aimed to investigate the clinical characteristics of patients with peripartum cardiomyopathy and the indications, timing, and mode of delivery.
Materials and methods
The study analyzed data of 36 pregnant and postpartum women with suspected PPCM, including 16 patients with a confirmed diagnosis of PPCM who were divided into those who developed PPCM before (n=10) and after (n=6) delivery.
The retrospective analysis included findings of instrumental investigations and laboratory tests for pregnant and postpartum women and fetuses to confirm the diagnosis, differentiate between them, and assess the severity of PPCM. Instrumental methods included transthoracic echocardiography with a Philips iU22 xMatrix ultrasound scanner, cardiac magnetic resonance imaging with gadolinium, cardiotocography, and maternal-fetal Doppler velocimetry. The laboratory testing included chemiluminescent microparticle immunoassay to detect troponin I and an electro-chemiluminescent microparticle immunoassay to detect N-terminal prohormone of B-type natriuretic hormone (NT-proBNP).
The study was approved by the local Research Ethics Committee (Almazov National Medical Research Centre, Ministry of Health of Russia).
Statistical analysis
Descriptive statistics were obtained using IBM SPSS Statistics software (version 28.0.0.0) and reported as median and interquartile range Me (Q1; Q3) because most variables did not meet the normality assumption (Kolmogorov–Smirnov test). Risks, relative risks (RRs), and their confidence intervals (CIs) were calculated for the study group concerning the group of all women in labor and delivery over the study period and the group of all women with somatic comorbidities [12, 13]. The critical level of significance when using Fisher's exact test was considered at p<0.05.
Results
Patients' age ranged from 19 to 46 years with a median of 34 years (23.5; 39). Two patients (2/16; 12.5%) had PPCM in previous pregnancies. Most patients were multigravida (12/16; 75%), 4/16 (25%) were primigravida; 7/16 (43.75%) were nulliparas and 9/16 (56.25%) were multiparas. On review of significant comorbidities, 7/16 (43.75%) were obese, 2/16 (12.5%) had severe anemia, one of them of autoimmune origin, and 6/16 (37.5%) had pre-eclampsia, of whom 1/16 (6.25%) had severe anemia.
The absolute risk of PPCM among all parturient women delivered in the Perinatal Centre of the Almazov NMRC between 2012 and 2021 was 0.068%. Among parturient women with somatic comorbidities and cardiovascular diseases, it was 0.074% and 0.156%, respectively. The risk of preeclampsia (including a severe one) among all parturient women was 12.7% (1.60%); among parturient women with somatic comorbidities and cardiovascular diseases, it was 13.8% (1.74%) and 29.1% (3.68%), respectively. The risk of preeclampsia in the PPCM group was 37.5%. The RR for preeclampsia in the PPCM group among all parturient women was statistically significant: RR=2.954, 95% CI [1.568; 5.566], and among those with comorbidities: RR=2.717, 95% CI [1.442; 5.120]. Among parturient women with cardiovascular diseases, RR=1.287, 95% CI [0.683; 2.425] and was not statistically significant. The RR for severe preeclampsia in the PPCM group was not significantly different from 1.0 relative to the two groups of parturient women.
PPCM manifested before and after labor in 10/16 (62.5%) and 6/16 (37.5%) women, respectively (Fig. 1).
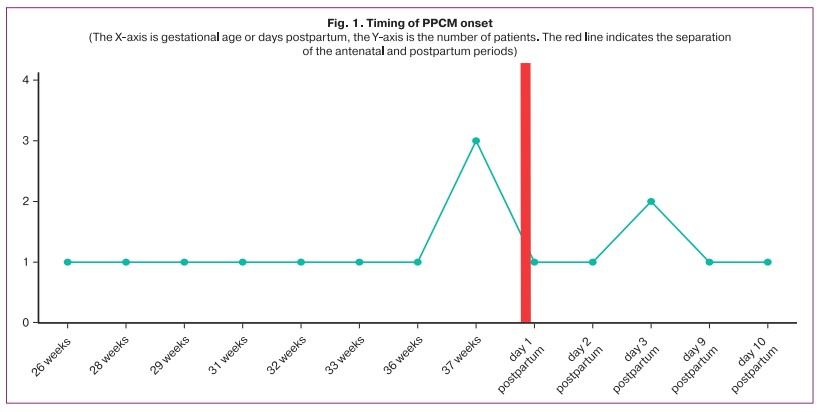
The most common manifestations of PPCM were left ventricular hypertension in 10/16 (62.5%), palpitations in 9/16 (56.25%), and dry cough in 3/16 (18.75%) patients. In 6/16 cases (37.5%), there was an acute onset of PPCM with dyspnea at rest.
According to the European Society of Cardiology classification (EOC, 2019) [14], 3/16 patients (18.75%) had mild, 5/16 (31.25%) moderate, and another 8/16 (50%) severe PPCM, respectively.
7/16 patients (43.75%) were admitted routinely to the pregnancy pathology unit, 4/16 (25%) were admitted to the adult perinatal anesthetic intensive care unit, 2/16 patients (12.5%) were admitted with regular labor to the labor ward; 3/16 patients (18.75%) were transferred to the central clinical intensive care unit from other maternity hospitals.
Saturation above 95% was observed in 13/16 patients (81.25%), and 3/16 patients (18.75%) required respiratory support.
Most patients had a fetal body size matching the gestational age (14/16; 87.5%). The grade 1 fetal growth restriction was diagnosed in 2/16 (12.5%) patients, the grade 1A fetoplacental blood flow insufficiency was diagnosed in 1/16 (6.25%), the grade 3 uteroplacental blood flow insufficiency was diagnosed in 1/16 (6.25%) in a patient with re-diagnosed PPCM and severe pre-eclampsia.
Left ventricular ejection fraction (LVEF) at presentation ranged from 19 to 45%, with a median of 38.5% (24.75; 40). The lowest LVEF was 11% in the patient whose case required transplantation.
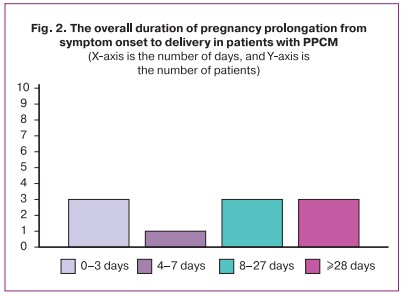
The median pregnancy prolongation (Fig. 2) from the onset of PPCM in patients with PPCM manifested before delivery (n=10) was 18 days (2; 49.5). The duration of pregnancy prolongation from admission to the hospital to delivery in patients with PPCM delivered for cardiovascular indications ranged from 0 to 7 days, with a median of 2 days (2; 6).
Transthoracic echocardiography showed pulmonary hypertension with elevated systolic pulmonary artery pressure of 47–62 mmHg in 7/16 patients (43.75%). Gadolinium enhancement cardiac magnetic resonance imaging in 16/16 (100%) patients showed dilatation of the left heart chambers with reduced left ventricular contractility and LVEF < 45%.
Assessment of biochemical parameters characterizing myocardial function revealed NT-proBNP levels ranging from 23.4 to ≥35000 pg/ml at the time of diagnosis, median being 591 pg/ml (109.8175; 1912.25); troponin I values ranged from 0 to 0.39 ng/ml, median being 0.02 ng/ml (0.0035; 0.06).
All patients with PPCM onset before delivery (n=10) underwent cesarean section, including 7/10 (70%) at full-term and 3/10 (30%) preterm. Three patients had obstetric indications, including emergency delivery due to grade II anterior asynclitism (1/10; 10%) and elective delivery (2/10; 20%) due to dichorionic diamniotic twins and uterine scar in patient G. and total placenta previa in patient Sh. Most patients (7/10; 70%) were delivered for cardiovascular indications, and a significant reduction in LVEF with decompensated HF was the cause of emergency delivery in 6/10 cases (60%), including 3/10 (30%) early delivery. One patient (10%) had elective delivery due to no emergency indications and stable hemodynamics in the setting of reduced LVEF (39%).
Among the postpartum PPCM patients (n=6), 2/6 (33.3%) had vaginal deliveries and 3/6 (50%) underwent emergency caesarean section; 1/6 (16.7%) woman underwent elective caesarean section. All surgical deliveries had obstetric indications. One patient had preterm birth due to a marginal placental abruption, multiple uterine fibroids, and regular labor; 5/6 patients (83.3%) had full-term delivery. One patient in this group (16.7%) had a placental abruption and antenatal fetal death outside the hospital.
Two patients (12.5%) underwent a hysterectomy. One patient (6.25%) had intraoperative bleeding with blood loss of 3700 ml. The second patient experienced a cardiac arrest during the operation, which required cardiopulmonary resuscitation, which resulted in 800 ml of intraoperative blood loss and ligation of the ascending branch of the uterine artery to achieve stable hemostasis. At the same time, the patient receiver extracorporeal membrane oxygenation (ECMO) with inevitable heparin administration, which led to recurrent bleeding and a total hysterectomy; the total blood loss was 1500 ml.
Surgical abdominal delivery was performed under regional anesthesia [7/16 epidural (43.75%) and 1/16 spinal (6.25%)] in conscious patients with spontaneous breathing. Five operations (31.25%) were performed under general anesthesia with endotracheal intubation. In all cases, invasive hemodynamic monitoring was used, including direct measurement of arterial pressure and central venous pressure. In 2/16 patients (12.5%), a Swan–Ganz catheter was placed in the pulmonary artery to measure pulmonary artery pressure and cardiac output.
Among patients with PPCM manifested before delivery (n=10), 5/10 (50%), 4/10 (40%), and 1/10 (10%) had Apgar scores of 8/9, 7/8, and 6/7, respectively. Among patients with PPCM manifested postpartum (n=6), 2/6 (33.3%), 1/6 (16.7%), and 2/6 (33.3%) had Apgar scores of 8/9, 8/8, and 7/8, respectively. One patient (16.7%) with placental abruption was 0/0 due to antenatal fetal death outside the hospital.
In the postoperative period, invasive hemodynamic monitoring continued in 5/16 patients (31.25%), including inotropic and vasoactive drugs (dopamine, dobutamine, epinephrine, norepinephrine). Five (31.25%) patients received a levosimendan infusion (Simdax, Orion, Finland) according to ESC recommendations (2018). The median length of stay in the intensive care unit was 6 (1; 9) days.
The majority (14/16; 87.5%) of cases ended favorably with the recovery of LVEF to values greater than 45% after one year; in one of the patients (6.25%), LVEF was assessed three months after the onset of PPCM and is currently 18%; one patient died. Thus, the mortality rate was 6.25%. Two (12.5%) patients with PPCM onset before delivery required mechanical circulatory support (ECMO via venoarterial circuit) in the postpartum period, and the ECMO was used for 19 and 54 days; one patient (6.25%) underwent orthotopic heart transplantation.
Seven (43.75%) patients were discharged from the Perinatal Center and were followed in outpatient settings; 8/10 (50%) patients were transferred to the cardiology department and subsequently discharged in satisfactory condition.
Discussion
The issue of elective delivery in patients with PPCM arises in all cases of this rare and complex pathology during pregnancy due to the severity and high fatality of PPCM. The differential and timely diagnosis of PPCM is an essential and crucial step in the approach to treatment and management [14], which determines the timing of delivery. PPCM most commonly manifests in the postpartum period [14]; however, a population-based study conducted in South Korea [6] reported that PPCM before delivery was diagnosed in 48%; the European Registry [10] reported that PPCM at the antenatal stage occurred in 34% of patients. In our study, PPCM manifested before delivery in 62.5% of patients, which, according to the guidelines for managing patients with PPCM [14], requires extra vigilance on the part of the obstetrician-gynecologist.
As the primary manifestations of PPCM are CH symptoms, the obstetrician may not pay due attention to the patient's complaints (dyspnea on exertion, palpitations), as these symptoms initially mimic physiological changes during pregnancy (fatigue, dyspnea on walking and exercise, weight gain) [2], especially in later pregnancy due to hypervolemia and increased fundal height, around the time when PCOS can manifest. Paroxysmal orthopnea and nocturnal dyspnea indicate left ventricular dysfunction and should be investigated further.
According to the literature [2, 10], PPCM most often manifests and is diagnosed in the presence of severe NYHA functional class III-IV HF. This observation is consistent with our findings, and it is likely to indicate that patients with initially milder manifestations are diagnosed only when HF progresses. Therefore, Sliwa K. [2] has proposed an algorithm for the work-up of a woman with suspected PPCM to guide the management of these patients (Fig. 3).
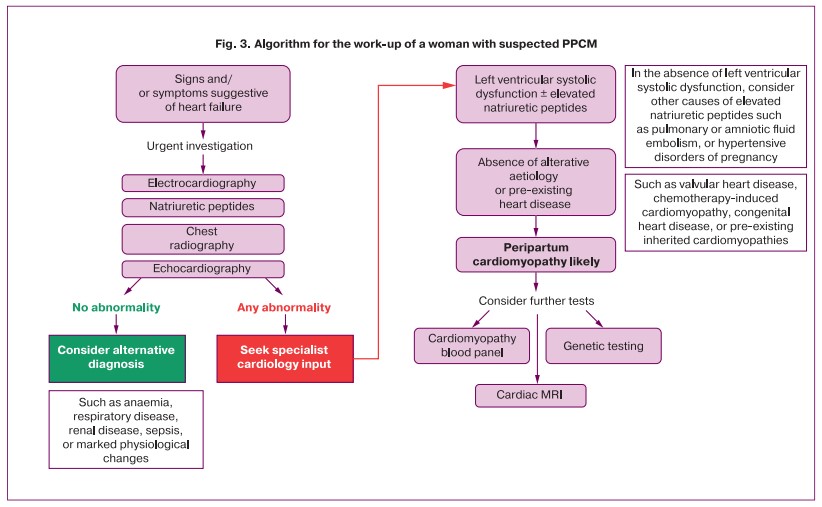
Although PPCM usually occurs late in pregnancy and postpartum [15], in one of our patients aged 19, PPCM developed in the second trimester of pregnancy. She had PPCM in her two previous pregnancies, and this time the patients had a fatal outcome. Notably, a woman's history of PPCM (particularly multiple episodes) complicates subsequent pregnancy planning. The modified WHO classification of maternal cardiovascular risk [16] indicates a significantly increased risk of maternal mortality or severe morbidity (mWHO III) in patients with the previous PPCM without residual left ventricular impairment. If there is any residual left ventricular impairment, as in this patient, pregnancy is contraindicated because of the extremely high risk of maternal death or severe complications,
According to Sliwa K. et al. (2021), the median LVEF at the time of diagnosis was 31% [2, 10]; in our study, the median LVEF was 38.5%, which is probably due to the extensive diagnostic facilities of our Center and the earlier diagnosis of PSCC. Gadolinium-enhanced cardiac MRI, recommended for the differential diagnosis of PPCM [3] primarily with inflammatory and ischemic genesis diseases, was performed in all our patients (100%) and revealed signs of left heart chamber myocardial dysfunction characteristic of PPCM. Gadolinium-enhanced cardiac MRI was conducted in the postpartum period, taking into account the limited excretion of gadolinium-based agents into breast milk.
Even though none of the laboratory biomarkers can be used in isolation to confirm the diagnosis of PBMCs, not being pathognomonic, measurement of NT-proBNP and troponin I was recommended to improve the diagnosis [14, 17], which was done in our patients. NT-proBNP levels exceeded the reference range in 75% of cases. Still, troponin I levels were elevated only in one patient (6.25%) to 0.39 ng/ml, confirming its low relevance for the isolated diagnosis of PPCM and its importance for differential diagnosis with the inflammatory and ischemic myocardial disease. The determination of 16-kDa prolactin, microRNA146a, and FMS-like tyrosine kinase levels in female patients are promising, but more studies are required [2].
Of note is the apparent contribution of pre-eclampsia as a risk factor for PPCM [18]. In our study group, the RR for pre-eclampsia in patients with PPCM was statistically significant (RR=2.954), consistent with other studies. According to a meta-analysis by Bello N. et al. (2013), the incidence of pre-eclampsia in patients with PPCM was more than 4-fold higher than in the population [19], confirming a close pathogenetic link between these diseases.
The decision about pregnancy prolongation, timing, and mode of delivery should be made jointly by cardiologists and obstetricians, and gynecologists [20]. In our study group, in patients with PPCM manifested before delivery, the prolongation of pregnancy was determined by the stability of maternal hemodynamics and the absence of cardiovascular indications for early or emergency delivery. Hemodynamics were assessed by saturation, blood pressure and pulse monitoring, echocardiography with LVEF measurement every three days, and response to treatment, which included diuretic therapy, beta-1-selective blockers, thromboembolism prevention, magnesia, and antihypertensive treatment, according to current guidelines [4, 5, 16, 21].
PPCM is associated with a significantly higher incidence of preterm delivery than the control group (25.4% versus 8.6%), according to Dhesi S et al. (2017) [22]. In our study, the maximum prolongation of pregnancy in patients with PPCM against the background of ongoing treatment in the hospital was seven days. However, 30% of patients delivered preterm due to destabilization of hemodynamics and decompensation, and 40% gave birth after 37 weeks of pregnancy.
Thus, if against the background of the therapy there was an increase in the functional class of CH, progressive reduction of LVEF, deterioration of laboratory parameters (including increased NT-proBNP, electrolyte imbalance), as well as hemodynamic instability and the need for inotropic support, the patient was delivered, regardless of gestational age, which is consistent with the experience of other researches [23].
The Russian Society of Cardiology (2018) has not identified cesarean section as a treatment of choice in the absence of obstetric indications [21]. Natural childbirth with epidural anesthesia and continuous hemodynamic monitoring is preferable in patients with stable hemodynamics. Besides, according to the observational study by Ruys T.P. et al. (2015), a planned cesarean section does not confer any advantage over planned vaginal delivery in terms of maternal outcome [24]. In our study, most patients had unstable hemodynamics with decompensated HF and worsening general condition; some had obstetric indications for operative delivery. Therefore, 100% of patients with PPCM manifested before delivery underwent cesarean delivery. According to Sliwa K. et al. (2020) [10], patients with PPCM before delivery are more likely to have cesarean section due to unstable hemodynamics, as in our study.
However, when choosing the mode of delivery, it should be borne in mind that surgical delivery in patients with PPCM is associated with a higher risk of bleeding, infection, and thromboembolic complications [4]. Besides, there is no evidence that elective cesarean section or labor induction is associated with improved perinatal outcomes and complete resolution of PPCM [25]. These observations are consistent with our results, as 20% of patients with PPCM onset before labor required ECMO in the postpartum period. There is also no substantial evidence that early delivery stops the progression of HF, improves LVEF [8], and reduces maternal risks [23]. After delivery, irrespective of its timing and mode, hemodynamic changes occur due to blood auto-transfusion of about 500 ml from the uteroplacental bed into the maternal systemic bloodstream, which increases preload and cardiac output [23]. Following delivery, removal of caval compression by the fetus, fluid mobilization, and resorption contributes to an increase in venous return. These factors should also be considered concerning the development of pulmonary edema in the postpartum period, control of water balance, and possible use of diuretics [4, 8].
A paradoxical finding in the dynamic assessment of the fetal state was that, despite increasing maternal HF, unstable hemodynamics, and low LVEF, there were only two cases of impaired uteroplacental blood flow and delayed fetal development. According to Maheu-Cadotte M.A. et al. (2019) [26], the severity of the maternal condition does not directly predict fetal and neonatal outcomes. However, PPCM is associated with increased adverse neonatal outcomes, including being born small for gestational age and lower Apgar scores at both 1 and 5 minutes [3, 23, 25]. However, among our patients with PPCM manifested before delivery, there was only one case with an Apgar score of 6/7 in a patient with repeat PPCM and severe pre-eclampsia who delivered at 26 6/7 weeks.
In the postpartum period, close monitoring of hemodynamic and urine output for 12–24 hours, preferably in the intensive care unit, is recommended by most authors [14, 23] and was also performed in 100% of our patients with PPCM manifested before labor. A levosimendan infusion was given in 31.25% of cases in the postpartum period. In a randomized clinical trial by Biteker M. et al. (2011) involving 24 women with PPCM, the addition of levosimendan to conventional therapy did not improve LVEF recovery in patients with PPCM [27]. However, in a newer study by Labbene I. et al. (2017), the use of levosimendan resulted in a significant rapid improvement in systolic function and stabilization of hemodynamics in patients with PPCM [28]. Besides, European guidelines recommend levosimendan as the preferred inotrope in patients with PPCM [16].
The prognosis of PPCM patients is more favorable compared to other cardiomyopathies (3). The rate of complete LVEF recovery varies, depending on the geographical region and definition of recovered LVEF (40, 45, 50%), which ranges from 28% in Haiti to 43% in Israel; 48% in Turkey, 47% in Germany, 55% in South Africa, 63% in Japan and 67% in Denmark; 23% to 72% in the US [3]. In our study, LVEF recovered to values above 45% in 87.5% of patients. This high rate is probably due to the integrated approach and multidisciplinary team involved in the treatment of patients with PPCM, as well as the dynamic postpartum follow-up. The time for recovery of left ventricular function also varies between countries [15], suggesting the importance of follow-up in outpatient settings, conducted in 93.75% of cases in our study.
According to the US registry (2004–2011), patients with PPCM require mechanical circulatory support in 1.5% of cases and cardiac transplantation in 0.5% [3]. According to Rasmusson K. et al. (2012), patients with PPCM have a higher graft rejection rate than the control group [29]. At the same time, according to a study by Bouabdallaoui N. et al. (2018), no significant difference was found concerning the incidence of transplant-related complications [30]. In our study, only one patient underwent bicaval orthotopic heart transplantation complicated by opportunistic infection, and two patients required ECMO. Both had high NT-proBNP values, severe PPCM with LVEF < 25% before delivery, and decompensated HF with cardiogenic pulmonary edema. One patient had massive blood loss (1500 ml) during cesarean section, and the other had severe pre-eclampsia with a poor response to antihypertensive therapy.
Conclusion
Peripartum cardiomyopathy is a rare pathology with a high complication rate and substantial maternal and neonatal morbidity and mortality. Based on the analysis of PPCM clinical cases managed at the Almazov NMRC, indications for delivery were evaluated. Cardiovascular indications were identified in 70% of patients who had decompensated HF despite the ongoing therapy. The severity of the patient condition was mainly attributable to the course of PPCM, and in 37.5% of the cases, it was contributed by pre-eclampsia. Management of pregnancy and childbirth of women with PPCM is determined on a case-by-case basis; therefore, a generalization of the experience, based on the clinical cases of 16 patients, is of great importance for the further development of obstetric clinical protocols.
References
- Knight M., Bunch K., Tuffnell D., Shakespeare J., Kotnis R., Kenyon S., Kurinczuk J.J., eds. Saving lives, improving mothers’ care: Lessons learned to inform maternity care from the UK and Ireland confidential enquiries into maternal deaths and morbidity 2016-18. MBRRACE-UK. 2020. Available at: https://www.npeu.ox.ac.uk/assets/downloads/mbrrace-uk/reports/maternal-report-2020/MBRRACE-UK_Maternal_Report_Dec_2020_v10_ONLINE_VERSION_1404.pdf
- Silwa K. Peripartum cardiomyopathy: from pathophysiology to management. Academic Press; 2021. 178p.
- Douglass E.J., Blauwet L.A. Peripartum cardiomyopathy. Cardiol. Clin. 2021; 39(1): 119-42. https://dx.doi.org/10.1016/j.ccl.2020.09.008.
- Davis M.B., Arany Z., McNamara D.M., Goland S., Elkayam U. Peripartum cardiomyopathy: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020; 75(2): 207-21. https://dx.doi.org/10.1016/j.jacc.2019.11.014.
- Honigberg M.C., Givertz M.M. Peripartum cardiomyopathy. BMJ. 2019; 364: k5287. https://dx.doi.org/10.1136/bmj.k5287.
- Lee S., Cho G.J., Park G.U., Kim L.Y., Lee T.-S., Kim D.Y. et al. Incidence, risk factors, and clinical characteristics of peripartum cardiomyopathy in South Korea. Circ. Heart Failure. 2018; 11(4): e004134. https://dx.doi.org/10.1161/CIRCHEARTFAILURE.117.004134.
- Zazerskaya I.E., Rudenko K.A., Talanina Ya.S., Karelkina E.V., Osipova N.A., Yakubov A.V. Risk Factors of Peripartum Cardiomyopathy. Doctor.Ru. 2021; 20(6): 20-5. (in Russian). https://dx.doi.org/10.31550/1727-2378-2021-20-6-20-25.
- Cruz M.O., Briller J., Hibbard J.U. New insights in peripartum cardiomyopathy. Obstet. Gynecol. Clin. North Am. 2018; 45(2): 281-98. https://dx.doi.org/10.1016/j.ogc.2018.02.002.
-
Kerpen K., Koutrolou-Sotiropoulou P., Zhu C., Yang J., Lyon J.A., Lima F.V., Stergiopoulos K. Disparities in death rates in women with peripartum cardiomyopathy between advanced and developing countries: A systematic review and meta-analysis. Arch. Cardiovasc. Dis. 2019; 112(3): 187-98.
https://dx.doi.org/10.1016/j.acvd.201.8.10.002.
- Sliwa K., Petrie M.C., van der Meer P., Mebazaa A., Hilfiker-Kleiner D., Jackson A.M. et al. Clinical presentation, management, and 6-month outcomes in women with peripartum cardiomyopathy: an ESC EORP registry. Eur. Heart J. 2020; 41(39): 3787-97. https://dx.doi.org/10.1093/eurheartj/ehaa455.
- Rudaeva E.V., Khmeleva I.A., Moses K.B., Moses V.G., Zakharov I.S., Elgina S.I. et al. Peripartum cardiomyopathy: epidemiology, pathophysiology, and management. Complex Issues of Cardiovascular Diseases. 2021; 10(1): 73-82. (in Russian)]. https://dx.doi.org/10.17802/2306-1278-2021-10-1-73-82.
- Lang T.A., Sesik M. How to describe statistics in medicine. A guide for authors, editors and reviewers. M.: Practical Medicine; 2016. 480 p. (in Russian).
- Malov S.V. Regression analysis: theoretical foundations and practical recommendations. Publishing House of St. Petersburg University; 2013. 276 p. (in Russian).
- Bauersachs J., König T., van der Meer P., Petrie M.C., Hilfiker-Kleiner D., Mbakwem A. et al. Pathophysiology, diagnosis and management of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Study Group on peripartum cardiomyopathy. Eur. J. Heart Fail. 2019; 21(7): 827-43. https://dx.doi.org/10.1002/ejhf.1493.
-
Sliwa K., Petrie M.C., Hilfiker-Kleiner D., Mebazaa A., Jackson A., Johnson M.R. et al. Long-term prognosis, subsequent pregnancy, contraception and overall management of peripartum cardiomyopathy: practical guidance paper from the Heart Failure Association of the European Society of Cardiology Study Group on Peripartum Cardiomyopathy. Eur. J. Heart Fail. 2018; 20(6): 951-62.
https://dx.doi.org/10.1002/ejhf.1178.
-
Regitz-Zagrosek V., Roos-Hesselink J.W., Bauersachs J., Blomström-Lundqvist C., Cífková R., De Bonis M. et al.; ESC Scientific Document Group. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur. Heart J. 2018; 39(34): 3165-241. https://dx.doi.org/10.1093/
eurheartj/ehy340.
-
Ersbøll A.S., Goetze J.P., Johansen M., Hauge M.G., Sliwa K., Vejlstrup N. et al. Biomarkers and their relation to cardiac function late after peripartum cardiomyopathy. J. Card. Fail. 2021; 27(2): 168-75. https://dx.doi.org/10.1016/
cardfail.2021.01.002.
- Ignatko I.V., Strizhakov L.A., Timokhina E.V., Afanasyeva N.V., Ryabova S.G. Peripartum cardiomyopathy and clinical masks of severe preeclampsia: Issues of differential diagnosis and management tactics. Obstetrics and Gynecology. 2017; 11: 114-22 (in Russian). https://dx.doi.org/10.18565/aig.2017.11.114-122.
- Bello N., Rendon I.S.H., Arany Z. The relationship between pre-eclampsia and peripartum cardiomyopathy: a systematic review and meta-analysis. J. Am. Coll. Cardiol. 2013; 62(18): 1715-23. https://dx.doi.org/10.1016/j.jacc.2013.08.717.
-
Zagelbaum N.K., Bhinder J., Gupta C.A., Frishman W.H., Aronow W.S. Peripartum cardiomyopathy incidence, risk factors, diagnostic criteria, pathophysiology, and treatment options. Rev. 2020; 28(3): 148-55.
https://dx.doi.org/10.1097/CRD.0000000000000249.
- Diagnosis and treatment of cardiovascular diseases during pregnancy 2018. National Guidelines. Russian Journal of Cardiology. 2018; 3: 91-134. (in Russian). https://dx.doi.org/10.15829/1560-4071-2018-3-91-134.
- Dhesi S., Savu A., Ezekowitz J.A., Kaul P. Association Between Diabetes During Pregnancy and Peripartum Cardiomyopathy: a population-level analysis of 309,825 women. Can. J. Cardiol. 2017; 33(7): 911-7. https://dx.doi.org/10.1016/j.cjca.2017.02.008.
- Ersbøll A.S., Damm P., Gustafsson F., Vejlstrup N.G., Johansen M. Peripartum cardiomyopathy: a systematic literature review. Acta Obstet. Gynecol. Scand. 2016; 95(11): 1205-19. https://dx.doi.org/10.1111/aogs.13005.
- Ruys T.P., Roos-Hesselink J.W., Pijuan-Domènech A., Vasario E., Gaisin I.R., Iung B. et al.; ROPAC investigators. Is a planned caesarean section in women with cardiac disease beneficial? Heart. 2015; 101(7): 530-6. https://dx.doi.org/10.1136/heartjnl-2014-306497.
- Arany Z., Elkayam U. Peripartum cardiomyopathy. Circulation. 2016; 133(14): 1397-409. https://dx.doi.org/10.1161/CIRCULATIONAHA.115.020491.
-
Maheu-Cadotte M.A., Pépin C., Lavallée A., Hupé C., Mailhot T., Duchaine C.,
Fontaine G.
CE: gestational hypertension, preeclampsia, and peripartum cardiomyopathy: A clinical review. Am. J. Nurs. 2019; 119(11): 32-40.https://dx.doi.org/10.1097/01.NAJ.0000605352.84144.a2.
- Biteker M., Duran N.E., Kaya H., Gündüz S., Tanboğa H.Î., Gökdeniz T. et al. Effect of levosimendan and predictors of recovery in patients with peripartum cardiomyopathy, a randomized clinical trial. Clin. Res. Cardiol. 2011; 100(7): 571-7. https://dx.doi.org/10.1007/s00392-010-0279-7.
-
Labbene I., Arrigo M., Tavares M., Hajjej Z., Brandão J.L., Tolppanen H.
et al. Decongestive effects of levosimendan in cardiogenic shock induced by postpartum cardiomyopathy. Anaesth. Crit. Care Pain Med. 2017; 36(1): 39-42. https://dx.doi.org/10.1016/j.accpm.2016.02.009.
- Rasmusson K., Brunisholz K., Budge D., Horne B.D., Alharethi R., Folsom J. et al. Peripartum cardiomyopathy: post-transplant outcomes from the United Network for Organ Sharing Database. J. Heart Lung Transplant. 2012; 31(2): 180-6. https://dx.doi.org/10.1016/j.healun.2011.11.018.
-
Bouabdallaoui N., Demondion P., Maréchaux S., Varnous S., Lebreton G., Mouquet F., Leprince P. Heart transplantation for peripartum cardiomyopathy: a single-center experience. Bras. Cardiol. 2018; 110(2): 181-7.
https://dx.doi.org/10.5935/abc.20180014.
Received 17.11.2021
Accepted 16.12.2021
About the Authors
Irina E. Zazerskaya, Dr. Med. Sci., Head of the Department of Obstetrics and Gynecology, Almazov National Medical Research Centre, Ministry of Health of the Russian Federation, +7(921)948-83-40, zazera@mail.ru, https://orcid.org/0000-0003-4431-3917, 197341, Russia, Saint-Petersburg, Akkuratova str., 2.Kseniia A. Rudenko, Clinical Resident, Research Technician at the Department of Obstetrics and Gynecology, Almazov National Medical Research Centre, Ministry of Health of the Russian Federation, +7(921)890-00-52, xeniaruru@yandex.ru, https://orcid.org/0000-0001-8498-7938, 197341, Russia, Saint-Petersburg, Akkuratova str., 2.
Natalia A. Osipova, Dr. Med. Sci., Associate Professor at the Department of Obstetrics, Gynecology and Reproductive Medicine, St. Petersburg University;
Head of Pathologic Pregnancy Department, Almazov National Medical Research Centre, Ministry of Health of the Russian Federation, naosipova@mail.ru, 197341, Russia, Saint-Petersburg, Akkuratova str., 2.
Elena V. Karelkina, Researcher at the Cardiomyopathy Research Laboratory, Almazov National Medical Research Centre, Ministry of Health of the Russian Federation, +7(921)917-60-57, ekarelkina@mail.ru, https://orcid.org/0000-0002-3655-9709, 197341, Russia, Saint-Petersburg, Akkuratova str., 2.
Olga B. Irtyuga, PhD, Associate Professor at the Department of Cardiology, Head of the Teaching of the Department of Cardiology, Almazov National Medical Research Centre of the Ministry of Health of the Russian Federation, +7(905)220-72-06, olgir@yandex.ru, https://orcid.org/0000-0002-8656-3191, 197341, Russia, Saint-Petersburg, Akkuratova str., 2.
Andrei E. Bautin, Dr. Med. Sci., Professor at the Department of Anesthesiology and Reanimatology, Head of the Research Laboratory of Anesthesiology and Reanimatology, Almazov National Medical Research Centre, Ministry of Health of the Russian Federation, +7(921)753-91-10, abautin@mail.ru, https://orcid.org/0000-0001-5031-7637, 197341, Russia, Saint-Petersburg, Akkuratova str., 2.
Corresponding author: Kseniia A. Rudenko, xeniaruru@yandex.ruAuthors' contributions: Zazerskaya I.E., Karelkina E.V. - concept and design of the study; Rudenko K.A. - data collection and analysis, statistical analysis; Rudenko K.A., Zazerskaya I.E., Karelkina E.V. - manuscript drafting; Bautin A.E., Irtyuga O.B., Osipova N.A. - manuscript editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: State Assignment No. 730000F. 99.1.BV10AA00006 (for 2021 and the planning period 2022 and 2023).
Acknowledgment: The authors express their gratitude to Victor A. Bart for his contribution to developing the study design and statistical analysis.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Zazerskaya I.E., Rudenko K.A., Osipova N.A., Karelkina E.V., Irtyuga O.B., Bautin A.E. Management of pregnancy and childbirth in women with peripartum cardiomyopathy.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 1:80-89 (in Russian)
https://dx.doi.org/10.18565/aig.2022.1.80-89



